Abstract
OBJECTIVE
To investigate glucose-dependent insulinotropic polypeptide (GIP) secretion in patients with type 2 diabetes and nondiabetic control subjects during oral glucose or meal tests.
RESEARCH DESIGN AND METHODS
Eligible trials were identified by The Cochrane Library, MEDLINE, Embase, and Web of Science. Data were retrieved and random-effects models for the primary meta-analysis, random-effects meta-regression, and subgroup and regression analyses were applied.
RESULTS
Random-effects meta-analysis of GIP responses in 23 trials during 28 different stimulation tests showed that patients with type 2 diabetes (n = 363) exhibited no significant differences (P = not significant) in peak plasma GIP, total area under the curve (tAUC), time-corrected tAUC (tAUC × min−1), and time-corrected incremental area under the curve (iAUC × min−1) in comparison with nondiabetic control subjects (n = 325) but had lower GIP responses as evaluated from iAUC (weighted mean difference, −648 pmol/L × min; 95% CI, −1,276 to −21). Fixed-effects models meta-analyses confirmed most of the results of the primary meta-analysis but showed iAUC × min−1 to be reduced and showed tAUC and tAUC × min−1 to be higher in diabetic patients. Random-effects meta-regression of the primary meta-analysis showed that age (peak GIP, tAUC, iAUC, and iAUC × min−1), BMI (tAUC, iAUC, and iAUC × min−1), and HbA1c (iAUC and iAUC × min−1) predicted some of the GIP outcomes. Post hoc subgroup analysis showed a negative influence of age and of HbA1c on GIP responses and showed a positive influence of BMI on GIP responses.
CONCLUSIONS
Our results suggest that patients with type 2 diabetes are characterized by preserved GIP secretion in response to oral glucose and meal tests. They also suggest that high BMI is associated with increased GIP responses but increasing age and HbA1c are associated with reduced GIP secretion.
Glucose-dependent insulinotropic polypeptide (GIP) is a 42-amino-acid hormone secreted by intestinal K cells, located predominantly in the proximal small intestine (duodenum and proximal jejunum), in response to luminal presence of nutrients (carbohydrate, protein, and fat) (1). After secretion, the two N-terminal amino acids of GIP are cleaved-off by the enzyme dipeptidyl peptidase 4 (DPP-4), and the hormone is then inactivated. DPP-4 is present in the intestinal and renal brush border membranes, in hepatocytes, in capillary endothelium, and it is also found in a soluble form in plasma. As a consequence of the ubiquity of DPP-4, only a small amount of the intestinally secreted GIP circulates in the systemic circulation as intact hormone (2). Intact 42-amino-acid peptide GIP [GIP (1–42)] is a potent stimulator of glucose-dependent insulin secretion in healthy humans (3,4). In contrast, in patients with type 2 diabetes the insulinotropic effect of GIP is severely impaired (5), presumably as a consequence of the diabetic state (6). Besides the renowned insulinotropic effect of GIP, the hormone also exerts glucagonotropic and adipogenic effects (7,8). Therefore, GIP could play a crucial role in determining some of the key pathophysiologic characteristics of the type 2 diabetic phenotype, e.g., impaired insulin secretion, hyperglucagonemia, and obesity. Moreover, changes in GIP secretion could contribute to the loss of incretin effect that invariably characterizes patients with type 2 diabetes (9). However, the vast amount of data regarding GIP secretion published during the past decades have been inconsistent and confusing, showing impaired, normal, or increased responses after oral glucose or mixed meals in patients with type 2 diabetes.
The aim of this meta-analysis was to systematically compile human GIP secretory data to compare the secretion of GIP in patients with type 2 diabetes and matched control individuals without diabetes.
RESEARCH DESIGN AND METHODS
Data sources and searches
Eligible trials were identified by electronic searches and manual searches in literature references. For the electronic searches, The Cochrane Library, MEDLINE, Embase, and Web of Science were reviewed. The search terms included “GIP,” “glucose-dependent insulinotropic polypeptide,” “gastric inhibitory peptide,” “secretion,” and “diabetes mellitus.” These terms were adjusted to fit the requirements specified in each database. A description of the full electronic search strategy is supplied in the Supplementary Data. No restrictions regarding the year of publication were applied. The last search update was 1 May 2012.
Study selection
Studies (6,10–30) investigating adult patients with type 2 diabetes and appropriate healthy control subjects (weight-matched nondiabetic subjects) reporting plasma total GIP responses after oral glucose tolerance test (OGTT) or meal test were included. Data from studies that used early nonspecific assays (5,31–39) were analyzed separately and were not included in the main meta-analyses. Studies that did not provide raw data (only figures) (40–42) or that dealt only with intact GIP (43–45) were excluded. Eligible trials were listed and all authors assessed the inclusion criteria independently. Excluded trials were listed with the reason for exclusion.
Data extraction and quality assessment
Two authors extracted data independently. GIP responses were reported as peak plasma levels or integrated responses (total area under curve [tAUC] or incremental area under curve [iAUC]). Also, time-corrected integrated responses for tAUC and iAUC (taking into account the duration of the individual meal test or OGTT in minutes) were assessed.
Data synthesis and analysis
Data analyses were performed using Stata version 11 (Stata Corp, College Station, TX) and Trial Sequential Analysis 2007 (Copenhagen Trial Unit, Copenhagen, Denmark). Random-effects models for the primary analysis were applied because clinical heterogeneity between trials was expected (because of different patient populations and intervention regimens). Results of the meta-analysis were expressed as weighted mean differences for continuous outcomes and relative risks for dichotomous outcomes, both with 95% CIs, and with I2 values as markers of intertrial heterogeneity. To test the robustness of the results after attributing less weight to small trials, the meta-analysis was repeated using fixed-effects models. Results of the fixed-effects meta-analysis were reported only if they differed from those of the random-effects models. Regression analysis of funnel plot asymmetry allowed assessment of publication bias and small study effects (Egger test). Random-effects meta-regression analysis was applied to investigate whether BMI, age, or HbA1c could predict the size of the estimated intervention effects. For variables predicting intervention effects, we performed post hoc subgroup analyses. Sensitivity analysis was performed to examine the strength of evidence.
RESULTS
Included studies
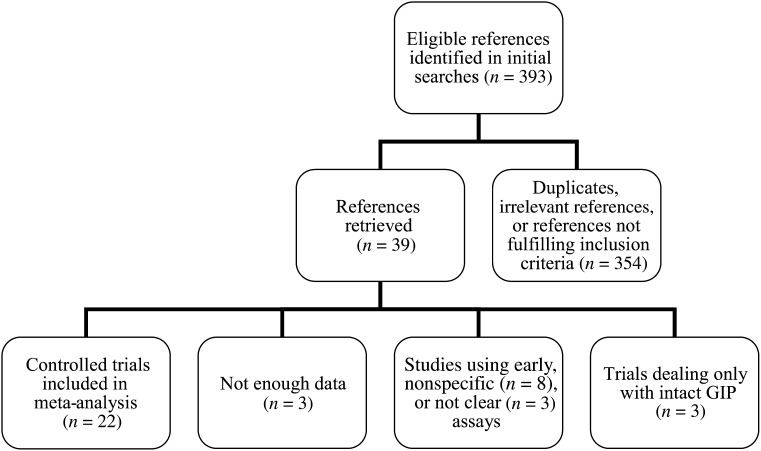
A flow chart for identification and selection of included studies is presented in Fig. 1. Of the retrieved references, 22 (6,10–30) (28 datasets) fulfilled our inclusion criteria (Supplementary Table 1). Total GIP was measured by antiserum R65 in 12 of the articles, by the ELISA kit (Millipore Corporation, Billerica, MA) in 7 articles, by the radioimmunoassay kit from (RIA kit; Phoenix Laboratories, Belmont, CA) in 2 articles, and by antibody S705, which recognizes 5-kD GIP, in 1 article. After contact with corresponding authors of retrieved articles, we received additional data that were not described in the published reports from four of the included trials (12,14,18,25). The included studies reported total GIP plasma responses from 15 OGTTs (one 25-g OGTT, one 40-g/m2 OGTT, four 50-g OGTTs, eight 75-g OGTTs, and one 125-g OGTT) and 13 mixed-meal tests (12 solid mixed-meal tests and 1 liquid mixed-meal test), ranging from 300 kcal to 833 kcal. Included studies were published as full paper articles between 1989 and 2012. Characteristics of the subjects and the diagnostic criteria for type 2 diabetes were similar across trials.
Figure 1.
Flow chart for identification and selection of included trials. Inclusion criteria included controlled studies of adult patients with type 2 diabetes (and control subjects without diabetes) evaluating postprandial or post–oral glucose GIP responses by providing peak plasma levels, integrated responses, or integrated incremental plasma responses of total GIP.
Subjects
We performed a random-effects meta-analysis of plasma GIP responses in the following 688 participants: 363 patients with type 2 diabetes (49% men; mean age, 54 years [range, 18–71]; BMI, 34 [range, 24–52]; fasting plasma glucose, 9.1 mmol/L [range, 5.3–12]; fasting plasma insulin, 101 pmol/L [range, 31–187]; HbA1c, 7.4% [range, 6.1–9.2] and 57 mmol/mol [range, 42.1–74.9]; fasting GIP, 18.8 pmol/L [range, 5.7–45]) and 325 nondiabetic controls (47% men; mean age, 50 years [range, 19–71]; BMI, 30 [range, 22–46]; fasting plasma glucose, 5.3 mmol/L [range, 4.6–6]; fasting plasma insulin, 62 pmol/L [range, 17–156]; HbA1c 5.4% [range, 3.6–6.5] and 31.1 mmol/mol [range, 9.3–42.1]; fasting GIP, 17.6 pmol/L [range, 4.5–49]).
Meta-analyses
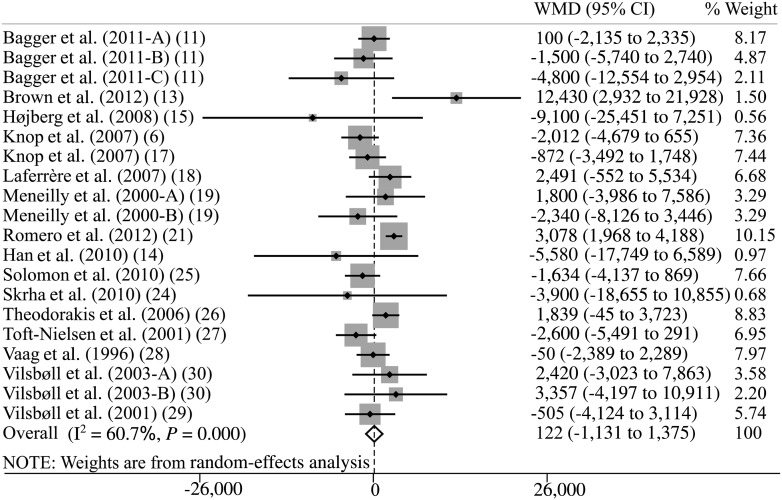
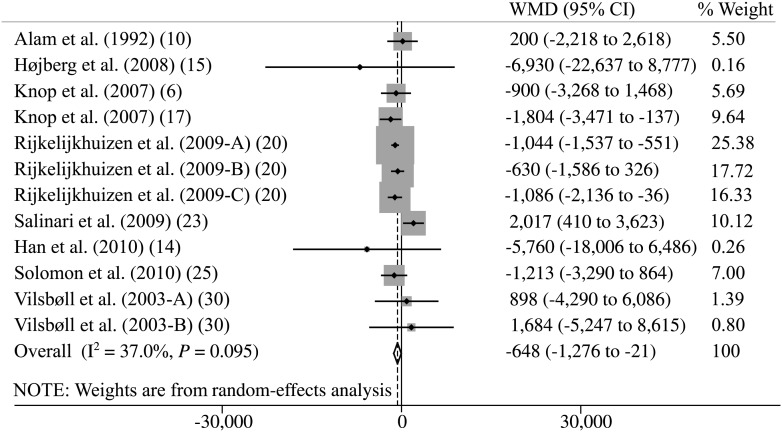
Random-effects meta-analysis of plasma GIP responses found that patients with type 2 diabetes exhibited increased fasting GIP levels (weighted mean difference, 2.6 pmol/L; 95% CI, 0.1–5.1), similar responses of total GIP in comparison with those of nondiabetic control subjects as evaluated from peak plasma concentrations (5.3 pmol/L; 95% CI, −7.6 to 18.2) (Supplementary Fig. 1), tAUC (122 pmol/L × min; 95% CI, −1,131 to 1,375; Fig. 2), time-corrected tAUC (tAUC × min−1; 4.1 pmol/L; 95% CI, −5.0 to 13.1), and time-corrected iAUC (iAUC × min−1; −4.2 pmol/L; 95% CI, −8.9 to 0.5). In contrast, we found lower responses of total GIP in patients with type 2 diabetes as evaluated from iAUC (−648 pmol/L × min; 95% CI, −1,276 to −21) (Fig. 3). Regression analysis did not show any clear evidence of bias or small study effects (Egger test, P > 0.1 for all analyses). A repeat of the initial meta-analysis with fixed-effects models (attributing less weight to smaller trials) confirmed most of the results of the primary meta-analysis but found lower GIP responses in patients with type 2 diabetes, as evaluated from iAUC × min−1 (−5 pmol/L; 95% CI, −7.6 to −2.5), and increased responses according to tAUC (938 pmol/L × min; 95% CI, 297–1,579) (Supplementary Fig. 2) and tAUC × min−1 (10 pmol/L; 95% CI, 6.3–13.7). We did not find any differences between the two stimuli investigated (OGTT and mixed-meal test) in GIP outcomes.
Figure 2.
Meta-analysis of total plasma GIP responses during an OGTT or meal test evaluated by tAUC (pmol/L × min) using random-effects model. WMD, weighted mean difference. Capital letters (A, B, C) indicate different GIP secretory stimuli in the same study.
Figure 3.
Meta-analysis of total plasma GIP responses during an OGTT or meal test evaluated by iAUC (pmol/L × min) using random-effects model. WMD, weighted mean difference. Capital letters (A, B, C) indicate different GIP secretory stimuli in the same study.
Random-effects meta-regression of the primary meta-analysis found that age predicted the outcomes peak plasma concentration, tAUC, iAUC, and iAUC × min−1; BMI predicted the outcomes tAUC, iAUC, and iAUC × min−1; and HbA1c predicted the outcomes iAUC and iAUC × min−1 (Supplementary Table 2).
Subgroup analyses
Post hoc subgroup analysis dividing the trials in two groups according to mean age of participants showed a negative influence of age on GIP responses in patients with type 2 diabetes evaluated by tAUC, iAUC, and iAUC × min−1 (Table 1). Subgroup 1 included trials with participants with mean age younger than 60 years (11,13–15,18,19,21,23,24,27,29,30); subgroup two included trials with participants with mean age 60 years or older (6,10,17,19,20,25,26,28).
Table 1.
Subgroup analysis
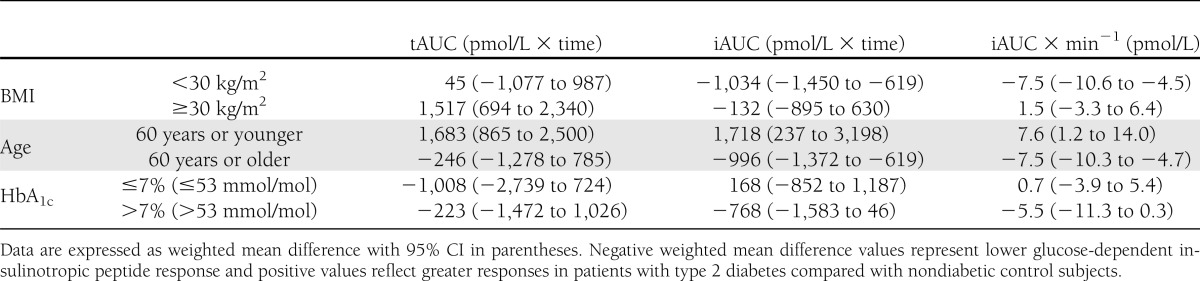
Post hoc subgroup analysis dividing the trials in two groups according to mean BMI levels showed a positive influence of BMI on tAUC, iAUC, and iAUC × min−1 in patients with type 2 diabetes (Table 1). Subgroup 1 included trials with participants with mean BMI <30 kg/m2 (6,11,14,17,20,24); subgroup two included trials with participants with mean BMI ≥30 kg/m2 (13,15,18,19,21,23,25–30).
Post hoc subgroup analysis dividing the trials in two groups according to mean HbA1c levels showed a negative influence of HbA1c levels on iAUC and iAUC × min−1 (Table 1). Subgroup one included trials with participants with mean HbA1c ≤7% (53 mmol/mol) (6,11,17,20,21,24,26); subgroup two included trials with mean HbA1c >7% (53 mmol/mol) (13–15,19,23,27,29,30).
Sensitivity analysis
Sensitivity analysis confirmed the results of random-effects meta-analysis, fixed-effects meta-analysis, and random-effects meta-regression, except that iAUC did not differ significantly between the groups in random-effects or fixed-effects meta-analyses (P > 0.05), and except that iAUC × min−1 did not differ significantly between the groups when using fixed-effects meta-analysis (P > 0.05).
Outdated assays
We performed a separate analysis including data from trials (32,34–36,39,46) using the older unspecific assays that recognize the 8-kD form of GIP. Random-effects and fixed-effects meta-analyses were performed with 332 participants (143 patients with type 2 diabetes and 179 healthy controls). The included studies reported total GIP plasma responses from 10 OGTTs (one 1-g/kg OGTT, one 40-g/m2 OGTT, two 50-g OGTTs, and six 75-g OGTTs) and 1 mixed-meal test. Random-effects meta-analysis of these plasma GIP responses found that patients with type 2 diabetes exhibited higher fasting GIP levels and higher responses of total GIP in comparison with those of nondiabetic control subjects as evaluated by peak plasma concentrations. Similar responses were observed in iAUC and iAUC × min−1 (Supplementary Table 3). A repeat of the initial meta-analysis with fixed-effects models confirmed the results of the primary meta-analysis and found significantly higher GIP responses in patients with type 2 diabetes as evaluated by iAUC and iAUC × min−1 (Supplementary Table 3).
CONCLUSIONS
The current study is the first extensive examination of human GIP secretory data from studies including patients with type 2 diabetes and matched healthy controls, and it provides evidence that patients with type 2 diabetes, in general, exhibit normal GIP secretion in response to OGTTs or meal tests. Moreover, high BMI, young age, and low HbA1c level seem to affect plasma GIP responses positively in patients with type 2 diabetes.
By combining heterogeneous studies, the present meta-analysis increased the numbers of observations and the statistical power of the individual trials and improved the estimates of the effect size of the intervention. The study population was characterized by wide ranges in age, BMI, and HbA1c levels, which in turn increases the validity of our meta-analysis. Another strength of the present approach is that data obtained using a broad spectrum of assays for the measurement of total GIP levels were included. Also, we were able to obtain unpublished data from four trials (12,14,18,25), which also added power to our analyses.
GIP is subject to degradation by the enzyme DPP-4 on its release (47). Therefore, total GIP (comprising the intact active form of GIP [GIP (1–42)] plus the DPP-4–inactivated metabolite GIP [GIP (3–42)]) represents the best and most reliable indicator of the overall secretory response of the K cells, increasing the possibility of detecting a small difference between responses in the peripheral circulation. Consequently, we excluded studies using only intact GIP, which is rapidly degraded by the enzyme DPP-4 on its release (elimination half-life ∼5 min in diabetic patients) (47), resulting in minor reliability of samples obtained from the systemic circulation.
Originally, GIP was detected as 5-kD and 8-kD forms in plasma (48). However, changes in the 8-kD form are small and inconsistent, and the main increase in the immunoreactive GIP after oral glucose or a meal is attributable to an increase in the 5-kD form (including both the intact hormone [GIP (1–42)] and the N-terminally cleaved GIP [GIP (3–42)]) (49), which is the best marker of secretion. Therefore, we excluded from the main analysis trials using the older unspecific assays that recognize the 8-kD form (5,31–39). Most of the studies excluded based on these considerations showed increased GIP secretion in diabetic patients and have generated the common notion that GIP secretion is higher in patients with diabetes than in matched nondiabetic subjects. To substantiate this notion, we performed a separate analysis including only data from trials using outdated assays (32,34–36,39,46); as expected, we found increased fasting GIP levels and poststimulatory (OGTT or meal test) peak GIP levels in patients with type 2 diabetes compared with nondiabetic control subjects. The iAUC and time-corrected iAUC also were higher in patients with diabetes when using fixed-effects models but not random-effect models. The reason for this discrepancy could be ascribed to the small number of trials included in this analysis and the significant differences in fasting plasma GIP values, which could mislead the interpretation of the overall response. The results of the main meta-analysis do not support these findings, although it cannot be ruled out that different inclusion criteria would have led to other conclusions. Nevertheless, the assays of the excluded trials are no longer employed in clinical research, whereas the assays used in the included studies in the main meta-analysis represent the current methods for the measurement of total GIP.
Significantly increased GIP release was only observed in two of the included studies (16,21). These studies evaluated GIP secretion in patients with newly diagnosed and well-regulated type 2 diabetes (16,21). This could explain why our fixed-effects model meta-analysis, which gives less weight to small trials, and some subgroup analyses found increased GIP responses evaluated by tAUC, iAUC, or time-corrected iAUC. However, our fixed-effects model should be interpreted with caution because of the large heterogeneity between the included studies. Consequently, the study effect size probably differs not only in the number of participants of each study (sampling error) but also in other unaccounted variables.
Many factors previously have been positively associated with GIP responses, including BMI (34,45), female sex (28), and metformin treatment (22). Unfortunately, we could not investigate the role of metformin or any other antidiabetic treatments because it was not clearly reported in most of the included studies. Romero et al. (21) (weighing heavily in our fixed-effects model analysis, showing higher GIP responses [tAUC] in patients with type 2 diabetes) included patients with type 2 diabetes characterized by younger age and higher BMI compared with those in the other trials. Interestingly, our meta-regression analysis revealed BMI as a predictor of GIP responses and post hoc subgroup analysis showed a positive influence of BMI on integrated plasma GIP responses. This could be a spurious finding because the groups were not well-matched according to BMI, i.e., the group of patients with type 2 diabetes had higher mean BMI compared with the group of healthy controls. However, we cannot rule out that this association, from a pathophysiological perspective, works in the opposite way, i.e., that high GIP responses result in increased BMI (perhaps via the adipogenic properties of GIP) (8), giving increased GIP levels a potentially important role in the development of obesity and insulin resistance (50). GIP has been shown to activate inflammatory responses in adipocytes by inducing secretion of inflammatory cytokines, chemokines, and proinflammatory nuclear factors (51). Thus, determining a causal role of GIP in the storage of fat and insulin resistance could be clinically pivotal for the management of obesity and insulin resistance disorders, including diabetes.
Our post hoc dichotomization of the compiled study populations according to age (younger than or older than 60 years) showed clear differences between subjects with type 2 diabetes and healthy control subjects. Thus, subgroup analysis revealed higher GIP secretion in younger patients with type 2 diabetes and lower GIP responses in elderly patients with type 2 diabetes, again when compared with controls without diabetes. It is well known that older age is a risk factor for impaired glucose tolerance, insulin resistance, and type 2 diabetes (52). Some studies have addressed how aging could differently affect GIP secretion in individuals with and without type 2 diabetes, but no clear conclusions have been made (19). Several things could explain our findings. First, contributing mechanisms could be defective activity of the autonomous nervous system, as seen in diabetic neuropathy and late-stage diabetes (53), or the slower gastric emptying, observed in some older patients with long-term diabetes (54). Both defective activity of the autonomous nervous system and reduced gastric emptying rate would probably correlate better with disease duration than age. However, because of inconsistently reported diabetes duration in the included studies, we were not able to perform subgroup analysis dividing the diabetic patients into groups according to duration of type 2 diabetes. Second, one could speculate whether the GIP response pattern could change with the development of type 2 diabetes from greater responses in younger patients (shorter duration of diabetes) to lower responses (K-cell “exhaustion”) when patients get older and have been compensating for progressive β-cell dysfunction (perhaps, partly, via increased GIP secretion) over a prolonged period of time. This idea is supported by the GIP results observed in the included studies with patients with a long duration of diabetes (17,24,27,30). A mechanism behind this phenomenon could stem from the possibility that hyperglycemia might lead to downregulation of GIP receptor expression on β-cells and GIP resistance (55). Unfortunately, the present data do not allow us to determine if GIP secretion differs in the early stages or in the late stages of type 2 diabetes.
Finally, the meta-regression analysis showed that HbA1c predicted GIP responses, and post hoc subgroup analysis showed that increasing levels of HbA1c are negatively associated with integrated incremental plasma GIP responses (iAUC and iAUC × min−1). Reduced iAUC was observed in patients with diabetes, who also were characterized by increased fasting plasma GIP levels (that contribute significantly to the calculation of iAUC) compared with healthy controls. The intrinsic variability related to iAUC and the small number of included articles using iAUC may explain why these data conflict with the overall preserved GIP response in diabetes.
In conclusion, patients with type 2 diabetes, in general, exhibit preserved GIP responses after OGTTs and meal tests in comparison with nondiabetic control subjects. High BMI, younger age, and low HbA1c level seem to affect plasma GIP responses positively in patients with type 2 diabetes. Thus, deterioration in GIP effect, rather than GIP secretion, seems to be the determining factor behind the loss of incretin effect that invariably characterizes patients with type 2 diabetes.
Acknowledgments
This study was supported by a grant from Fondazione della Societá Italiana di Diabetologia.
No potential conflicts of interest relevant to this article were reported.
S.C. contributed to study design, researched data, contributed to discussion, and wrote the manuscript. M.C. researched data, contributed to discussion, and reviewed and edited the manuscript. J.J.H. contributed to study design and discussion and reviewed and edited the manuscript. B.L. contributed to study design and discussion and reviewed and edited the manuscript. L.L.G. contributed to study design, researched data, contributed to discussion, and reviewed and edited the manuscript. T.V. contributed to study design and discussion and reviewed and edited the manuscript. F.K.K. designed the study, researched data, contributed to discussion, and reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Parts of this study were presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank S.J. Han (Department of Endocrinology and Metabolism, Ajou University School of Medicine, Wonchon-dong, Yeongtong-gu, Suwon, Gyeonggi-do, Republic of Korea), D.J. Kim (Department of Endocrinology and Metabolism, Ajou University School of Medicine, Wonchon-dong, Yeongtong-gu, Suwon, Gyeonggi-do, Republic of Korea), T.P.J. Solomon (Department of Pathobiology, Cleveland Clinic, Cleveland, Ohio), and J.P. Kirwan (Department of Pathobiology, Cleveland Clinic, Cleveland, Ohio) for contributing unpublished data from their studies for further analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0465/-/DC1.
References
- 1.Deacon CF, Ahrén B. Physiology of incretins in health and disease. Rev Diabet Stud 2011;8:293–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept 2005;128:117–124 [DOI] [PubMed] [Google Scholar]
- 3.Deacon CF, Plamboeck A, Rosenkilde MM, de Heer J, Holst JJ. GIP-(3-42) does not antagonize insulinotropic effects of GIP at physiological concentrations. Am J Physiol Endocrinol Metab 2006;291:E468–E475 [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Bartels E, Ørskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab 1993;76:912–917 [DOI] [PubMed] [Google Scholar]
- 5.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knop FK, Vilsbøll T, Højberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 2007;56:1951–1959 [DOI] [PubMed] [Google Scholar]
- 7.Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011;60:3103–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asmar M, Simonsen L, Madsbad S, Stallknecht B, Holst JJ, Bülow J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes 2010;59:2160–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holst JJ, Knop FK, Vilsbøll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011;34(Suppl. 2):S251–S257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam MJ, Kerr JI, Cormican K, Buchanan KD. Gastric inhibitory polypeptide (GIP) response in diabetes using a highly specific antiserum. Diabet Med 1992;9:542–545 [DOI] [PubMed] [Google Scholar]
- 11.Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsbøll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:737–745 [DOI] [PubMed] [Google Scholar]
- 12.Bose M, Teixeira J, Olivan B, et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes 2010;2:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care 2012;35:959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SJ, Kim HJ, Choi S-E, Kang Y, Lee KW, Kim DJ. Incretin secretion and serum DPP-IV activity in Korean patients with type 2 diabetes. Diabetes Res Clin Pract 2010;89:e49–e52 [DOI] [PubMed] [Google Scholar]
- 15.Højberg PV, Vilsbøll T, Zander M, et al. Four weeks of near-normalization of blood glucose has no effect on postprandial GLP-1 and GIP secretion, but augments pancreatic B-cell responsiveness to a meal in patients with type 2 diabetes. Diabet Med 2008;25:1268–1275 [DOI] [PubMed] [Google Scholar]
- 16.Jones IR, Owens DR, Luzio S, Williams S, Hayes TM. The glucose dependent insulinotropic polypeptide response to oral glucose and mixed meals is increased in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1989;32:668–677 [DOI] [PubMed] [Google Scholar]
- 17.Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia 2007;50:797–805 [DOI] [PubMed] [Google Scholar]
- 18.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meneilly GS, Demuth HU, McIntosh CH, Pederson RA. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet Med 2000;17:346–350 [DOI] [PubMed] [Google Scholar]
- 20.Rijkelijkhuizen JM, McQuarrie K, Girman CJ, et al. Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism 2010;59:502–511 [DOI] [PubMed] [Google Scholar]
- 21.Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc 2012;26:2231–2239 [DOI] [PubMed] [Google Scholar]
- 22.Ryskjaer J, Deacon CF, Carr RD, et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur J Endocrinol 2006;155:485–493 [DOI] [PubMed] [Google Scholar]
- 23.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009;32:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skrha J, Hilgertová J, Jarolímková M, Kunešová M, Hill M. Meal test for glucose-dependent insulinotropic peptide (GIP) in obese and type 2 diabetic patients. Physiol Res 2010;59:749–755 [DOI] [PubMed] [Google Scholar]
- 25.Solomon TPJ, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010;33:1561–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006;290:E550–E559 [DOI] [PubMed] [Google Scholar]
- 27.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 28.Vaag AA, Holst JJ, Vølund A, Beck-Nielsen HB. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol 1996;135:425–432 [DOI] [PubMed] [Google Scholar]
- 29.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001;50:609–613 [DOI] [PubMed] [Google Scholar]
- 30.Vilsbøll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 2003;88:2706–2713 [DOI] [PubMed] [Google Scholar]
- 31.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986;29:46–52 [DOI] [PubMed] [Google Scholar]
- 32.Korosi J, McIntosh CH, Pederson RA, et al. Effect of aging and diabetes on the enteroinsular axis. J Gerontol A Biol Sci Med Sci 2001;56:M575–M579 [DOI] [PubMed] [Google Scholar]
- 33.Crockett SE, Mazzaferri EL, Cataland S. Gastric inhibitory polypeptide (GIP) in maturity-onset diabetes mellitus. Diabetes 1976;25:931–935 [DOI] [PubMed] [Google Scholar]
- 34.Salera M, Giacomoni P, Pironi L, et al. Gastric inhibitory polypeptide release after oral glucose: relationship to glucose intolerance, diabetes mellitus, and obesity. J Clin Endocrinol Metab 1982;55:329–336 [DOI] [PubMed] [Google Scholar]
- 35.Coxe JS, O’Dorisio TM, Cataland S, Crockett SE. Gastric inhibitory polypeptide hypersecretion in diabetes mellitus: effect of sulfonylurea treatment. J Clin Endocrinol Metab 1981;52:1002–1005 [DOI] [PubMed] [Google Scholar]
- 36.Mazzaferri EL, Starich GH, Lardinois CK, Bowen GD. Gastric inhibitory polypeptide responses to nutrients in Caucasians and American Indians with obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985;61:313–321 [DOI] [PubMed] [Google Scholar]
- 37.Ross SA, Brown JC, Dupré J. Hypersecretion of gastric inhibitory polypeptide following oral glucose in diabetes mellitus. Diabetes 1977;26:525–529 [DOI] [PubMed] [Google Scholar]
- 38.Osei K, Falko JM, O’Dorisio TM, Fields PG, Bossetti B. Gastric inhibitory polypeptide responses and glucose turnover rates after natural meals in type II diabetic patients. J Clin Endocrinol Metab 1986;62:325–330 [DOI] [PubMed] [Google Scholar]
- 39.Reynolds C, Tronsgard N, Gibbons E, Blix PM, Rubenstein AH. Gastric inhibitory polypeptide response to hyper- and hypoglycemia in insulin-dependent diabetics. J Clin Endocrinol Metab 1979;49:255–261 [DOI] [PubMed] [Google Scholar]
- 40.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]
- 41.Fukase N, Manaka H, Sugiyama K, et al. Response of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide to glucose ingestion in non-insulin dependent diabetes mellitus. Effect of sulfonylurea therapy. Acta Diabetol 1995;32:165–169 [DOI] [PubMed] [Google Scholar]
- 42.Groop PH, Fyhrquist F, Groop LC. Effect of serial test meals on plasma immunoreactive GIP in non-insulin dependent diabetic patients and non-diabetic controls. Scand J Clin Lab Invest 1985;45:115–122 [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Yabe D, Nohtomi K, et al. Intact glucagon-like peptide-1 levels are not decreased in Japanese patients with type 2 diabetes. Endocr J 2010;57:119–126 [DOI] [PubMed] [Google Scholar]
- 44.Kozawa J, Okita K, Imagawa A, et al. Similar incretin secretion in obese and non-obese Japanese subjects with type 2 diabetes. Biochem Biophys Res Commun 2010;393:410–413 [DOI] [PubMed] [Google Scholar]
- 45.Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 2008;57:1340–1348 [DOI] [PubMed] [Google Scholar]
- 46.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986;63:492–498 [DOI] [PubMed] [Google Scholar]
- 47.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 2000;85:3575–3581 [DOI] [PubMed] [Google Scholar]
- 48.Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept 1984;9:35–46 [DOI] [PubMed] [Google Scholar]
- 49.Krarup T, Holst JJ, Larsen KL. Responses and molecular heterogeneity of IR-GIP after intraduodenal glucose and fat. Am J Physiol 1985;249:E195–E200 [DOI] [PubMed] [Google Scholar]
- 50.Nasteska D, Harada N, Yamane S, et al. Lowering GIP secretion has beneficial role in reducing obesity and insulin resistance without impairing glucose tolerance and osteogenesis. Diabetologia 2012;55(Suppl. 1):S80 [Google Scholar]
- 51.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg 2004;70:1–4; discussion 4–5 [PubMed] [Google Scholar]
- 52.Gong Z, Muzumdar RH. Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol 2012;2012:320482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrofsky J, Berk L, Al-Nakhli H. The influence of autonomic dysfunction associated with aging and type 2 diabetes on daily life activities. Exp Diabetes Res 2012;2012:657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Harding PE, Shearman DJ. Changes in gastric emptying rates with age. Clin Sci (Lond) 1984;67:213–218 [DOI] [PubMed] [Google Scholar]
- 55.Xu G, Kaneto H, Laybutt DR, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 2007;56:1551–1558 [DOI] [PubMed] [Google Scholar]