Abstract
OBJECTIVE
To compare efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled with metformin.
RESEARCH DESIGN AND METHODS
Adults with diabetes inadequately controlled (HbA1c 7–10%) with metformin were randomized to lixisenatide 20 μg once daily (n = 318) or exenatide 10 μg twice daily (n = 316) in a 24-week (main period), open-label, parallel-group, multicenter study. The primary objective was a noninferiority assessment of lixisenatide versus exenatide in HbA1c change from baseline to week 24.
RESULTS
Lixisenatide once daily demonstrated noninferiority in HbA1c reduction versus exenatide twice daily. The least squares mean change was −0.79% (mean decrease 7.97 to 7.17%) for lixisenatide versus −0.96% (mean decrease 7.96 to 7.01%) for exenatide, and treatment difference was 0.17% (95% CI, 0.033–0.297), meeting a predefined noninferiority upper CI margin of 0.4%. Responder rate (HbA1c <7.0%) and improvements in fasting plasma glucose were comparable. Both agents induced weight loss (from 94.5 to 91.7 kg and from 96.7 to 92.9 kg with lixisenatide and exenatide, respectively). Incidence of adverse events (AEs) was similar for lixisenatide and exenatide, as was incidence of serious AEs (2.8 and 2.2%, respectively). Discontinuations attributable to AEs occurred in 33 lixisenatide (10.4%) and 41 exenatide (13.0%) patients. In the lixisenatide group, fewer participants experienced symptomatic hypoglycemia (2.5 vs. 7.9%; P < 0.05), with fewer gastrointestinal events (especially nausea; 24.5 vs. 35.1%; P < 0.05).
CONCLUSIONS
Add-on lixisenatide once daily in type 2 diabetes inadequately controlled with metformin demonstrated noninferior improvements in HbA1c, with slightly lower mean weight loss, lower incidence of hypoglycemia, and better gastrointestinal tolerability compared with exenatide twice daily.
The glucagon-like peptide-1 (GLP-1) receptor system has become an attractive target for type 2 diabetes therapies (1–5). GLP-1 receptor agonists increasingly have become established as effective therapeutic options in type 2 diabetes management (6,7).
Glucose-lowering effects of GLP-1 receptor agonists are mediated by glucose-dependent stimulation of insulin release and inhibition of glucagon secretion, which decreases prandial blood glucose excursion and hepatic glucose production (1–5). Notably, GLP-1 receptor agonists achieve physiological blood glucose–insulin response with a low risk of hypoglycemia (as a result of their glucose-dependent action) (8), delay gastric emptying, and are associated with beneficial effects on weight and appetite reduction (9).
Currently available GLP-1 receptor agonists include twice-daily and once-weekly formulations of exenatide, a once-daily formulation of liraglutide, and a once-daily formulation of lixisenatide. Both exenatide and liraglutide have been shown to improve glycemic control associated with beneficial effects on weight and a low risk of hypoglycemia (10,11). However, although exenatide and liraglutide share the same basic mechanisms, each has a distinct pharmacokinetic profile and molecular structure, with potential clinical implications in terms of efficacy against fasting plasma glucose (FPG) and postprandial plasma glucose, and in terms of regimen burden and safety. This has been demonstrated in a 26-week, randomized, parallel-group, open-label trial in adults with inadequately controlled type 2 diabetes who were assigned to receive additional liraglutide 1.8 mg once daily or additional exenatide 10 µg twice daily (11). Liraglutide reduced mean FPG more than did exenatide (−29.0 mg/dL vs. −10.8 mg/dL; P < 0.0001), whereas exenatide reduced postprandial plasma glucose increment after breakfast and dinner more than did liraglutide (breakfast: estimated treatment difference, 23.9 mg/dL; P < 0.0001; dinner: estimated treatment difference, 18.2 mg/dL; P = 0.0005) (11). These findings suggest that liraglutide and exenatide should not be used interchangeably, but instead should be prescribed on an individual basis according to the glycemic requirements of each patient.
Lixisenatide is a once-daily prandial GLP-1 receptor agonist for the treatment of type 2 diabetes that was approved by the European Medicines Agency in February 2013 (12,13). It is a 44–amino-acid peptide that is amidated at the COOH terminal amino acid and shares some structural elements with the GLP-1 receptor agonist exenatide; the primary difference is the addition of six lysine residues at the C terminus (13). A 13-week, randomized, double-blind, placebo-controlled, dose-ranging study that evaluated the dose-dependent effects of lixisenatide (5, 10, 20, or 30 µg once daily or twice daily) found that lixisenatide 20 µg administered once daily provided the best efficacy-to-tolerability ratio, with no additional benefits with any of the twice-daily doses (14). Lixisenatide 20 μg once daily subsequently has been shown to significantly improve glycemic control, with low rates of hypoglycemia and beneficial weight effects, when administered as monotherapy (15), as add-on therapy to oral agents (14,16–18), and in combination with basal insulin with or without oral antidiabetic therapy (19–21).
In the current study, we report the results from a head-to-head study (GetGoal-X) that compared the benefit/risk profile of lixisenatide once daily versus exenatide twice daily in patients with type 2 diabetes inadequately controlled with metformin monotherapy.
RESEARCH DESIGN AND METHODS
Study design
This was a 24-week, phase III, randomized, parallel-group, open-label, multicenter, multinational, noninferiority study followed by a long-term safety extension of at least 52 weeks (data not reported here). The study was conducted at 122 centers in 18 countries (Argentina, Austria, Brazil, Colombia, Denmark, Finland, Germany, Greece, Hungary, Italy, the Netherlands, Norway, Poland, Puerto Rico, Russian Federation, Spain, Sweden, and United States) from June 2008 to November 2010. The study was approved by the local Institutional Review Boards or Ethics Committees and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants gave written informed consent.
Participants
Men and women aged 21–84 years with type 2 diabetes receiving ≥1.5 g/day metformin and with HbA1c 7–10% (between 53 and 86 mmol/mol) were included in the study. The main exclusion criteria were as follows: use of oral or injectable glucose-lowering agents other than metformin within 3 months before the time of screening; FPG at screening >13.9 mmol/L (250 mg/dL); history of unexplained pancreatitis, chronic pancreatitis, pancreatectomy, stomach/gastric surgery, or inflammatory bowel disease; history of metabolic acidosis, including diabetic ketoacidosis, within 1 year before screening; history within the previous 6 months of myocardial infarction, stroke, or heart failure requiring hospitalization; and clinically relevant history of gastrointestinal disease, with prolonged nausea and vomiting during the previous 6 months.
Randomization
After a screening period of up to 2 weeks, participants were randomized in a 1:1 ratio to receive either lixisenatide once daily or exenatide twice daily using a centralized randomization through an interactive voice-response system. Treatment numbers were allocated using an interactive voice-response system managed by S-CLINICA and according to a predefined randomization list.
The following stepwise dose increases were used in both groups to a maintenance dose of 20 μg/day: lixisenatide 10 μg once daily for 1 week, 15 μg once daily for 1 week, and then 20 μg once daily; and exenatide 5 μg twice daily for 4 weeks and then 10 μg twice daily. Treatments were administered within 1 h before the morning meal (lixisenatide) or before the morning and evening meals (exenatide). Participants were stratified by screening values of HbA1c (<8%, ≥8%) and BMI (<30 kg/m2, ≥30 kg/m2).
Efficacy and safety outcomes
All efficacy parameters were assessed in the prespecified modified intent-to-treat population, which consisted of all randomized participants who received at least one dose of open-label investigational product and had both a baseline assessment and at least one postbaseline assessment for any primary or secondary efficacy variables. The primary efficacy end point was the absolute change in HbA1c from baseline to week 24. HbA1c was measured at a National Glycohemoglobin Standardization Program Level 1–certified central laboratory using a high-performance liquid chromatography method. The secondary efficacy measures included the percentage of participants attaining HbA1c <7.0% or ≤6.5% at week 24 and changes in FPG and body weight from baseline to week 24.
The safety population comprised all randomized participants exposed to at least one dose of the investigational product. Safety and tolerability were assessed by review of adverse events (AEs) and, in particular, treatment-emergent AEs, occurrence of symptomatic hypoglycemia, and clinical laboratory data. Symptomatic hypoglycemia was defined as symptoms consistent with hypoglycemia, with accompanying blood glucose <3.3 mmol/L (60 mg/dL) and/or prompt recovery with oral carbohydrate, glucagon, or intravenous glucose. Severe hypoglycemia was defined as symptomatic hypoglycemia in which the subject required the assistance of another person and that was associated with either a plasma glucose level <2.0 mmol/L (36 mg/dL) or, if no plasma glucose measurement was available, prompt recovery with intravenous glucose, glucagon, or oral carbohydrate administered by a third party. Laboratory tests were performed for hematology, creatinine, microalbuminuria, and serum chemistry, including amylase, lipase, and calcitonin. The impact of gastrointestinal tolerability on quality of life was evaluated by the Patient Assessment of Upper Gastrointestinal Disorders–Quality of Life (PAGI-QOL) questionnaire.
Statistical analyses
The primary end point was analyzed using ANCOVA with treatment group, screening strata for HbA1c and BMI, and country as fixed effects, and with baseline HbA1c as covariate. Differences in the least squares (LS) mean change in HbA1c between lixisenatide and exenatide and two-sided 95% CIs were estimated within the framework of ANCOVA. Noninferiority between lixisenatide and exenatide was assessed based on a predefined noninferiority criterion (≤0.4% for the upper limit of the 95% CI). The 0.4% margin was selected in accordance with the Committee for Medicial Products for Human Use (CHMP)/International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)/363/96 and CHMP/ICH/364/96 (22,23), as well as in accordance with the Food and Drug Administration 2008 guidelines on statistical principles for clinical trials (24). It is commonly used and accepted in noninferiority studies of compounds developed for the treatment of type 2 diabetes (25,26).
A sample size of 600 (300 participants in each group) was calculated to ensure that the upper limit of the two-sided 95% CI for the adjusted LS mean difference between lixisenatide and exenatide would not exceed 0.4% HbA1c with 96% power (assuming that the SD was 1.3 and the true difference between lixisenatide and exenatide was zero). The last observation carried forward (LOCF) procedure was used to handle missing assessments or early discontinuation during the study treatment period. No predefined formal statistical tests were performed for any secondary efficacy end points. Exploratory analyses of continuous secondary end points were performed using an approach and ANCOVA model similar to those described for the primary analysis.
RESULTS
Demographics, baseline characteristics, and patient disposition
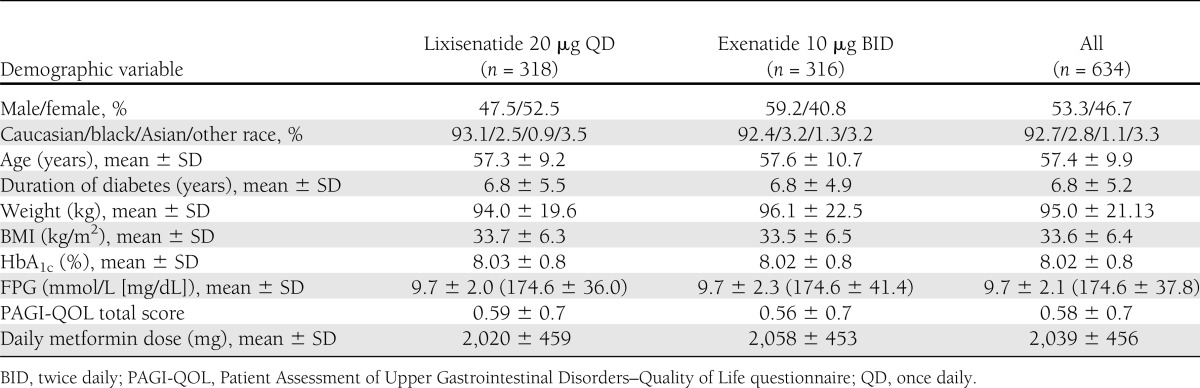
A total of 1,243 patients were screened and 639 eligible patients were randomized to study treatment (Supplementary Fig. 1). The main reason for screening failure was an HbA1c value outside of the defined protocol range at the screening visit. Before database lock, it was decided to exclude five patients from all analyses because of serious noncompliance with the protocol. In total, 634 patients were included in the analyses. Demographic and baseline characteristics of the lixisenatide (n = 318) and exenatide (n = 316) treatment groups were comparable, apart from a slight sex imbalance (Table 1). The majority of patients (92.7%) in the lixisenatide and exenatide groups were Caucasian and the mean age of the study population was 57.4 years. The mean duration of known diabetes was ∼6.8 years.
Table 1.
Demographic and baseline characteristics (safety population)
The majority of patients (n = 548 [86.4%]) completed the 24-week main treatment period (Supplementary Fig. 1). In total, 86 participants (41 [12.9%] lixisenatide, 45 [14.2%] exenatide) discontinued treatment prematurely. A total of 74 patients had AEs that led to premature treatment discontinuation: 33 (10.4%) in the lixisenatide group and 41 (13.0%) in the exenatide group. In the lixisenatide group, 93% of patients (n = 295) demonstrated tolerance and continued with the target total daily dose of 20 μg at week 24 compared with 85% (n = 268) in the exenatide group.
Efficacy
HbA1c.
Lixisenatide once daily achieved the primary efficacy objective of noninferiority to exenatide twice daily in terms of HbA1c reduction from baseline to week 24 (Fig. 1A). Mean (±SD) HbA1c decreased from 7.97% (±0.82) to 7.17% (±0.96%) with lixisenatide and from 7.96% (±0.77) to 7.01% (±0.88) with exenatide. The LS mean (±SE) HbA1c reduction at week 24 (LOCF) was −0.79% (±0.05) for lixisenatide versus −0.96% (±0.05) for exenatide, and the LS mean change difference between the two groups was 0.17% (95% CI, 0.033–0.297). Thus, the upper limit of the 95% CI met the predefined noninferiority margin of 0.4%. A stricter margin of 0.3% also would have been met if this threshold had been chosen. A similar proportion of patients in each group achieved HbA1c goals of <7.0% at week 24 (48.5% lixisenatide and 49.8% exenatide); the number with HbA1c ≤6.5% was 28.5% in the lixisenatide group compared with 35.4% in the exenatide group (Supplementary Fig. 2).
Figure 1.
Efficacy outcomes from baseline to LOCF. Mean change in HbA1c from baseline to week 24 (A), mean FPG (mmol/L) by visit (B), and changes in absolute body weight over 24 weeks (C). BID, twice daily; QD, once daily.
Fasting plasma glucose.
Lixisenatide once daily and exenatide twice daily provided comparable reductions in FPG from baseline (Fig. 1B). Mean (±SD) FPG decreased from 9.7 (±2.0) to 8.4 (±2.0) mmol/L (174.6 ± 36.0 to 151.2 ± 36.0 mg/dL) with lixisenatide (n = 315) and from 9.7 (±2.3) to 8.2 (±2.1) mmol/L (174.6 ± 41.4 to 147.6 ± 37.8 mg/dL) with exenatide (n = 315). The LS mean (±SE) FPG reduction at week 24 (LOCF) was −1.22 (±0.12) mmol/L (−21.9 ± 2.16 mg/dL) for lixisenatide compared with –1.45 (±0.12) mmol/L (−26.1 ± 2.16 mg/dL) for exenatide, and the LS mean change difference between the two groups was 0.23 mmol/L (4.14 mg/dL; 95% CI, −0.052 to 0.522).
Body weight.
Body weight decreased from baseline in both the lixisenatide once-daily group and exenatide twice-daily group. Mean (±SD) body weight decreased from 94.5 (±19.4) to 91.7 (±18.9) kg with lixisenatide and from 96.7 (±22.8) to 92.9 (±22.3) kg with exenatide (Fig. 1C). The LS mean (±SE) body weight reduction at week 24 (LOCF) was −2.96 (±0.23) kg for lixisenatide compared with −3.98 (±0.23) kg for exenatide. The LS mean change difference between the two groups was 1.02 kg (95% CI, 0.456–1.581). Overall, 25.1% of lixisenatide-treated patients and 31.4% of exenatide-treated patients had ≥5% weight loss from baseline to week 24.
Safety and tolerability
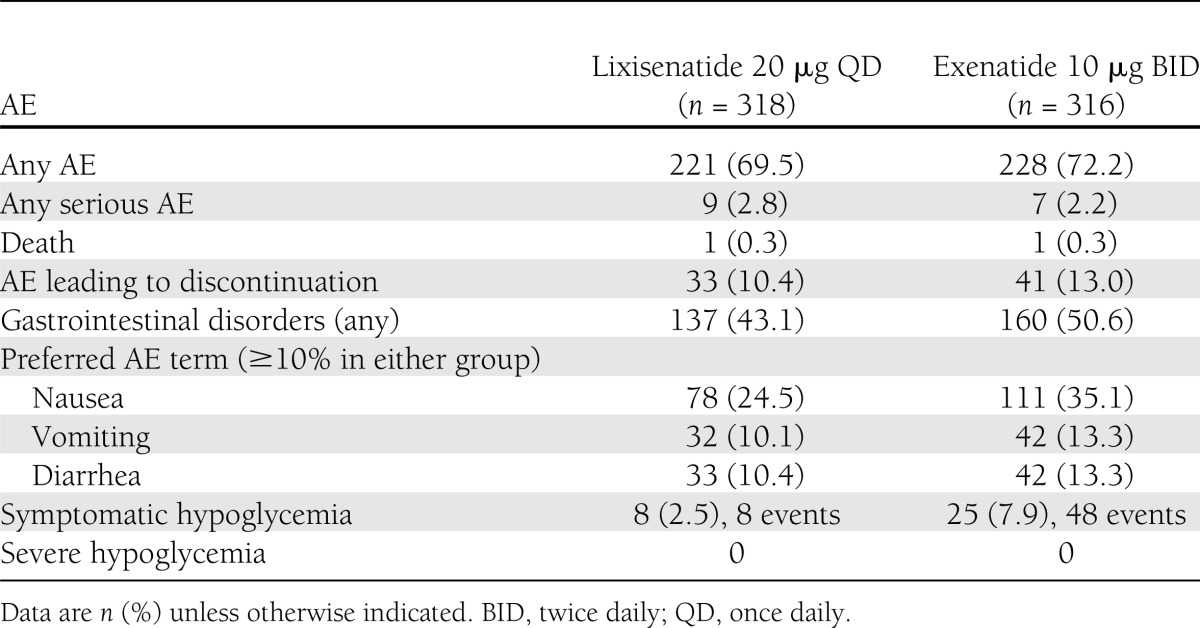
During the 24-week main treatment period, the overall rates of AEs and serious AEs were similar in the lixisenatide once-daily group and exenatide treatment twice-daily group (Table 2). Discontinuations attributable to AEs (mainly because of gastrointestinal events) were slightly higher in the exenatide treatment group. The most common AEs in both groups were gastrointestinal in nature, mainly nausea (Table 2). Gastrointestinal events were less frequent in the lixisenatide once-daily group (Table 2), with significantly fewer participants reporting nausea compared with the exenatide twice-daily group (24.5% vs. 35.1%, respectively; P < 0.05). In general, events of nausea were reported more frequently during the first 3 weeks of treatment in the lixisenatide group and during the first 5 weeks of treatment in the exenatide group; most of the events of nausea resolved during the first 8 weeks of treatment. Overall, gastrointestinal symptoms led to treatment discontinuation for 20 participants (6.3%) in the lixisenatide group and 24 participants (7.6%) in the exenatide group; specifically, nausea and vomiting led to treatment discontinuation for 16 (5.0%) and 20 (6.3%) participants, respectively.
Table 2.
Safety profile during the 24-week, double-blind treatment period
Mean total Patient Assessment of Upper Gastrointestinal Disorders–Quality of Life score improved slightly from 0.60 ± 0.7 to 0.49 ± 0.64 with lixisenatide and from 0.56 ± 0.7 to 0.50 ± 0.67 with exenatide, with no significant between-group difference (LS mean change: −0.09 vs. −0.06, respectively; LS mean change difference: −0.03%; 95% CI, −0.111 to 0.043).
Significantly fewer participants experienced symptomatic hypoglycemia in the lixisenatide once-daily group compared with the exenatide twice-daily group (2.5 vs. 7.9%; P < 0.05) and no severe hypoglycemic events were reported during the 24-week treatment period (Table 2). The eight participants experiencing symptomatic hypoglycemia in the lixisenatide group each reported a single event; the 25 participants experiencing symptomatic hypoglycemia in the exenatide group reported a total of 48 events. Injection site reactions were reported for 27 participants (8.5%) in the lixisenatide group and five participants (1.6%) in the exenatide group. Three participants (0.9%) in the lixisenatide group had injection site reactions that led to discontinuation of study treatment (injection site hypersensitivity, injection site pain, and injection site reaction). However, none of the injection site reactions was serious or considered severe by the investigator. No allergic reactions adjudicated as possibly related to study treatment were observed in any of the treatment groups.
An increase of blood calcitonin (≥20 ng/L) was reported as a treatment-emergent AE for one participant (0.3%) in each treatment group. No participant had a calcitonin value ≥50 ng/L over 24 weeks of study treatment, and none of the events was serious or considered by the investigator to be severe. No clinically relevant changes in creatinine, aspartate aminotransferase, and alanine aminotransferase were observed in either treatment group.
A slight decrease in blood pressure (both systolic and diastolic) was observed in both treatment groups from baseline to week 24. The mean decreases in systolic blood pressure between baseline and end of treatment were –2.9 mmHg in the lixisenatide group and –2.5 mmHg in the exenatide group; for diastolic blood pressure, the mean decreases were –1.8 mmHg and –1.3 mmHg, respectively. There were no clinically relevant changes in heart rate from baseline to the last treatment value in either treatment group (mean change of 0.1 beats per minute in the lixisenatide group and mean change of –0.1 beats per minute in the exenatide group). No cases of pancreatitis were reported.
CONCLUSIONS
In this head-to-head comparison of two GLP-1 receptor agonists, lixisenatide administered once daily achieved the primary efficacy objective of noninferiority to exenatide administered twice daily in terms of HbA1c reduction at 24 weeks. Approximately 50% of participants in each group achieved the HbA1c target of <7.0%, and both agents provided similar reductions in FPG. There were reductions in body weight in both groups. Overall, the rates of AEs and serious AEs were comparable in the lixisenatide once-daily group and exenatide twice-daily group. However, gastrointestinal events and symptomatic hypoglycemia were reported less frequently with lixisenatide.
Previous randomized controlled trials with GLP-1 receptor agonist therapy (exenatide twice daily or liraglutide once daily) have investigated glycemic control for subjects in a similar setting (metformin monotherapy failure) (27–29). In the study by DeFronzo et al. (27), exenatide 10 µg twice daily reduced HbA1c by 0.8% (from 8.2 to 7.4%) over 30 weeks. In the study by Nauck et al. (28), which also included subjects previously receiving oral combination therapy who were switched to metformin monotherapy at the start of the study, liraglutide at 1.2 mg and at 1.8 mg once daily reduced HbA1c from ∼8.4 to ∼7.4% over 26 weeks. In a second liraglutide study, 1.2 mg and 1.8 mg liraglutide once daily reduced HbA1c from ∼8.4 to ∼7.2% and from ∼8.4 to ∼7.0%, respectively, over 26 weeks (29). Thus, in the studies to date in subjects receiving metformin monotherapy, add-on therapy with a GLP-1 receptor agonist consistently achieves HbA1c levels in the range of 7.0–7.4%. In the current study, final values of 7.0–7.2% were observed over a similar time scale, and a similar proportion of patients from both groups with high baseline HbA1c achieved a goal of <7%. It appears that despite apparent differences in study population, baseline characteristics, and HbA1c reductions, the final HbA1c values appear fairly similar in most studies with different GLP-1 receptor agonists. Nevertheless, only additional head-to-head studies would allow a robust comparison.
The noninferiority of lixisenatide versus exenatide was demonstrated for the prespecified CI margin of 0.4% and also was accomplished with the even stricter CI margin of 0.3% indicated in the more recent European Medicines Agency guideline (30). In the current analysis, exenatide twice daily had a slightly better effect on glucose control compared with lixisenatide once daily within the established noninferiority margin. However, the degree of clinical benefit from this small difference is unlikely to be clinically relevant because a similar proportion of patients in each group achieved HbA1c <7%. Any potential benefit of a small HbA1c difference has to be weighed against the more convenient once-daily injection of lixisenatide, the increased risk of mild hypoglycemia, and the initial increased incidence of gastrointestinal AEs with exenatide.
Clinical studies to date suggest that gastrointestinal symptoms, particularly nausea and vomiting, represent the main tolerability issue associated with GLP-1 receptor agonist therapy (31). Accordingly, in the current study, nausea was the most common AE with lixisenatide once daily, and gastrointestinal symptoms were the main reason for treatment discontinuation. Nausea was experienced by 25% of participants administered lixisenatide once daily compared with 35% receiving therapy with exenatide twice daily, representing ∼30% lower incidence in favor of lixisenatide. Improved tolerability with lixisenatide was reflected by the fact that more lixisenatide-treated subjects tolerated the target dose of 20 µg/day. The safety profile of lixisenatide in this study also compares favorably with that of the study of exenatide add-on to metformin, which reported nausea in 45% of subjects over 30 weeks (27). Higher rates have been reported in studies with other background therapies (51% in combination with a sulfonylurea) (32,33). In studies with liraglutide added to metformin monotherapy, the range of nausea incidence was 16–21% for 1.2 mg once daily and was 19–27% for 1.8 mg once daily over 26 weeks (28,29). A higher incidence (40%) was reported for the combination with metformin plus rosiglitazone (34). As in previous studies with GLP-1 receptor agonists, gastrointestinal symptoms with both lixisenatide once daily and exenatide twice daily in the current study occurred predominantly at the start of therapy and subsequently subsided. Consequently, these events had no negative impact on quality of life at week 24.
The incidence of hypoglycemia in previous studies with GLP-1 receptor agonists added to metformin monotherapy has been in the range of 3–5% of subjects over 26 to 30 weeks (27–29). These rates are generally of a magnitude similar to those reported in the current study, although we found a higher incidence with exenatide twice daily (7.9%) such that approximately threefold fewer subjects reported hypoglycemic events with lixisenatide once daily (2.5%). To further investigate the difference in the incidence of hypoglycemia between lixisenatide and exenatide, we looked at the timing of the occurrence of the hypoglycemic events during the day. In the exenatide group, most of the hypoglycemic events were observed during the hours after the morning and evening injections; however, with lixisenatide, hypoglycemic events mostly occurred during the hours after the morning injection.
The results of the current study demonstrated that as add-on therapy in the context of inadequate control with metformin monotherapy, administration of lixisenatide in a convenient once-daily regimen is not inferior to exenatide twice daily in terms of glycemic efficacy. It has marginally lower beneficial effect on weight but may provide some additional advantages in terms of fewer hypoglycemic and gastrointestinal events and has the potential for better adherence because of one less injection per day. These characteristics have the potential to improve compliance and reduce dropouts because of poor gastrointestinal tolerability during the initial phase of treatment initiation. Ultimately, the potential clinical utility of lixisenatide once daily will depend on the specific needs and preferences of the individual patient based on the overall efficacy, tolerability, and dosing frequency profile during the shared decision-making process with a health care professional. This also may involve consideration of the patient’s underlying pathophysiology and the need to target postprandial glucose in those with marked postprandial hyperglycemia. In conclusion, these results highlight the efficacy–tolerability profile of lixisenatide once daily and its potential as a new option for the treatment of type 2 diabetes inadequately controlled with metformin monotherapy.
Acknowledgments
The study was funded by Sanofi, the manufacturer of lixisenatide. The investigators and representatives from Sanofi were responsible for the study design, protocol, statistical analysis plans, analysis, and reporting of the results. Final responsibility for the decision to submit the manuscript for publication was made jointly by all authors. Editorial support was funded by Sanofi. This study was sponsored by Sanofi.
J.R. has served on scientific advisory boards and received honoraria or consulting fees or grants/research support from insulin and GLP-1 receptor agonist manufacturers Amylin, Eli Lilly, GlaxoSmithKline, Hoffmann-La Roche, Intarcia, Novo Nordisk, and Sanofi. D.R. has been a member of advisory boards and a speaker at symposia for Bristol-Myers Squibb, Eli Lilly, Medtronic, Merck Serono, MSD, Novartis, Novo Nordisk, and Sanofi. G.B. and P.M. are full-time employees of the sponsor Sanofi. J.E.G. has served on scientific advisory boards or received honoraria or consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
J.R. was involved in the development of the protocol, clinical conduct of the study, and analysis of the data, and led the writing of the manuscript. D.R. was involved in analysis of the data, development and review of the study manuscript, and approval of the final submitted article. L.K. was a study investigator and was involved in the interpretation of the data, development and review of the study manuscript, and approval of the final submitted article. L.M. was a study investigator and was involved in the interpretation of the data, development and review of the study manuscript, and approval of the final submitted article. G.B. was involved in the development of the protocol, analysis of the data, development and review of the manuscript, and approval of the final submitted article. P.M. was involved in development of the protocol, clinical conduct of the study, analysis of the data, development and review of the study manuscript, and approval of the final submitted article. J.E.G. was involved in the analysis and interpretation of the data, development and review of the study manuscript, and approval of the final submitted article. J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were previously presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and at the 47th European Association for the Study of Diabetes Annual Meeting, Lisbon, Portugal, 12–16 September 2011.
The authors thank all of the investigators, coordinators, the Detroit Medical Center, the Allergic Reaction Assessment Committee, Covance, and patients who took part in this study. The authors thank Frances Gambling, BA (Medicus International), for editorial support in preparing the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2709/-/DC1.
Clinical trial reg. no. NCT00707031, clinicaltrials.gov.
References
- 1.Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab 2012;14:675–688 [DOI] [PubMed] [Google Scholar]
- 2.Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001;25:781–792 [DOI] [PubMed] [Google Scholar]
- 3.Komatsu R, Matsuyama T, Namba M, et al. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide. Diabetes 1989;38:902–905 [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–744 [DOI] [PubMed] [Google Scholar]
- 5.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab 1996;81:327–332 [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 8.Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 2002;87:1239–1246 [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 10.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 12.Christensen M, Knop FK. Once-weekly GLP-1 agonists: How do they differ from exenatide and liraglutide? Curr Diab Rep 2010;10:124–132 [DOI] [PubMed] [Google Scholar]
- 13.Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: A new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010;164:58–64 [DOI] [PubMed] [Google Scholar]
- 14.Ratner RE, Rosenstock J, Boka G, DRI6012 Study Investigators Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010;27:1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE, EFC6018 GetGoal-Mono Study Investigators Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 2013;36:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolli G, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Hanefield M. Efficacy and safety of lixisenatide once-daily versus placebo in patients with T2DM insufficiently controlled on metformin (GetGoal-F1). Poster presented at 47th Annual Meeting of the European Association for the Study of Diabetes, 12–16 September 2011, Lisbon, Portugal. Diabetologia 2011;54(Suppl. 1):1–542 [Abstract 78] [Google Scholar]
- 18.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in patients with type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 30 April 2013 [Epub ahead of print] doi: 10.1111/dom.12121. [DOI] [PubMed] [Google Scholar]
- 19.Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle MC, Forst T, Aronson R, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care 2013;36:2497–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seino Y, Min KW, Niemoeller E, Takami A, EFC10887 GETGOAL-L Asia Study Investigators Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency. ICH Topic E9 Statistical Principles for Clinical Trials. Note for Guidance on Statistical Principles for Clinical Trials (CPMP/ICH/363/96) [Internet], 1998. Available from http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002928.pdf Accessed 12 December 2012
- 23.European Medicines Agency. ICH Topic E10 Choice of Control Group in Clinical Trials. Note for Guidance on Choice of Control Group in Clinical Trials (CPMP/ICH/364/96) [Internet], 2001. Available from http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002925.pdf Accessed 12 December 2012
- 24.US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for Industry. E9 Statistical principles for Clinical Trials [Internet], 1998. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073137.pdf Accessed 12 December 2012
- 25.European Medicines Agency. Committee for proprietary medicinal products. Note for guidance on statistical principles for clinical trials. CPMP/ICH/363/96 [Internet], 1998. Available from http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002928.pdf Accessed 12 December 2012
- 26.Food and Drug Administration. Guidance for Industry. Diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention [Internet], 2008. Available from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071624.pdf Accessed 12 December 2012
- 27.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 28.Nauck M, Frid A, Hermansen K, et al. LEAD-2 Study Group Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–1456 [DOI] [PubMed] [Google Scholar]
- 30.European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. CPMP/EWP/1080/00 Rev. 1 [Internet], 2012. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf Accessed 12 December 2012
- 31.Aroda VR, Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev 2011;27:528–542 [DOI] [PubMed] [Google Scholar]
- 32.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 33.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 34.Zinman B, Gerich J, Buse JB, et al. LEAD-4 Study Investigators Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]



