Abstract
OBJECTIVE
To investigate the quality of type 2 diabetes care according to sex.
RESEARCH DESIGN AND METHODS
Clinical data collected during the year 2009 were extracted from electronic medical records; quality-of-care indicators were evaluated. Multilevel logistic regression analysis was applied to estimate the likelihood of women versus men to be monitored for selected parameters, to reach clinical outcomes, and to be treated with specific classes of drugs. The intercenter variability in the proportion of men and women achieving the targets was also investigated.
RESULTS
Overall, 415,294 patients from 236 diabetes outpatient centers were evaluated, of whom 188,125 (45.3%) were women and 227,169 (54.7%) were men. Women were 14% more likely than men to have HbA1c >9.0% in spite of insulin treatment (odds ratio 1.14 [95% CI 1.10–1.17]), 42% more likely to have LDL cholesterol (LDL-C) ≥130 mg/dL (1.42 [1.38–1.46]) in spite of lipid-lowering treatment, and 50% more likely to have BMI ≥30 kg/m2 (1.50 [1.50–1.54]). Women were less likely to be monitored for foot and eye complications. In 99% of centers, the percentage of men reaching the LDL-C target was higher than in women, the proportion of patients reaching the HbA1c target was in favor of men in 80% of the centers, and no differences emerged for blood pressure.
CONCLUSIONS
Women show a poorer quality of diabetes care than men. The attainment of the LDL-C target seems to be mainly related to pathophysiological factors, whereas patient and physician attitudes can play an important role in other process measures and outcomes.
Gender medicine integrates aspects of biology, sociology, ethnicity, and culture responsible for different responses to care in women and men (1). Gender medicine applied to the field of diabetes care is particularly relevant because women with diabetes, regardless of menopausal status, have a four- to sixfold increase in the risk of developing coronary artery disease, whereas men with diabetes have a two- to threefold increase in risk (2). Women with diabetes have a poorer prognosis after myocardial infarction and a higher risk of death overall from cardiovascular disease than men with diabetes (3,4).
This greater excess coronary risk may be explained by more adverse cardiovascular risk profiles among women with diabetes (5). Compared with men, women with diabetes have higher prevalent abdominal obesity, increased risk of hypertension, and a more severe type of dyslipidemia (low levels of HDL cholesterol [HDL-C], small particle size of LDL cholesterol [LDL-C], and high levels of triglycerides). Furthermore, polycystic ovary syndrome is an important correlate of insulin resistance and metabolic syndrome (2).
Besides innate differences in sex physiology, disparities between sexes in the treatment of major cardiovascular risk factors also still exist, attributed to an underestimation of patient risk (6,7). Documenting these disparities and identifying their determinants in a specific health care setting can help caregivers provide higher standards of care and apply evidence-based therapies for diabetes care and prevention of cardiovascular disease (8–11).
In Italy, a continuous improvement effort implemented by a network of diabetes clinics has been promoted since 2006 (12,13). The initiative, which involves approximately one-third of all the diabetes outpatient clinics operating within the national health care system, allows the monitoring of a large set of process and outcome indicators and the use of specific classes of drugs, with the aim of examining strengths and limitations of the current diabetes care.
We used the data of the Italian Association of Clinical Diabetologists (Associazione Medici Diabetologi [AMD]) Annals to 1) evaluate whether sex differences in pharmacological and nonpharmacological treatment of diabetes exist in Italy and 2) investigate the role of biological and cultural factors in determining different outcomes for men and women.
RESEARCH DESIGN AND METHODS
The AMD Annals initiative
Since 2006, the AMD has promoted a continuous quality improvement initiative called AMD Annals. In this context, AMD identified a set of indicators to be used for benchmarking activities (9,10). Quality indicators include process measures evaluating diagnostic, preventive, and therapeutic procedures performed by the participating centers and outcome indicators measuring favorable and unfavorable modifications in patient health status. Furthermore, the use of antidiabetic, antihypertensive, and lipid-lowering drugs is evaluated. Centers share the same software for data extraction from electronic medical records. Data are collected annually in a standardized format (AMD data file) and centrally analyzed anonymously. The entire project is conducted without allocation of extra resources or financial incentives but simply through a physician-led effort, made possible by the commitment of the specialists involved.
Quality-of-care indicators
Process measures are expressed as percentages of patients monitored at least once during the previous 12 months for the following parameters: HbA1c, blood pressure (BP), lipid profile (LDL-C or total and HDL-C and triglycerides), renal function, foot examination, and eye examination.
Intermediate outcome measures include the proportion of patients with satisfactory values as well as the percentage of those with unacceptably high values. Outcomes are considered satisfactory if HbA1c levels are ≤7.0% (≤53 mmol/mol), BP values are <130/80 mmHg, LDL-C levels are <100 mg/dL, and BMI is <27 kg/m2. Unsatisfactory outcomes include HbA1c levels >8.0%, BP values ≥140/90 mmHg, LDL-C levels ≥130 mg/dL, BMI ≥30 kg/m2, presence of micro/macroalbuminuria, and glomerular filtration rate (GFR) ≤60 mL/min. Seven indicators of treatment intensity/appropriateness were also measured to take into consideration the use of pharmacological treatments in relation to the achievement of the targets: no insulin despite HbA1c >9.0%, (>75 mmol/mol), no lipid-lowering agents despite LDL-C ≥130 mg/dL, no antihypertensive treatments despite BP ≥140/90 mmHg, no ACE-I and/or angiotensin receptor blockers (ARBs) despite micro/macroalbuminuria, HbA1c >9.0% (>75 mmol/mol) in spite of insulin treatment, LDL-C ≥130 mg/dL in spite of lipid-lowering treatment, and BP ≥140/90 mmHg in spite of antihypertensive treatment.
Finally, a quality-of-care summary score (Q score) was calculated. The Q score has been developed and validated in two previous studies (14,15). It is based on a combination of process and outcome indicators relative to HbA1c, BP, LDL-C, and microalbuminuria. The score ranges between 0 and 40; the higher the score, the better the quality of care. Previous studies (14,15) documented that the risk of developing a new cardiovascular event was 80% higher in patients with a score <15 and 20% higher in those with a score between 15 and 25, as compared with those with a score >25.
Sample selection and data analyses
Clinical data collected during the year 2009 were extracted from electronic medical records. Only patients with a diagnosis of type 2 diabetes were selected. In cases of multiple records collected during the year for the same patient, the last available value was included in the quality-of-care profiling. The denominators for the different quality indicators vary according to the availability of the information in the index year (Fig. 1). No missing imputation was performed.
Figure 1.
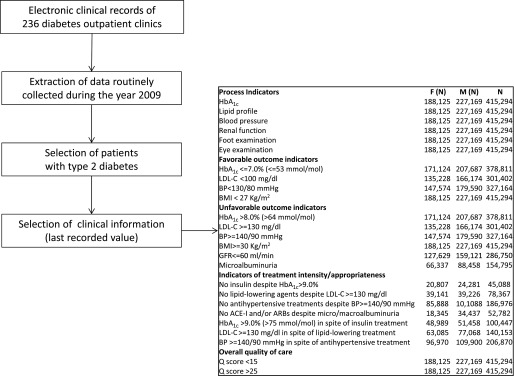
Flowchart of data selection and sample size for each indicator (n).
Because normal ranges for glycated hemoglobin varied among the different centers, to allow direct comparison, the percentage change with respect to the upper normal value (actual value/upper normal limit) was estimated and multiplied by 6.0. LDL-C was estimated by the Friedewald equation. Microalbuminuria was defined as albumin excretion rate ≥20 μg/min, albumin-creatinine ratio >2.5 (men) or >3.5 (women) mg/mmol, or microalbuminuria >30 mg/L. GFR was calculated with the Modification of Diet in Renal Disease (MDRD) formula (16).
Statistical analyses
Patient characteristics and quality indicators according to sex were described as mean and SD or frequencies. Between-group statistical tests were not applied; in fact, due to the large sample size, even trivial differences reached statistical significance.
To take into consideration unbalanced characteristics of the two sexes, the likelihood of women as compared with men (reference class) to be monitored for specific clinical parameters, to reach specific clinical outcomes, and to be treated with specific classes of drugs has been investigated through multilevel logistic regression analyses, adjusted for age, diabetes duration, BMI, and, in a separate model, clustering effect; the participating diabetes outpatient clinics accounted for the clusters. Results are expressed as odds ratio (OR) and 95% CI. Analyses were performed on the whole sample as well as in two different age-groups, i.e., <75 vs. ≥75 years of age.
For each indicator, we estimated the intraclass correlation coefficient (ICC) to evaluate the extent to which the indicator varied between centers as compared with within-center variation, taking patient case mix into account (17). The higher the ICC, the greater the influence of the center level on the quality-of-care indicator.
The intercenter variability in the difference between men and women achieving the targets of HbA1c, LDL-C, and BP was also investigated using multilevel models adjusted for age, diabetes duration, BMI, and clustering effect.
RESULTS
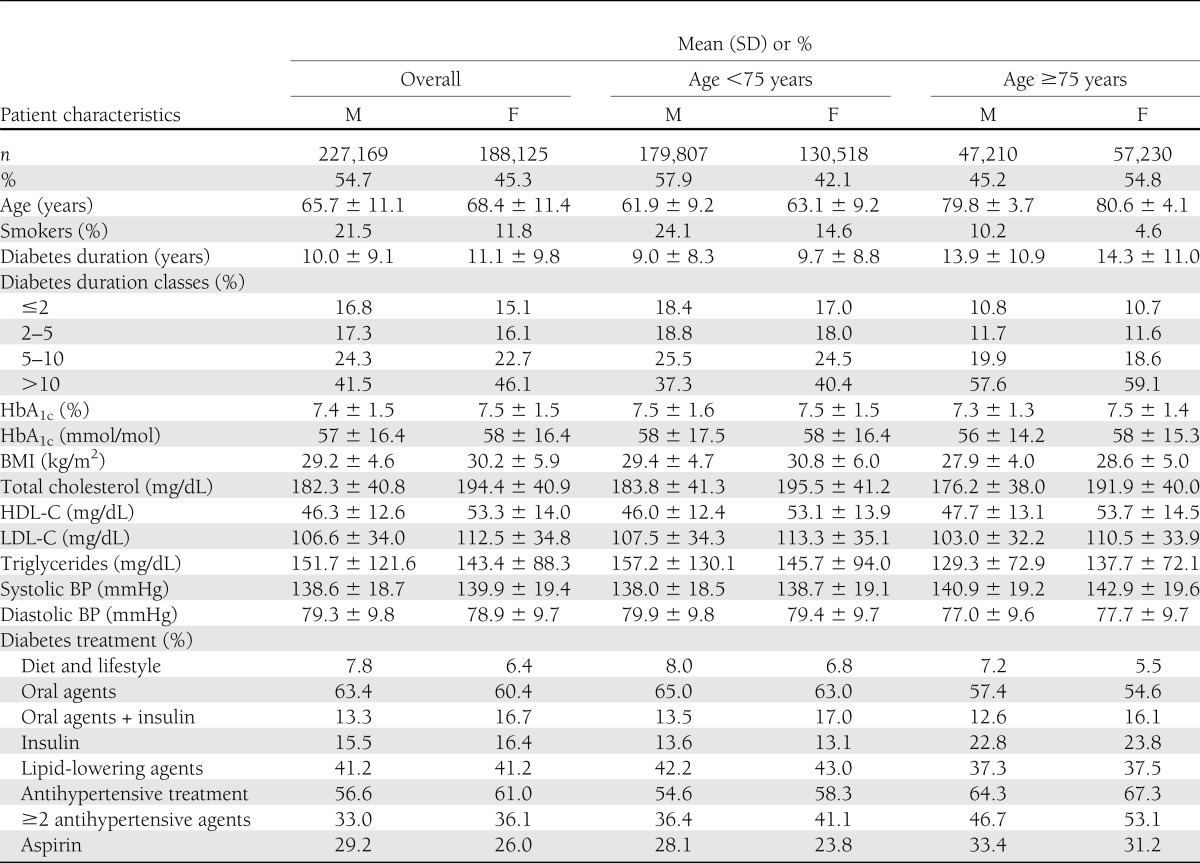
Overall, 415,294 patients with type 2 diabetes referred to 236 diabetes outpatient centers during the year 2009 were evaluated; of them, 188,125 (45.3%) were women and 227,169 (54.7%) were men. Patient characteristics according to sex are summarized in Table 1. The data show some substantial differences in the between-sex clinical characteristics. Women had an older age (30.5% of women vs. 20.8% of men were >75 years of age), had a slightly higher diabetes duration (11.1 ± 9.8 vs. 10.0 ± 9.1 years), and were more obese (average BMI 29.2 ± 4.6 kg/m2 in men vs. 30.2 ± 5.9 kg/m2 in women). Smoking was more prevalent among men. Similar proportions of women and men were treated with insulin, lipid-lowering agents, and antihypertensive drugs (Table 1); when evaluating the different classes of drugs and their combinations, no difference was found (Supplementary Table 1), except for a wider use of diuretics in women than in men (35.9 vs. 27.6%, respectively). The analyses by age class showed similar patterns in individuals below and over 75 years of age (Table 1).
Table 1.
Patient characteristics according to sex
All process indicators were slightly but systematically more favorable in men than in women. Adjustment for patient case mix and clustering attenuated sex differences; despite that, women still showed a lower likelihood to be monitored for diabetes complications, particularly foot and eye complications.
As for intermediate outcomes, the proportion of individuals reaching satisfactory HbA1c, LDL-C, BP, and BMI values was systematically lower for women than for men. Adjustment for patient case mix and clustering effect confirms that women were 14% less likely than men to reach the HbA1c target, 27% less likely to reach the LDL-C target, and 20% less likely to reach the BMI target. On the other hand, the likelihood of reaching the BP target was slightly higher in women than in men. These findings are mirrored by those relative to unfavorable outcomes. Fully adjusted models show that women were 11% more likely than men to have HbA1c levels >8.0% (>64 mmol/mol), 41% more likely to have LDL-C levels ≥130 mg/dL, 50% more likely to have a BMI ≥30 kg/m2, and 32% more likely to have a GFR ≤60 mL/min. On the other hand, women were 48% less likely than men to have micro/macroalbuminuria. No major differences were detected for BP.
Data on the use of drugs show mixed results. The likelihood of not being treated with insulin in the presence of elevated HbA1c values or with antihypertensive agents in the presence of elevated BP values was lower in women than in men, whereas no sex difference emerged in the use of lipid-lowering drugs in the presence of elevated LDL-C levels. Women had a 10% higher probability than men of not being treated with ACE-I or ARBs in the presence of micro/macroalbuminuria. Women also showed a 14% higher likelihood of having HbA1c levels >9.0% (>75 mmol/mol) despite insulin treatment and a 42% higher likelihood of having LDL-C levels ≥130 mg/dL despite lipid-lowering treatment (Table 2).
Table 2.
Quality indicators of diabetes care according to sex
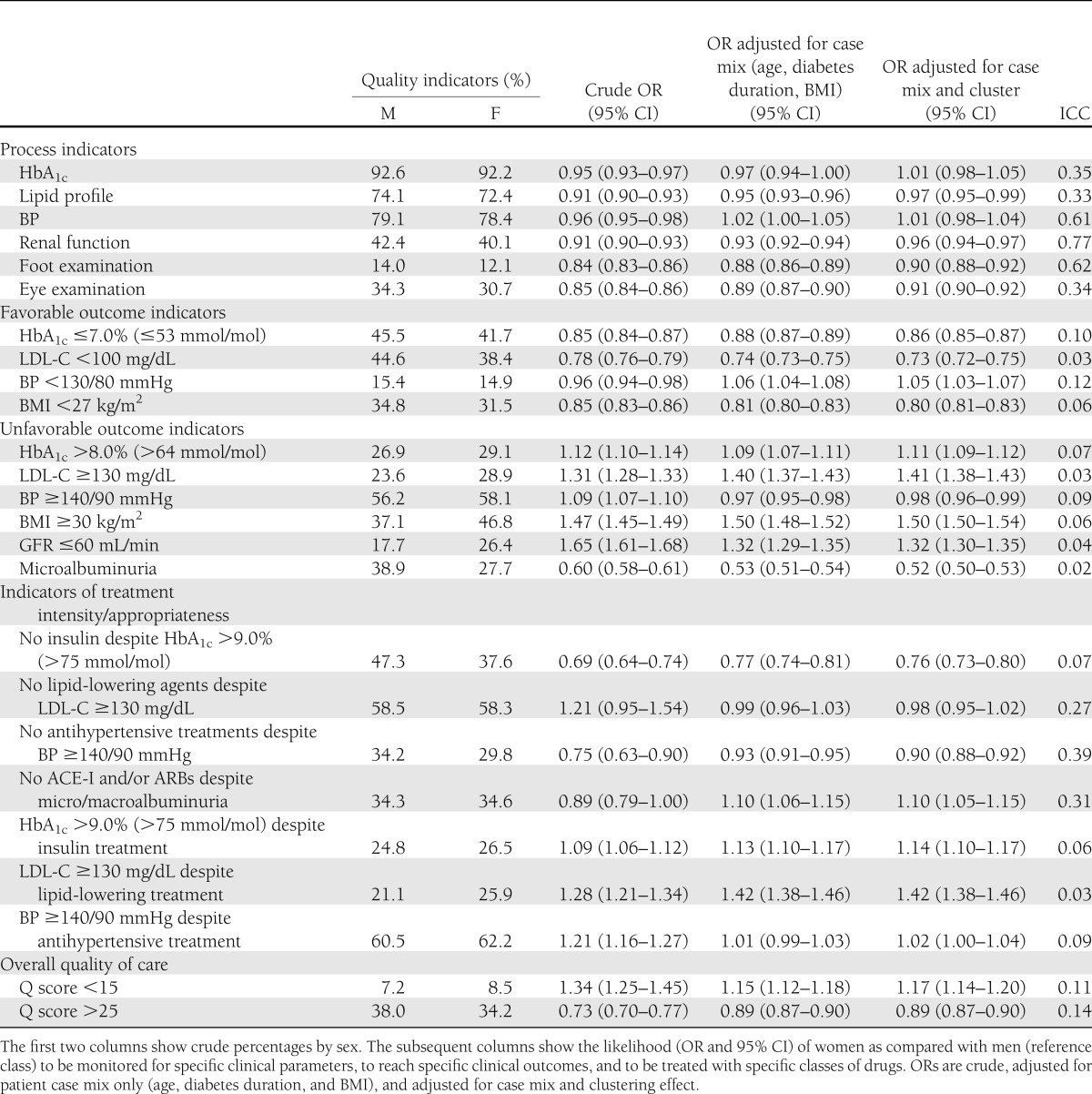
The analysis of overall quality of care, as summarized by the Q score, shows that women had a 17% greater likelihood of having a score <15 and an 11% lower likelihood of having a score >25 as compared with men. The contribution of between-center variability was substantial for process measures and moderate for intermediate outcomes, as documented by intraclass correlations (Table 2).
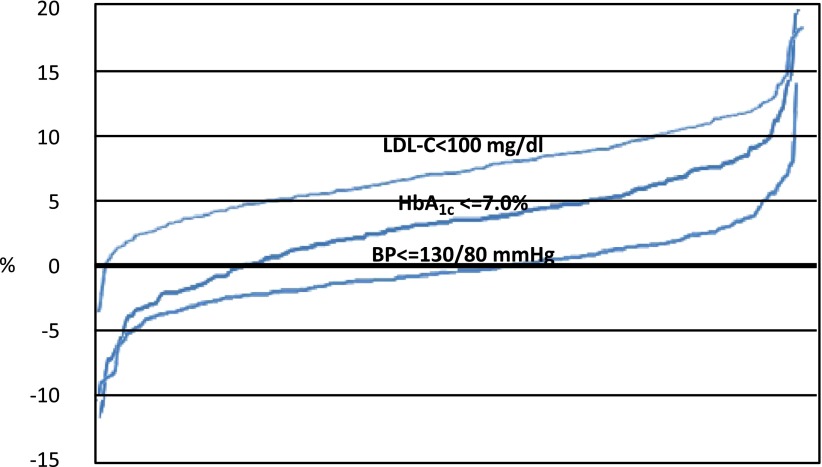
Finally, we examined the between-center variability in the proportion of men and women reaching the desired therapeutic targets (Fig. 2). In almost all the centers, the percentage of men reaching the LDL-C target was higher than in women, with differences exceeding 5% in most of the centers. Similarly, the proportion of patients reaching the HbA1c target was in favor of men in the vast majority of the centers. As for the BP target, no clear trend in favor of men or women emerged.
Figure 2.
Intercenter variability in the percentage difference of men and women achieving the main clinical therapeutic targets. (A high-quality color representation of this figure is available in the online issue.)
Supplementary Table 2 reports the results of the analyses performed separately in individuals below and over 75 years of age. These analyses show that sex disparities are more pronounced in older people, particularly with reference to the attainment of HbA1c and BP targets. In fact, elderly women were 27% less likely than men to reach an HbA1c level ≤7.0% (OR 0.73 [95% CI 0.71–0.75]) and 17% less likely to reach a BP target <130/80 mmHg (0.83 [0.79–0.87]).
CONCLUSIONS
Data from AMD Annals show that in Italy, despite equity of access to specialist care and universal coverage of health care costs, sex disparities are still present. Between-sex differences in the prevalence and treatment of cardiovascular risk factors are less pronounced in Italy than in other countries, not only those where cultural barriers or deprivation can be responsible for lower levels of care provided to women (18,19) but also in other European countries (20,21) or in the U.S. (22–24). In Spain, data from electronic databases of primary care showed a slightly better metabolic control in women than in men, in contrast with AMD Annals, although BP and lipid control were in favor of men (10). In the context of a pay-for-performance initiative in the U.K., women were 13% less likely to be monitored for HbA1c, BP, lipid profile, and smoking and 25% less likely to reach the recommended therapeutic target than men, although no major differences in metabolic control were detected (11). In the vast majority of studies, women were less likely to reach the recommended targets as well as receive treatment and monitoring. Constantly, the wider gap was related to the lipid target; women had higher LDL-C levels than men and were less likely to be receiving lipid-lowering therapy (25,26).
Our findings call for a revision of clinical practice. In fact, it is alarming that the likelihood to reach specific clinical outcomes is systematically unfavorable for women as compared with men. The likelihood of receiving poorer quality of care is partly reduced but remains consistently higher for women, even after taking into consideration the baseline differences in patient characteristics (especially the higher proportion of elderly patients, a longer diabetes duration, and the higher BMI in women) and the effect of clustering. To this respect, our study confirms that not taking patient case mix and clustering effect into consideration may lead to biased results (27,28). On the other hand, when the analyses were performed separately in individuals below and over 75 years of age, sex disparities were still documented in younger people and were further increased in elderly patients.
Our findings do not seem to be explained by a lower propensity of physicians to treat women. In fact, the proportion of women treated with insulin, statins, or antihypertensive agents was equal or even greater than that of men. Also, women were more likely than men to be treated with insulin and antihypertensive agents and equally likely to be treated with lipid-lowering drugs in the presence of elevated values. The greater difficulty in reaching the targets can be related to the use of a lower aggressive approach (i.e., prescription of lower doses), poorer compliance of women, or between-sex physiopathological differences. For example, data on sex differences in carbohydrate metabolism showed that during submaximal endurance exercise, women oxidize more lipid and less carbohydrate as metabolic substrates than men (29). In addition, prior research demonstrated the existence of sex differences in drug responses, due to differences in pharmacodynamics and pharmacokinetics (30). On this issue, a study documented that statin therapy after an acute myocardial infarction is associated with reduced rates of all-cause and cardiac mortality, but the degree of risk reduction is lower for women than for men (31). Furthermore, women experience a higher incidence of adverse drug reactions than men. Sex-specific differences in the pharmacokinetics and pharmacodynamics of drugs are still unclear (32). Another element calling for possible intrinsic physiopathological sex differences is the impairment in the renal function that follows different pathways with a higher prevalence of microalbuminuria in men and more frequently reduced GFR in women (33), as also documented in our study.
Apart from biomedical differences, the documented differences could also be explained in terms of behavioral factors. Information on psychosocial characteristics and adherence to treatments is lacking in AMD Annals. Nevertheless, in the AMD BEnchmarking Network for Clinical and Humanistic outcomes in Diabetes (BENCH-D) study (34), women showed a higher prevalence of depression, as documented by a two times higher likelihood than men of being in the lowest quartile of the WHO-5 well-being index score; the study also showed that lower levels of psychological well-being were associated with lower levels of satisfaction with treatment, diabetes empowerment, and self-care attitudes and with a worse perception of barriers to medication. All these factors can negatively affect compliance with medical recommendations.
Recently, a new conceptual model has been developed to explain sex differences in health: neither exclusively biomedical explanations nor exclusively social explanations. The model intends to bridge and integrate these dichotomous perspectives as the key to establishing new policies to increase opportunities and provide incentives for pursuing health (35). The existence of mixed mechanisms is also suggested in our study by the analysis of between-center variability. For LDL-C, in almost all the centers (233 out of 236), the proportion of men reaching the target was higher than that of women. Such a systematic difference can hardly be explained by the attitudes of the physicians or the patients, and the existence of pathophysiological differences between sexes may play an important role. On the other hand, for the attainment of the HbA1c, men were more likely than women to reach the target of ≤7.0% in 80% of the centers (190 out of 236 centers), thus suggesting the existence of a mixed effect of physiological mechanisms and attitudes. As for BP targets, the direction and magnitude of the between-center difference was variable, thus suggesting that different physician- and patient-related factors, rather than physiopathology, may play a crucial role.
Our study has strengths and limitations. The main strengths are the sample size and the data source, which are largely representative of the quality of diabetes care provided to both sexes in Italy. A limitation is that we missed information on drug doses, sociodemographic and socioeconomic characteristics, and micro- and macrovascular diabetes complications, which could aid in understanding the reasons for the results obtained. Also, our findings refer to patients attending specialist centers and cannot be generalized to patients cared for by general practitioners.
In conclusion, our data strongly suggest that the greater difficulty in reaching LDL-C targets in women is mainly related to pathophysiological factors, whereas patient and physician attitudes can play an important role in other process measures and outcomes. These findings underline the need for diversifying the care and specializing the support provided to men and women based on sociodemographic, clinical, and psychological characteristics.
Further research is required to improve the knowledge about mechanisms underlying these differences. The regular evaluation of quality-of-care indicators with initiatives such as the AMD Annals is the first fundamental step to identify the main areas of interventions and monitor the desirable increase in equity during the time.
Acknowledgments
The statistical analysis was supported by LifeScan and Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
The sponsors did not participate in the design or conduct of this study; the collection, management, analysis, or interpretation of data; the writing, preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
M.C.R. and A.Ni. wrote the manuscript. M.R.C., S.G., V.M., M.F.M., A.Na., C.S., and C.G. critically revised the manuscript for intellectual content and interpreted data. G.L. and F.P. performed data analysis. All authors were involved in the concept and design of the initiative and the final approval of the manuscript to be submitted for publication. C.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data were presented as a poster at the International Diabetes Federation (IDF) World Diabetes Congress, Dubai, UAE, 4–8 December 2011.
The authors thank the participating diabetes outpatient centers for their contribution. The authors acknowledge LifeScan and Eli Lilly and Company for having supported this data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0184/-/DC1.
A complete list of the members of the AMD Annals Study Group can be found in the Supplementary Data online.
References
- 1.McGregor AJ, Choo E. Gender-specific medicine: yesterday’s neglect, tomorrow’s opportunities. Acad Emerg Med 2012;19:861–865 [DOI] [PubMed] [Google Scholar]
- 2.Legato MJ, Gelzer A, Goland R, et al. Writing Group for The Partnership for Gender-Specific Medicine Gender-specific care of the patient with diabetes: review and recommendations. Gend Med 2006;3:131–158 [DOI] [PubMed] [Google Scholar]
- 3.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004;27:2898–2904 [DOI] [PubMed] [Google Scholar]
- 4.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002;162:1737–1745 [DOI] [PubMed] [Google Scholar]
- 6.Bugiardini R, Yan AT, Yan RT, et al. Canadian Acute Coronary Syndrome Registry I and II Investigators Factors influencing underutilization of evidence-based therapies in women. Eur Heart J 2011;32:1337–1344 [DOI] [PubMed] [Google Scholar]
- 7.Poon S, Goodman SG, Yan RT, et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J 2012;163:66–73 [DOI] [PubMed] [Google Scholar]
- 8.Jarvie JL, Foody JM. Recognizing and improving health care disparities in the prevention of cardiovascular disease in women. Curr Cardiol Rep 2010;12:488–496 [DOI] [PubMed] [Google Scholar]
- 9.Ekström N, Miftaraj M, Svensson AM, et al. Glucose-lowering treatment and clinical results in 163 121 patients with type 2 diabetes: an observational study from the Swedish national diabetes register. Diabetes Obes Metab 2012;14:717–726 [DOI] [PubMed] [Google Scholar]
- 10.Guthrie B, Emslie-Smith A, Morris AD. Which people with type 2 diabetes achieve good control of intermediate outcomes? Population database study in a UK region. Diabet Med 2009;26:1269–1276 [DOI] [PubMed] [Google Scholar]
- 11.Vinagre I, Mata-Cases M, Hermosilla E, et al. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care 2012;35:774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi MC, Nicolucci A, Arcangeli A, et al. Associazione Medici Diabetologi Annals Study Group Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care 2008;31:2166–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolucci A, Rossi MC, Arcangeli A, et al. AMD-Annals Study Group Four-year impact of a continuous quality improvement effort implemented by a network of diabetes outpatient clinics: the AMD-Annals initiative. Diabet Med 2010;27:1041–1048 [DOI] [PubMed] [Google Scholar]
- 14.De Berardis G, Pellegrini F, Franciosi M, et al. QuED (Quality of Care and Outcomes in Type 2 Diabetes) Study Group Quality of diabetes care predicts the development of cardiovascular events: results of the QuED study. Nutr Metab Cardiovasc Dis 2008;18:57–65 [DOI] [PubMed] [Google Scholar]
- 15.Rossi MC, Lucisano G, Comaschi M, et al. AMD-QUASAR Study Group Quality of diabetes care predicts the development of cardiovascular events: results of the AMD-QUASAR study. Diabetes Care 2011;34:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 2012;156:785–795 [DOI] [PubMed] [Google Scholar]
- 17.Kaplan SH, Griffith JL, Price LL, Pawlson LG, Greenfield S. Improving the reliability of physician performance assessment: identifying the “physician effect” on quality and creating composite measures. Med Care 2009;47:378–387 [DOI] [PubMed] [Google Scholar]
- 18.Alberti H, Alberti B. The influence of gender on the primary care management of diabetes in Tunisia. Pan Afr Med J 2009;3:2. [PMC free article] [PubMed] [Google Scholar]
- 19.Njelekela MA, Mpembeni R, Muhihi A, et al. Gender-related differences in the prevalence of cardiovascular disease risk factors and their correlates in urban Tanzania. BMC Cardiovasc Disord 2009;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouni-Berthold I, Berthold HK, Mantzoros CS, Böhm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care 2008;31:1389–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kautzky-Willer A, Kamyar MR, Gerhat D, et al. Sex-specific differences in metabolic control, cardiovascular risk, and interventions in patients with type 2 diabetes mellitus. Gend Med 2010;7:571–583 [DOI] [PubMed] [Google Scholar]
- 22.Bird CE, Fremont AM, Bierman AS, et al. Does quality of care for cardiovascular disease and diabetes differ by gender for enrollees in managed care plans? Womens Health Issues 2007;17:131–138 [DOI] [PubMed] [Google Scholar]
- 23.Ferrara A, Mangione CM, Kim C, et al. Translating Research Into Action for Diabetes Study Group Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2008;31:69–74 [DOI] [PubMed] [Google Scholar]
- 24.Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care 2005;28:514–520 [DOI] [PubMed] [Google Scholar]
- 25.Vimalananda VG, Miller DR, Palnati M, Christiansen CL, Fincke BG. Gender disparities in lipid-lowering therapy among veterans with diabetes. Womens Health Issues 2011;21(Suppl.):S176–S181 [DOI] [PubMed] [Google Scholar]
- 26.Kim C, Kerr EA, Bernstein SJ, Krein SL. Gender disparities in lipid management: the presence of disparities depends on the quality measure. Am J Manag Care 2006;12:133–136 [PubMed] [Google Scholar]
- 27.Greenfield S, Kaplan SH, Kahn R, Ninomiya J, Griffith JL. Profiling care provided by different groups of physicians: effects of patient case-mix (bias) and physician-level clustering on quality assessment results. Ann Intern Med 2002;136:111–121 [DOI] [PubMed] [Google Scholar]
- 28.De Berardis G, Pellegrini F, Franciosi M, et al. QuED Study Quality of care and outcomes in type 2 diabetic patients: a comparison between general practice and diabetes clinics. Diabetes Care 2004;27:398–406 [DOI] [PubMed] [Google Scholar]
- 29.Tarnopolsky MA, Ruby BC. Sex differences in carbohydrate metabolism. Curr Opin Clin Nutr Metab Care 2001;4:521–526 [DOI] [PubMed] [Google Scholar]
- 30.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res 2007;55:81–95 [DOI] [PubMed] [Google Scholar]
- 31.Karp I, Chen SF, Pilote L. Sex differences in the effectiveness of statins after myocardial infarction. CMAJ 2007;176:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol 2008;64:999–1004 [DOI] [PubMed] [Google Scholar]
- 33.Solini A, Penno G, Bonora E, et al. Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care 2012;35:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolucci A, Rossi MC, Gentile S, et al.; BENCH-D Study Group. Correlates of psychological well-being in individuals with type 2 diabetes. Abstract presented at the 72nd Scientific Sessions of the American Diabetes Association, 8–12 June 2012, Philadelphia, Pennsylvania
- 35.Bird CE, Rieker PP. Gender and Health: The Effects of Constrained Choices and Social Policies. New York, Cambridge University Press, 2008 [Google Scholar]