Abstract
OBJECTIVE
Haptoglobin (Hp) genotype (Hp 1-1, 1-2, or 2-2) is associated with risk for type 2 diabetes complications, but its relationship with cognitive compromise, a growing concern in type 2 diabetes, has rarely been studied. This study investigated whether Hp genotype is associated with cognitive function in cognitively normal elderly diabetic subjects.
RESEARCH DESIGN AND METHODS
Relationships of Hp genotype with episodic memory, semantic categorization, attention/working memory and executive function, and an overall cognitive score were examined in subjects from the Israel Diabetes and Cognitive Decline (IDCD) study.
RESULTS
In the present analysis, 812 subjects participated (84 with Hp 1-1, 335 with Hp 1-2, and 393 with Hp 2-2 genotypes). Average was 72.9 years of age (SD 4.7), and Mini-Mental State Exam (MMSE) was 28.0 (SD 1.8). Compared with subjects with Hp 1-2 genotype, Hp 1-1 subjects performed significantly worse in semantic categorization (F = 7.03; P = 0.008) and the overall cognitive score (F = 5.57; P = 0.02). A separate stepwise multiple regression analysis demonstrated that compared with subjects with Hp 2-2 genotype, Hp 1-1 subjects performed significantly worse in semantic categorization (F = 4.18; P = 0.04) and the overall cognitive score (F = 4.70; P = 0.03). The contribution of cardiovascular risk factors to cognition was significantly higher in subjects with Hp 1-1 genotype compared with Hp 2 carriers (Hp 1-2 and Hp 2-2) in the semantic categorization (P = 0.009) and attention/working memory (P = 0.002) cognitive domains.
CONCLUSIONS
Compared with Hp 2 carriers, those with Hp 1-1 genotype present lower cognitive performance. Stronger relationships between cardiovascular risk factors and cognition in the latter group may suggest an underlying vascular mechanism.
The prevalence of type 2 diabetes is steadily rising in the Western world, reaching 40% by 85 years of age (1). Type 2 diabetes, and even prediabetic stages, (2) has consistently been shown to be a risk factor for cognitive decline, mild cognitive impairment (MCI) (3), and dementia (4), both vascular dementia (5,6) and Alzheimer disease (5,6). Yet, strategies for prevention of dementia in type 2 diabetes are not available since it is still unknown what factors and underlying mechanisms within type 2 diabetes cause the increased risk.
The haptoglobin (Hp) genotype has been associated with cardiovascular complications in numerous studies (7–11) in type 2 diabetes but less so in type 2 diabetes–free individuals (12). Hp is a glycoprotein synthesized in the liver and found in abundance in the plasma. There are two classes of functional alleles (1 and 2) that form three possible phenotypes (1-1, 1-2, and 2-2). Hp binds to free hemoglobin (Hb) released from blood cells as part of red cell turnover (13), thus inhibiting the considerable oxidative tissue damage resulting from free Hb (through heme iron) (14). The Hp-Hb complex is rapidly cleared from the bloodstream by the CD163 scavenger receptor expressed in monocytes/macrophages (15). Hp phenotypes differ in chemical and clinical properties (16). For example, Hp alleles differ in their ability to clear free Hb from the plasma; Hp(2-2)-Hb complexes are cleared less efficiently from the plasma than non–Hp(2-2)-Hb complexes (14). Thus, subjects with Hp 2-2 are more prone to oxidative stress (17).
Previous case-control and longitudinal studies have demonstrated that the different Hp genotypes are associated with clinical advantages or disadvantages depending on specific diseases and the body system involved (16). Hp 2-2 phenotype is associated with an increased incidence of micro- (7) and macrovascular (8) complications in type 2 diabetic subjects, such that compared with non–Hp 2-2 subjects, those with Hp 2-2 suffer from higher rates of cardiovascular disease (CVD) (8) and nonfatal myocardial infarction (MI) (9–11).
The role of the Hp type in cerebrovascular disease and cognitive decline with aging is presently unclear. No consistent or significant relationship has been previously shown between clinically evident large watershed or hemorrhagic stroke and the Hp type in type 2 diabetic subjects. Recent studies (18,19), however, suggest that the Hp 1-1 type may be associated with an increased prevalence of small lacunar strokes, identified as cerebral deep white matter lesions (WMLs). Therefore, in this study, we examined the relationship of Hp type with cognitive function in a large cohort of the elderly with type 2 diabetes participating in the Israel Diabetes and Cognitive Decline (IDCD) study and hypothesized that the cognitive profile of Hp 1-1 would be inferior to that of Hp 2 allele carriers based on the recent evidence of the disadvantageous brain microvascular profile (18,19).
RESEARCH DESIGN AND METHODS
This study was approved by the Sheba Medical Center and Maccabi Healthcare Services (MHS) institutional review board committees.
Sample
This study consists of elderly (≥65 years of age) type 2 diabetic subjects who are engaged in the IDCD, a longitudinal investigation assessing the relationship of long-term type 2 diabetes characteristics and cognitive decline. The study is ongoing. Longitudinal follow-up began recently, so the present results are based on baseline data only. Subjects were randomly selected from the ∼11,000 type 2 diabetic individuals that are in the diabetes registry of MHS. MHS is the second largest HMO in Israel. The MHS diabetes registry is an integral part of the MHS electronic patient record system and was established in 1999 to facilitate disease management and to improve treatment. Entry criteria to the registry are any of the following: 1) HbA1c >7.25%, 2) glucose >200 mg/dL on two exams >3 months apart, 3) purchase of diabetic medication twice within 3 months supported by an HbA1c >6.5% or glucose >125 mg/dL within half a year, or 4) diagnosis of type 2 diabetes (ICD-9 code) by a general practitioner, internist, endocrinologist, ophthalmologist, or type 2 diabetes advisor, supported by a HbA1c >6.5% or glucose >125 mg/dL within half a year. These criteria have been validated by 20 physicians in MHS against their own practice records (20). Additionally, age-specific prevalence rates were similar to those of a diabetes registry of another large HMO in Israel (21). The diabetes registry has collected detailed laboratory, medication, and diagnosis information since 1998 (20).
Eligibility criteria for the study
Subjects are eligible for the study if they are listed in the MHS diabetes registry, live in the central area of Israel, are diagnosed as suffering from type 2 diabetes, are 65 years of age or above, are diagnosed as cognitively normal at baseline (based on a multidisciplinary weekly consensus conference), do not suffer from major medical, psychiatric, or neurological conditions that affect cognitive performance, have three or more HbA1c measurements in the diabetes registry, and speak Hebrew fluently and have contact with an informant for at least 10 h per week. The latter criterion was implemented to ensure obtaining of data regarding the existence of functional impairments secondary to cognitive changes as well as information on changes in behavior, both of which are important for diagnosis of cognitive status, especially in cases that are on the border between normal cognition and MCI or early dementia.
Subject recruitment process
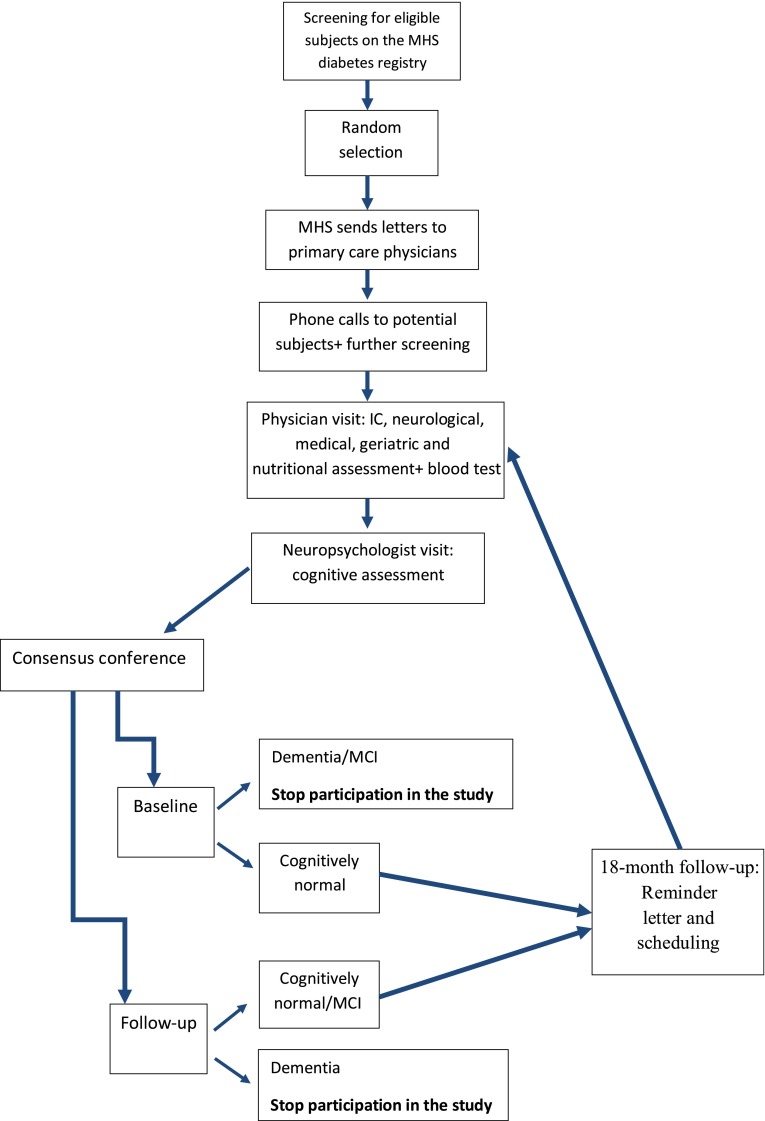
The MHS team performs a thorough screening of the diabetes registry and the MHS electronic patient record in order to identify potential subjects, excluding anyone with an ICD code for dementia or its subtypes, treated with prescribed cholinesterase inhibitors, or with a major psychiatric or neurological condition (such as schizophrenia or Parkinson disease) that could affect cognitive performance (Fig. 1). After random selection of subjects, letters are sent by MHS to the primary care physicians, asking for permission to contact each patient regarding the study. If the doctors agree, letters are sent to the subjects briefly describing the study and saying that they will be contacted by phone in the following 2-week period. Then the MHS team calls the subjects and asks for their participation after determining that they are fluent in Hebrew and have a family member or caregiver whom they see at least 10 h per week who is willing to be an informant for the study. Subjects who are willing to participate in the study are assessed at their residence (or they come to the Sheba Medical Center Memory Clinic, according to their preference) in two phases. First, the subject is visited by a study physician who, after the subject signs the informed consent form, performs medical, neurological, geriatric, and nutritional assessments (food frequency questionnaire) and draws blood for inflammatory markers (IL-6 and CRP) and Hp and apolipoprotein E (APOE) genotypes. In the second phase (optimally 2 weeks after the physician visit), the subject is visited by a neuropsychologist who administers a cognitive battery (described below) and questionnaires to the subject and informant for cognitive and functional impairment and for depression and behavioral disturbances characteristic of dementia. All subject cognitive data are discussed by a multidisciplinary consensus conference team in order to define the subject’s cognitive status (as cognitively normal, MCI, or dementia and their subtypes). If the subject is cognitively normal at baseline, the IDCD has follow-up interviews at 18-month intervals. If the subject has dementia, there is no additional follow-up. Subjects who are diagnosed as MCI at baseline are not included in the study; however, subjects who convert from cognitive normal status to MCI during follow-up continue their participation in the study until conversion to dementia.
Figure 1.
Study flowchart. IC, informed consent.
Cognitive assessment
The Clinical Dementia Rating scale.
The Clinical Dementia Rating scale assesses, through an interview with the subject and an informant, the severity of cognitive and functional impairment in six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care (22).
Mini-Mental State Exam.
The Mini-Mental State Exam (MMSE) is a 30-item questionnaire assessing orientation, concentration, memory, and language (23).
Outcome measures.
The outcome measures were based on a comprehensive neuropsychological battery. All neuropsychological test scores were transformed into z scores. Factor analysis revealed four cognitive domains, which were then scored as totals of z scores: episodic memory factor (included word list immediate and delayed recall and recognition from the Consortium to Establish a Registry for Alzheimer's Disease [CERAD] neuropsychological battery) (24), semantic categorization factor (25) (included the letter [26] and category fluency [24] and similarities [27]), attention/working memory factor (included the diamond cancellation and digit span forward and backward tests) (28), and an executive factor (included the Trail Making Test, Parts A and B [24] and the Digit Symbol Test [27]). Finally, we computed an overall measure summing the scores of all four domains (overall cognitive score).
Diagnosis of MCI and dementia
Diagnosis of MCI was based on the Peterson criteria (21). Dementia diagnosis was based on the DSM criteria (29).
Hp typing
Blood samples for Hp typing were taken during the physician assessment. Immediately after the bloods were drawn into an EDTA-containing vacutainer tube, the tubes were placed in an ice box until handling at Sheba Medical Center. Within up to 6 h from phlebotomy (as recommended), the bloods were centrifuged and serum was stored at −70°C until determination. Hp typing was performed on stored plasma samples by polyacrylamide gel electrophoresis as previously described (30). In brief, 10 µL of Hb-enriched plasma was subjected to electrophoresis in a nondenaturing gel, and the gel was subsequently immersed in solution containing a congener of benzidine with a precipitate forming in the gel corresponding to the location of Hb-Hp complexes. The Hp type of the sample was determined by the banding pattern of the Hp-Hb complexes with each of the three Hp types having a characteristic banding fingerprint. Previous work established a 1:1 correspondence between this method and a PCR-based method for Hp genotyping (30).
Covariates
The sociodemographic covariates were age at the time of cognitive assessment, years of education, sex, and number of follow-up years in the registry. The cardiovascular covariates were BMI, creatinine, total cholesterol, triglycerides, diastolic and systolic blood pressure, and HbA1c. For the cardiovascular covariates, we used the average of all the subject’s measurements available in the MHS diabetes registry.
Additional data from the MHS diabetes registry
Microalbumin levels in the urine were used to reflect microvascular disease, whereas diagnosis of MI was used to reflect macrovascular disease.
Statistical analyses
Our a priori hypothesis was that Hp 1-1 would have lower cognitive performance; we therefore compared this group to each of the other two genotypes (Hp 1-2 and Hp 2-2). For each of the five cognitive measures separately, ANCOVA compared Hp 1-1 to Hp 1-2 and Hp 1-1 to Hp 2-2, controlling for demographic factors (age, years of education, sex, and number of follow-up years in the registry) and cardiovascular factors (BMI, creatinine, total cholesterol, triglycerides, diastolic and systolic blood pressure, and HbA1c).
RESULTS
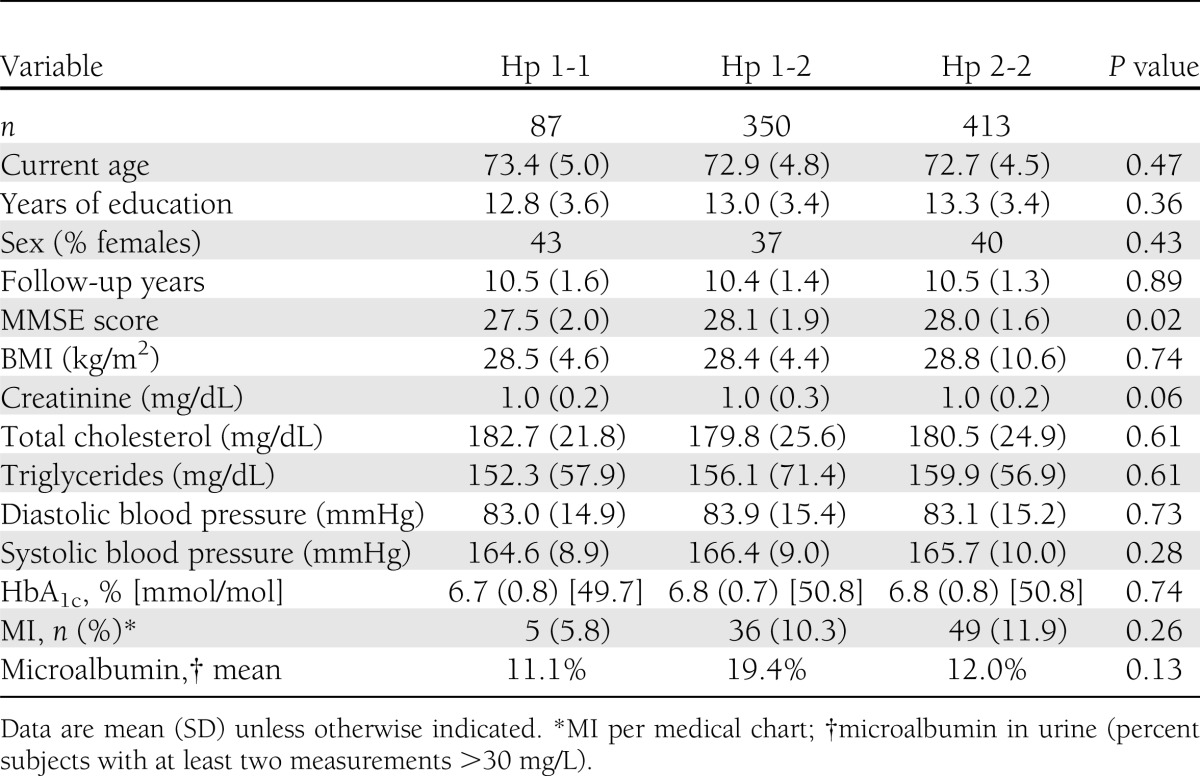
One thousand two hundred and eighty-eight subjects passed the preliminary screening, expressed consent to participate, were approached by a study physician, and signed informed consent. Of these 1,288 subjects, 282 (21.1%) were excluded from the study due to incompatibility with eligibility criteria (based on physician assessment), 109 (8.5%) refused to continue their participation in the study, and 897 remained active participants. The present analysis consists of 850 subjects who had an Hp genotype available, 87 with Hp 1-1, 350 with Hp 1-2, and 413 with Hp 2-2. Of these, 84, 335, and 393, respectively, had full data on demographic, cardiovascular, and cognitive data to allow comparisons. Table 1 describes the sample by Hp type. The entire sample's average was 72.9 years of age (SD 4.7) and 39% were women. Average education was 13.1 years (SD 3.4). Subjects were in the diabetes registry for 10.5 years (SD 1.4) on average, and their MMSE score was 28.0 (SD 1.8), consistent with a cognitively normal status in a sample with a broad education range. The mean HbA1c was 6.8% (SD 0.8), consistent with good diabetes control. The three groups of Hp genotypes did not differ significantly in any characteristic except for the MMSE, for which the Hp 1-1 had poorer performance (P = 0.02).
Table 1.
Sample characteristics
Cognitive function by Hp genotype
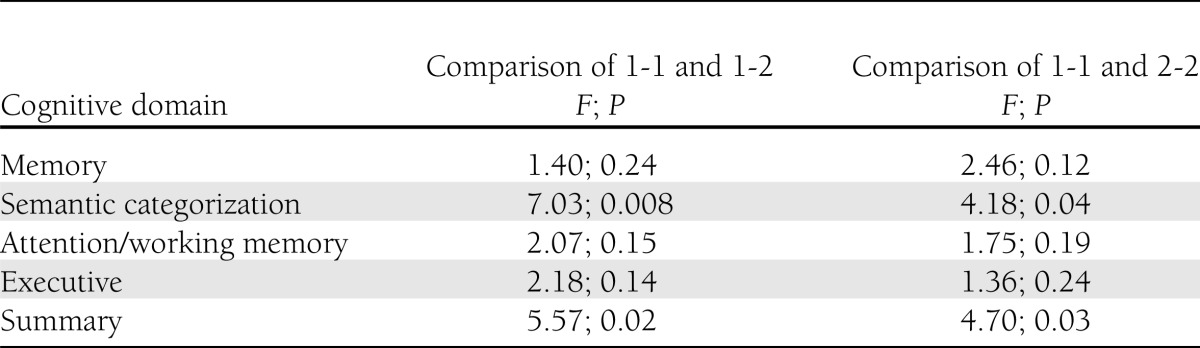
As depicted in Table 2, compared with Hp 1-2 and Hp 2-2, cognitive function (presented as z scores) of Hp 1-1 genotype was lower in all categories. Compared with subjects with the Hp 1-2 genotype, Hp 1-1 subjects performed significantly worse in semantic categorization (F = 7.03; P = 0.008) and overall cognition (F = 5.57; P = 0.02) (Table 3). Similarly, compared with subjects with Hp 2-2 genotype, Hp 1-1 subjects performed significantly worse in semantic categorization (F = 4.18; P = 0.04) and overall cognition (F = 4.70; P = 0.03) (Table 3).
Table 2.
Means and SDs of z scores of cognitive performance by cognitive domain and by Hp genotype
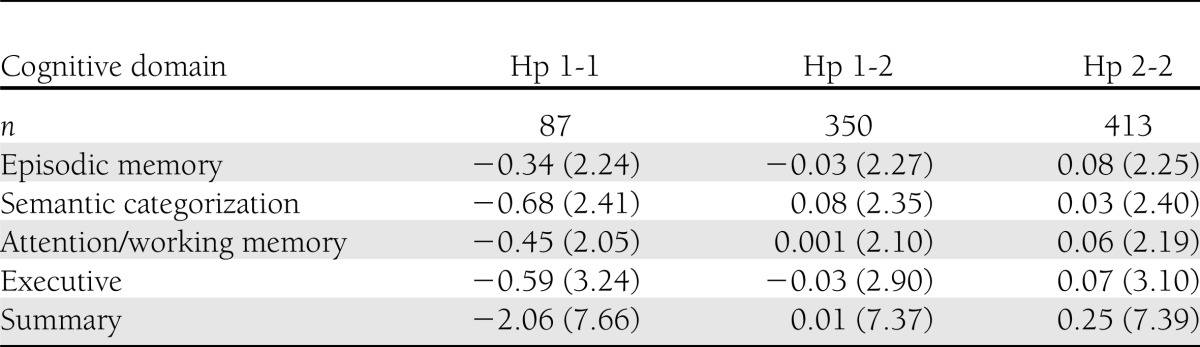
Table 3.
Comparison of cognitive function in Hp genotypes
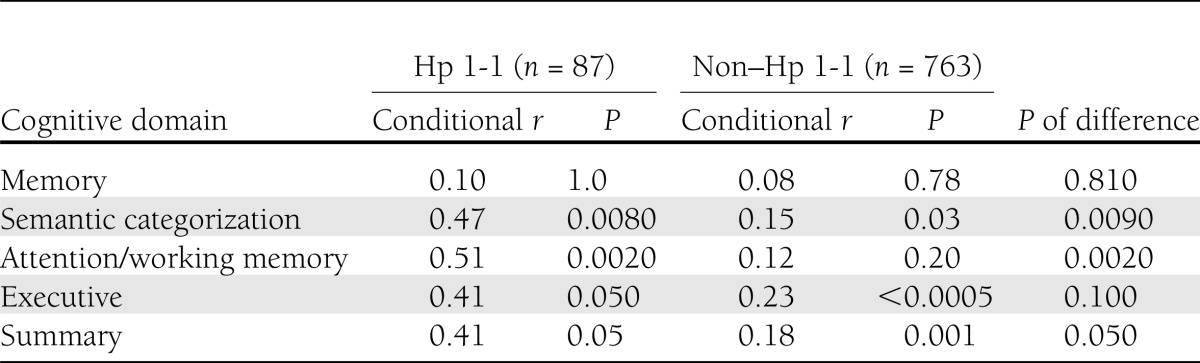
Since the results of the comparisons of Hp 1-1 with each of the Hp 2 carriers on cognitive functions were substantially similar (Tables 2 and 3), and in order to minimize multiple comparisons, in a secondary analysis, examining the relationship of cardiovascular risk factors and cognition by Hp genotype, we compared Hp 1-1 to Hp 2 carriers (Hp 1-2 and Hp 2-2). For each of the cognitive domains and overall cognition, for Hp 1-1 and Hp 2 carrier groups separately, stepwise multiple regressions evaluated the collective association with the seven cardiovascular risk factors, controlling for the demographic predictors. Since the sample sizes of Hp 1-2 and Hp 2-2 were at least eight times as large as the sample size for the Hp 1-1 group, the difference in power makes P values inappropriate when comparing the usefulness of cardiovascular predictors to cognition in the two Hp groups. A conditional r showed the proportion of variation not explained by the demographic predictors that was explained by the cardiovascular predictors. The distribution of each conditional r was approximately normal (31), so a Student t test (for normal distributions with different SDs) was used to compare them. For descriptive purposes, ANOVA and χ2 were used to compare the three Hp groups to describe demographic and cardiovascular characteristics. Table 4 shows the conditional r (the proportion of variation not explained by the demographic predictors that was explained by the cardiovascular predictors) in the two groups. In the Hp 1-1 group, the association of cardiovascular risk factors with cognition was significant for semantic categorization (P = 0.008), attention/working memory (P = 0.002), executive function (P = 0.05), and overall cognitive score (P = 0.05). In the Hp 2 carrier group, the association of cardiovascular risk factors with cognition was significant for semantic categorization (P = 0.03), executive function (P < 0.0005), and the overall cognitive score (P = 0.001). Compared with Hp 2 carriers, the conditional r was significantly higher in subjects with Hp 1-1 genotype for the semantic categorization (P = 0.009), attention/working memory (P = 0.002), and overall cognitive score (0.05). Cardiovascular risk factors were not associated with the episodic memory domain in either genotype.
Table 4.
Associations of cardiovascular risk factors with cognitive domains by Hp genotype
CONCLUSIONS
The current study reveals two important findings. First, compared with Hp 2 carriers (both Hp 1-2 and Hp 2-2), subjects with Hp 1-1 genotype performed significantly worse in the semantic categorization domain and the overall cognitive score (reflecting lower performance in the summary of all cognitive domains). Second, in both Hp 1-1 and the Hp 2 carriers, cardiovascular risk factors were associated with cognition with significantly stronger associations in the Hp 1-1 group for semantic categorization, attention/working memory, and overall cognition. Cardiovascular risk factors were not associated with the episodic memory domain in either group.
To the best of our knowledge, this is the first study to report an association between Hp type and cognition in type 2 diabetic subjects. In subjects with post-traumatic brain injury, a similar association was demonstrated, such that subjects with Hp 1-1 genotype had lower scores on verbal IQ and motor tests at 1 and 12 months after injury (32).
Previous studies have shown that Hp 2-2 genotype is associated with a less favorable cardiovascular profile. Since the latter is associated with cognitive function (33), Hp 2 allele could be expected to be associated with lower cognitive performance. However, the role of Hp in vascular pathology may differ according to the vascular bed (brain vs. periphery); the frequency of Hp 1-1 genotype was significantly higher and frequency of Hp 2 allele was significantly lower in a cohort of subjects with first symptomatic lacunar stroke due to small vessel disease compared with their frequency in healthy controls (18). Consistent with this finding, in hypertensive subjects with asymptomatic small vessel disease, Hp 1-1 was associated with larger volumes of deep WMLs when adjusting for age, sex, brain volume, 24-h mean arterial pressure, duration of hypertension, and previous antihypertensive treatment (19). Lacunar strokes and WML have been consistently associated with compromised cognitive functioning (34). These studies are consistent with the present neuropsychological findings and may explain the different role of Hp genotype in the brain compared with its role in the periphery.
Several mechanisms have been suggested to underlie the pathogenesis of small vessel disease (which includes WML and lacunar infarcts) (35) in the brain of Hp 1-1 carriers. Among these are endothelial damage, compromised ability to form new blood vessels, and ineffective functioning of the blood-brain barrier (18). These hypotheses are based on findings pointing to the association of Hp 1-1 with decreased activity of epithelial progenitor cells, which are involved in endothelial repair (35), a process that may attenuate the trajectories of damaged epithelium (35). In contrast, Hp 2-2 has been demonstrated in vitro to be superior in the promotion of new blood vessel formation compared with other Hp phenotypes (36).
An alternative explanation for our findings is a possible interaction of Hp genotype with hypertension, a risk factor for dementia by itself (37). Though groups stratified by Hp genotype did not differ in mean systolic or diastolic blood pressure values, the negative association between cardiovascular risk factors (including blood pressure) and cognition was consistently stronger in Hp 1-1 compared with Hp 2 carriers. In line with our findings, higher levels of Hp in hypertensive men were associated in a previous study with higher risk for stroke (38) and other sequelae of cardiovascular risk factors (39). These levels vary by genotype, with Hp 1-1 values being highest (39). The mechanisms for these associations are not fully understood.
The interaction of Hp genotype and cardiovascular risk factors was associated with lower cognitive performance in semantic categorization, attention/working memory, and overall cognitive score, with a similar trend for the executive function but not episodic memory domains. These findings may suggest that in type 2 diabetic subjects, a vascular mechanism (34) is involved in cognition rather than a hippocampal-related mechanism, which manifests typically in episodic memory impairment (40). Accordingly, the association of diabetes with performance for executive functions and overall cognition, but not episodic memory, has been demonstrated by some (41,42), but not all (43), previous studies.
This report is based on cross-sectional data; thus, we cannot rule out that the present results reflect an artifact of the selection of subjects. Hp 2 carriers, who tend to have more CVD (8,44), might have had higher mortality, morbidity, or cognitive impairment that precluded their participation in the study. Some previous cross-sectional studies have also demonstrated higher prevalence of CVD in Hp 1-1 carriers, but in longitudinal follow-up, Hp 2-2 was consistently associated with more incident events (44). The longitudinal follow-up of this sample is essential to shed light on this potential limitation and the interpretation of the current results.
Strengths of this study include the large sample, validated diabetes diagnosis for each subject, strong validity for risk factor levels and medical diagnosis, and thorough cognitive evaluation. The main limitation of this study is lack of brain imaging, thus limiting our ability to distinguish the vascular contribution to the association of Hp with cognition. The Hp phenotype is not available for subjects from the MHS diabetes registry who were excluded from the study, and the results reported here are cross-sectional (44). An additional limitation is lack of measurement of crystallized intelligence, preventing the evaluation of the contribution of this factor to our results. Nevertheless, we did control for education, which is a major contributor to crystallized intelligence. Women are slightly underrepresented in the study, potentially affecting generalizability of the results. Israel has a strong family-oriented culture, so a major role in grandparenting has been the primary reason of refusal of women to participate in the study. Subjects who do not have contact with a caregiver for 10 weekly hours were excluded to optimize diagnosis of cognitive status. This exclusion, however, may limit applicability.
In summary, in our sample of subjects with type 2 diabetes, cognitive function is associated with Hp genotype, being worse for Hp 1-1 compared with Hp 2-2 and Hp 1-2 genotypes. The cognitive domains affected are semantic categorization and overall cognitive score. Episodic memory is not affected. Furthermore, cardiovascular risk factors are associated with cognitive function in different domains (but not episodic memory) in all genotypes, but more strongly in the Hp 1-1.
Acknowledgments
This study was supported by the American Federation for Aging Research (Young Investigator Award 2011 to R.R.-S.), the Alzheimer’s Association (NIRG-11-205083 to R.R.-S.), the National Institute on Aging (R01-AG-034087 to M.S.-B. and P50-AG-05138 to M.S.), the Helen Bader Foundation, and the Irma T. Hirschl Scholar Award (to M.S.-B.). A.L. received a grant from the National Institutes of Health (R01-DK085226).
No potential conflicts of interest relevant to this article were reported.
R.R.-S. and M.S.-B. collaborated in study design, data collection, data research, and manuscript writing. A.H. researched the data and contributed to the discussion. J.S. performed statistical analysis and reviewed the manuscript. E.G.-B. reviewed the manuscript. M.S. reviewed the manuscript and contributed to the discussion. R.P., K.K., and H.H. searched the data. A.L. and J.M.S. reviewed the manuscript and contributed to the discussion. R.R.-S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Thomas VS, Darvesh S, MacKnight C, Rockwood K. Estimating the prevalence of dementia in elderly people: a comparison of the Canadian Study of Health and Aging and National Population Health Survey approaches. Int Psychogeriatr 2001;13(Suppl. 1):169–175 [DOI] [PubMed] [Google Scholar]
- 2.Cavalieri M, Ropele S, Petrovic K, et al. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care 2010;33:2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–575 [DOI] [PubMed] [Google Scholar]
- 4.Schnaider Beeri M, Goldbourt U, Silverman JM, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology 2004;63:1902–1907 [DOI] [PubMed] [Google Scholar]
- 5.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010;75:1195–1202 [DOI] [PubMed] [Google Scholar]
- 6.Peila R, Rodriguez BL, Launer LJ, Honolulu-Asia Aging Study Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262 [DOI] [PubMed] [Google Scholar]
- 7.Costacou T, Ferrell RE, Ellis D, Orchard TJ. Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes 2009;58:2904–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy AP, Hochberg I, Jablonski K, et al. Strong Heart Study Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol 2002;40:1984–1990 [DOI] [PubMed] [Google Scholar]
- 9.Levy AP, Gerstein HC, Miller-Lotan R, et al. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care 2004;27:2767. [DOI] [PubMed] [Google Scholar]
- 10.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care 2003;26:2628–2631 [DOI] [PubMed] [Google Scholar]
- 11.Suleiman M, Kapeliovich MR, Roguin A, et al. Haptoglobin type and 30-day mortality in diabetic individuals presenting with acute myocardial infarction. Diabetes Care 2003;26:2699–2700 [DOI] [PubMed] [Google Scholar]
- 12.Lioupis C, Barbatis C, Drougou A, et al. Association of haptoglobin genotype and common cardiovascular risk factors with the amount of iron in atherosclerotic carotid plaques. Atherosclerosis 2011;216:131–138 [DOI] [PubMed] [Google Scholar]
- 13.Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet 1982;12:189–261, 453–454 [DOI] [PubMed] [Google Scholar]
- 14.Bamm VV, Tsemakhovich VA, Shaklai M, Shaklai N. Haptoglobin phenotypes differ in their ability to inhibit heme transfer from hemoglobin to LDL. Biochemistry 2004;43:3899–3906 [DOI] [PubMed] [Google Scholar]
- 15.Vardi M, Levy AP. Is it time to screen for the haptoglobin genotype to assess the cardiovascular risk profile and vitamin E therapy responsiveness in patients with diabetes? Curr Diab Rep 2012;12:274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996;42:1589–1600 [PubMed] [Google Scholar]
- 17.Asleh R, Blum S, Kalet-Litman S, et al. Correction of HDL dysfunction in individuals with diabetes and the haptoglobin 2-2 genotype. Diabetes 2008;57:2794–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staals J, Pieters BM, Knottnerus IL, et al. Haptoglobin polymorphism and lacunar stroke. Curr Neurovasc Res 2008;5:153–158 [DOI] [PubMed] [Google Scholar]
- 19.Staals J, Henskens LH, Delanghe JR, et al. Haptoglobin phenotype correlates with the extent of cerebral deep white matter lesions in hypertensive patients. Curr Neurovasc Res 2010;7:1–5 [DOI] [PubMed] [Google Scholar]
- 20.Heymann AD, Chodick G, Halkin H, et al. The implementation of managed care for diabetes using medical informatics in a large Preferred Provider Organization. Diabetes Res Clin Pract 2006;71:290–298 [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194 [DOI] [PubMed] [Google Scholar]
- 22.Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer’s Disease. Aging (Milano) 1996;8:379–385 [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 24.Beeri MS, Schmeidler J, Sano M, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology 2006;67:1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernaeus SE, Almkvist O. Word production: dissociation of two retrieval modes of semantic memory across time. J Clin Exp Neuropsychol 1998;20:137–143 [DOI] [PubMed] [Google Scholar]
- 26.Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia: Manual of instructions (NCCEA). Rev. ed. Victoria, BC, University of Victoria, 1977 [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. San Antonio, The Psychological Corporation, 1981 [Google Scholar]
- 28.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, The Psychological Corporation, 1987 [Google Scholar]
- 29.American Psychiatric Association , Ed. Diagnostic and Statistical Manual of Mental Disorders IV. Washington, DC, American Psychiatric Association, 2010 [Google Scholar]
- 30.Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem 2002;48:1377–1382 [PubMed] [Google Scholar]
- 31.Olkin I, Finn JD. Correlations redux. Psychol Bull 1995;118:155–164 [Google Scholar]
- 32.Anderson GD, Temkin NR, Dikmen SS, et al. Haptoglobin phenotype and apolipoprotein E polymorphism: relationship to posttraumatic seizures and neuropsychological functioning after traumatic brain injury. Epilepsy Behav 2009;16:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Jacobs D, Andrews H, et al. Cardiovascular risk and memory in non-demented elderly women. Neurobiol Aging 2010;31:1250–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jokinen H, Gouw AA, Madureira S, et al. LADIS Study Group Incident lacunes influence cognitive decline: the LADIS study. Neurology 2011;76:1872–1878 [DOI] [PubMed] [Google Scholar]
- 35.Rouhl RP, van Oostenbrugge RJ, Damoiseaux JG, et al. Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr Neurovasc Res 2009;6:32–41 [DOI] [PubMed] [Google Scholar]
- 36.Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest 1993;91:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension 2012;59:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engström G, Lind P, Hedblad B, Stavenow L, Janzon L, Lindgärde F. Long-term effects of inflammation-sensitive plasma proteins and systolic blood pressure on incidence of stroke. Stroke 2002;33:2744–2749 [DOI] [PubMed] [Google Scholar]
- 39.Van Vlierberghe H, Langlois M, Delanghe J. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 2004;345:35–42 [DOI] [PubMed] [Google Scholar]
- 40.Henneman WJ, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology 2009;72:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spauwen PJ, Köhler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of type 2 diabetes on 12-year cognitive change: results from the Maastricht Aging Study. Diabetes Care 2013;36:1554–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen M, Pedersen KK, Bruunsgaard H, et al. Cognitive functions in middle aged individuals are related to metabolic disturbances and aerobic capacity: a cross-sectional study. PLoS ONE 2012;7:e51132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okereke OI, Kang JH, Cook NR, et al. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc 2008;56:1028–1036 [DOI] [PubMed] [Google Scholar]
- 44.Levy AP, Asleh R, Blum S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal 2010;12:293–304 [DOI] [PubMed] [Google Scholar]