Abstract
OBJECTIVE
Copeptin, a surrogate marker for arginine vasopressin, has been associated with cardiovascular (CV) events and mortality in patients with type 2 diabetes complicated by end-stage renal disease or acute myocardial infarction. For stable outpatients, these associations are unknown. Our aim was to investigate whether copeptin is associated with CV and all-cause mortality in patients with type 2 diabetes treated in primary care.
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes participating in the observational Zwolle Outpatient Diabetes Project Integrating Available Care (ZODIAC) study were included. Cox regression analyses with age as time scale were used to assess the relationship of baseline copeptin with CV and all-cause mortality.
RESULTS
We included 1,195 patients (age 67 ± 12 years, 44% male). Median baseline copeptin concentration was 5.4 (interquartile range [IQR] 3.1–9.6) pmol/L. After a median follow-up of 5.9 (IQR 3.2–10.1) years, 345 patients died (29%), with 148 CV deaths (12%). Log2 copeptin was associated with CV (hazard ratio 1.17 [95% CI 0.99–1.39]; P = 0.068) and all-cause mortality (1.22 [1.09–1.36]; P = 0.001) after adjustment for age, sex, BMI, smoking, systolic blood pressure, total cholesterol to HDL ratio, duration of diabetes, HbA1c, treatment with ACE inhibitors and angiotensin receptor blockers, history of CV diseases, log serum creatinine, and log albumin to creatinine ratio; however, copeptin did not substantially improve risk prediction for CV (integrated discrimination improvement 0.14% [IQR −0.27 to 0.55%]) and all-cause mortality (0.77% [0.17–1.37%]) beyond currently used clinical markers.
CONCLUSIONS
We found copeptin to be associated with CV and all-cause mortality in patients with type 2 diabetes treated in primary care. Intervention studies should show whether the high CV risk in type 2 diabetes can be reduced by suppression of vasopressin, for example by reducing salt intake.
The prevalence of type 2 diabetes and its complications are increasing worldwide (1). One of the major complications in type 2 diabetes is cardiovascular disease (CVD), and CVD is the main cause of morbidity and mortality in this patient group (2).
Arginine vasopressin (AVP), or antidiuretic hormone, is a hormone that exerts cardiovascular (CV) and renal effects (3). Several studies have reported that AVP levels are elevated in animals and patients with diabetes (4–7). Increased levels of AVP may have long-term deleterious effects. AVP acts through three different vasopressin receptors, the V1a, V2, and V3 (or V1b) receptors, which mediate vasoconstriction, stimulate water retention, and facilitate secretion of ACTH, respectively (3). High concentrations of plasma AVP are known to stimulate V1a receptors preferentially (8), which may contribute to the CV complications in type 2 diabetes.
Despite the pivotal role of AVP in CVD, technical difficulties related to AVP’s small size, short plasma half-life, and association with platelets in the circulation have hindered the large-scale clinical use of AVP as a biomarker (3,9,10). Vasopressin is synthesized from a polypeptide precursor that contains AVP, neurophysin II, and copeptin (3). Copeptin, or COOH-terminal proarginine vasopressin, is released in equimolar amounts to AVP during precursor processing and has been found to be a stable and sensitive surrogate marker for AVP (11,12).
A recent study of Fenske et al. (8) showed copeptin levels to be strongly associated with CV events and mortality in patients with type 2 diabetes and end-stage renal disease. Copeptin was also found to be associated with CV events in patients with acute myocardial infarction and type 2 diabetes (13). To our knowledge, however, these associations have not been demonstrated for stable, ambulatory patients with type 2 diabetes. This is of particular interest, because it could point to a new modifiable system for treatment and prevention of CV events and mortality in type 2 diabetes (8,14). Our primary objective was to assess the association of baseline plasma copeptin level with CV and all-cause mortality in a population of patients with type 2 diabetes treated in primary care. Our secondary aim was to investigate the additional predictive value of copeptin for risk prediction of CV and all-cause mortality in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study group
The Zwolle Outpatient Diabetes Project Integrating Available Care (ZODIAC) study was initiated in 1998 in the Zwolle region of the Netherlands. The design and details of this study have been published elsewhere (15,16). In this study, general practitioners were assisted by hospital-based diabetes specialist nurses in their care of patients with type 2 diabetes. In the first year, 1,143 patients with type 2 diabetes were included in this prospective cohort study. In 2001, additional 546 patients with type 2 diabetes enrolled, for a combined cohort of 1,689 patients (17). Baseline plasma copeptin values were measured in 1,257 patients (74%). In this study, we included 1,195 patients(95%) with complete data. The ZODIAC study was approved by the local medical ethics committee, and all patients provided informed consent.
Data collection and measurements
Baseline data, which were collected in 1998 and 2001, consisted of a full medical history, including a history of CVD, use of medication, and tobacco consumption. Patients were considered to have a history of CVD if they had a history of angina pectoris, myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass grafting, stroke, or transient ischemic attack. Laboratory and physical assessment data were collected annually and included nonfasting lipid profile, HbA1c, serum creatinine (SCr), urinary albumin to creatinine ratio (ACR), and blood pressure. SCr was measured by a kinetic colorimetric Jaffe method (Modular P Analyzer; Roche Almere, the Netherlands), ACR was measured by immunonephelometry (Behring Nephelometer; Behring Diagnostics, Mannheim, Germany), and blood pressure was measured twice with a Welch Allyn sphygmomanometer in the supine position after at least 5 min of rest. The creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation was used to obtain the estimated glomerular filtration rate (eGFR) (18).
Copeptin was measured in plasma samples collected at baseline and kept frozen at −80°C until analysis in 2010. Morgenthaler et al. (12) showed that prolonged frozen storage and repeated freeze-thaw cycles have no effect on copeptin values. Plasma copeptin was measured with a sandwich immunoassay (B.R.A.H.M.S. GmbH, Hennigsdorf, Germany), which was based on the assay described by Morgenthaler et al. (12). Measurement of copeptin was performed in batches. The lower detection limit was 0.4 pmol/L, the interassay coefficient of variation was <6% for copeptin concentrations >6 pmol/L, and the functional assay sensitivity (20% interassay coefficient of variation) was <1 pmol/L.
Clinical end points
In this study, we examined the association between baseline copeptin concentration and two coprimary clinical end points: CV mortality and all-cause mortality. In 2009, vital status and cause of death were retrieved from records maintained by the hospital and the general practitioners for the first 1,143 patients. For the additional 546 patients, vital status and cause of death were retrieved in 2005. Cause of death was coded according to the ICD-9. CV death was defined as any death in which the principal cause of death was CV in nature (ICD-9 codes 390–459).
Statistical analyses
Statistical analyses were performed with SPSS version 18.0 for Windows (IBM Corp., Armonk, NY) and STATA version 11 (StataCorp LP, College Station, TX). Results are expressed as mean ± SD or median (interquartile range [IQR]) for normally distributed and nonnormally distributed data, respectively. Nominal data are presented as the total number of patients with percentage (n [%]). A two-sided P < 0.05 was considered to indicate statistical significance.
For illustrative purposes, the study population was subdivided into tertiles according to baseline copeptin concentration, and data are presented accordingly to visualize associations with copeptin. Because copeptin concentrations are significantly higher in men than in women (19), tertiles were sex-stratified. P values for differences in copeptin tertiles were assessed with ANOVA for normally distributed continuous data, the Kruskal-Wallis test for nonnormally distributed data, and the χ2 test for nominal data. Multivariable linear regression analyses were used to investigate whether baseline copeptin concentration was associated with the clinical parameters. Because copeptin values were nonnormally distributed, logarithmic transformation (base 2) was applied to fulfill the criteria for linear regression analyses.
We investigated whether there were differences in baseline characteristics of patients with and without copeptin measurement. P values for differences between patients with and without copeptin measurement were assessed with the independent sample t test for normally distributed continuous data, the Mann-Whitney U test for nonnormally distributed data, and the χ2 test for nominal data.
Cox regression analyses were used to test whether there were interactions between copeptin and clinical parameters including age, sex, history of CVD, and duration of diabetes. We used Cox regression analyses with age as time scale in which we accounted for left truncation (delayed entry) to analyze the risk of CV and all-cause mortality during follow-up. We applied log2 transformation of copeptin values so the hazard ratios (HRs) derived from Cox regression analyses were expressed as an increase in risk per doubling of baseline copeptin values. Various models were built to adjust for possible confounders. First, the univariable association of log2 copeptin with CV and all-cause mortality was investigated. Second, the model was adjusted for age and sex. Finally, the model was additionally adjusted for CV risk factors and medication that could potentially influence copeptin secretion (BMI, smoking, systolic blood pressure, total cholesterol to HDL ratio, duration of diabetes, HbA1c, history of CVD, use of ACE inhibitors or angiotensin receptor blockers, log SCr, and log ACR). The assumption of proportional hazards for baseline predictors was investigated by inspecting the Schoenfeld residuals. As sensitivity analyses, we repeated the Cox regression analyses with follow-up time as time scale. Furthermore, we investigated the effect of inclusion of time-dependent covariates (age, systolic blood pressure, total cholesterol to HDL ratio, duration of diabetes, HbA1c, SCr, and ACR) in Cox regression analyses. In addition, Cox regression analyses were used to test whether an association existed between the presence or absence of a copeptin measurement and CV and all-cause mortality in the combined cohort of 1,689 patients.
Discrimination, a measure to evaluate how well a model distinguishes between patients who died and those who survived while taking follow-up time into account, was assessed with the Harrell's C statistic (20). The additional value of copeptin for the risk prediction of CV and all-cause mortality was assessed in terms of integrated discrimination improvement (IDI) and net reclassification improvement (NRI) (21). The IDI can be interpreted as the difference between model-based probabilities for events and nonevents for the models with and without copeptin. The NRI is calculated by assessing the net improvement in risk classification (<10, 10–20, 20–30, and >30%) for events and nonevents separately. Calibration, a measure to evaluate how well predicted probabilities agree with observed risks, was assessed with the Grønessby and Borgan goodness-of-fit likelihood ratio test (22).
RESULTS
Patient characteristics
A total of 1,195 patients with type 2 diabetes were included in this study. Mean age of the study population was 67 ± 12 years, and 524 patients (44%) were male. Median copeptin concentration was 5.4 (IQR 3.1–9.6) pmol/L. Median copeptin concentration was significantly higher in men than in women (7.4 [4.5–11.5] vs. 4.1 [2.6–7.3] pmol/L, respectively; P < 0.0001). Baseline patient characteristics are presented as sex-stratified tertiles in Table 1. Variables that were significantly different between tertiles of copeptin concentrations were age, history of CVD, BMI, total cholesterol to HDL ratio, duration of diabetes, HbA1c, ACR, SCr, and eGFR (Table 1). Multivariable linear regression analyses showed that sex (b = −0.57; P < 0.001), age (b = 0.01; P < 0.001), BMI (b = 0.03; P < 0.001), HbA1c (b = 0.11; P < 0.001), systolic blood pressure (b = −0.004; P < 0.001), log ACR (b = 0.21; P < 0.001), and log SCr (b = 3.69; P < 0.001) were associated with baseline copeptin concentrations.
Table 1.
Baseline patient characteristics of the study population presented as sex-stratified tertiles of copeptin concentration
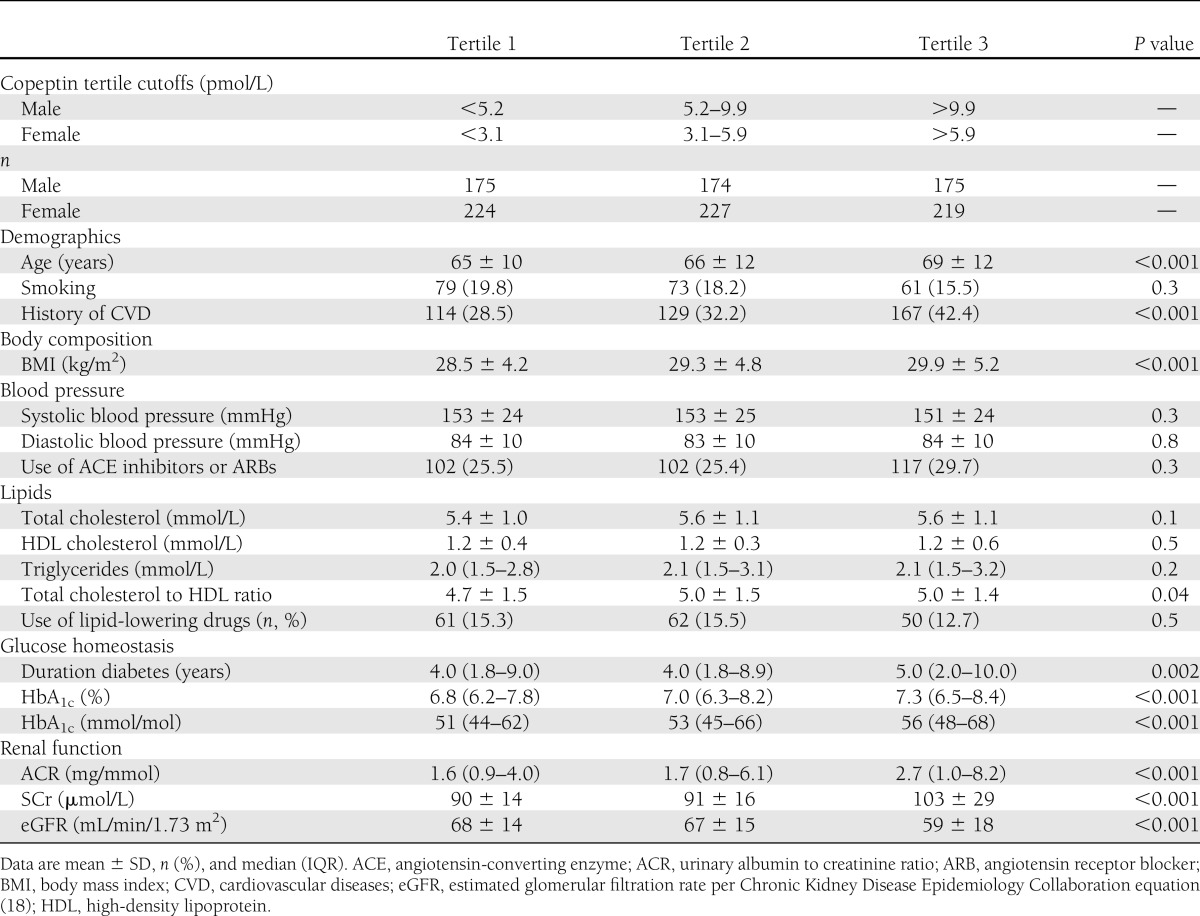
In addition, we investigated whether there were differences in baseline characteristics of patients with and without copeptin measurement (Supplementary Table 1). Only baseline serum creatinine values were slightly higher in patients without copeptin measurements (98 ± 23 µmol/L) than in patients with copeptin measurements (95 ± 22 µmol/L, P = 0.04). We found no other significant differences between baseline characteristics of patients with and without copeptin measurements.
Copeptin as a predictor of mortality
After a follow-up period of 10 years for the patients entering the study in 1998 and 3 years for those included in 2001, 345 of 1,195 included patients had died (29%), with 148 deaths (12%) attributable to CV causes. All-cause deaths (152 males [29%] vs. 193 females [29%]; P = 0.9) and CV deaths (64 males [12%] vs. 84 females [13%]; P = 0.9) were not more common among male subjects than among female subjects. Furthermore, there was no difference in median follow-up time for male and female subjects (5.5 [IQR 3.1–10.1] vs. 6.2 [3.2–10.1] years, respectively; P = 0.4). The median baseline copeptin concentration for survivors (4.9 [IQR 3.0–8.5] pmol/L) was significantly lower than both the median copeptin level of patients who had died of CV causes (7.9 [3.9–13.8] pmol/L) and the median copeptin level of patients who had died of all causes during follow-up (7.3 [3.7–13.0] pmol/L; P < 0.0001).
Cox regression analyses were used to investigate whether there were interactions between copeptin and clinical parameters including age, sex, history of CVD, and duration of diabetes. We found no significant interactions between copeptin and these clinical parameters.
In univariable Cox regression analyses with age as time scale, log2 copeptin was significantly associated with CV mortality (HR 1.55 [95% CI 1.34–1.80]; P < 0.001) and all-cause mortality (1.39 [1.26–1.53]; P < 0.001). The association of copeptin with all-cause mortality remained significant after adjustment for the various confounders (Table 2). The Schoenfeld residuals showed no substantial deviations, supporting the assumption of proportional hazards.
Table 2.
Association of baseline log2 copeptin concentrations with CV and all-cause mortality in Cox regression analyses with age as time scale
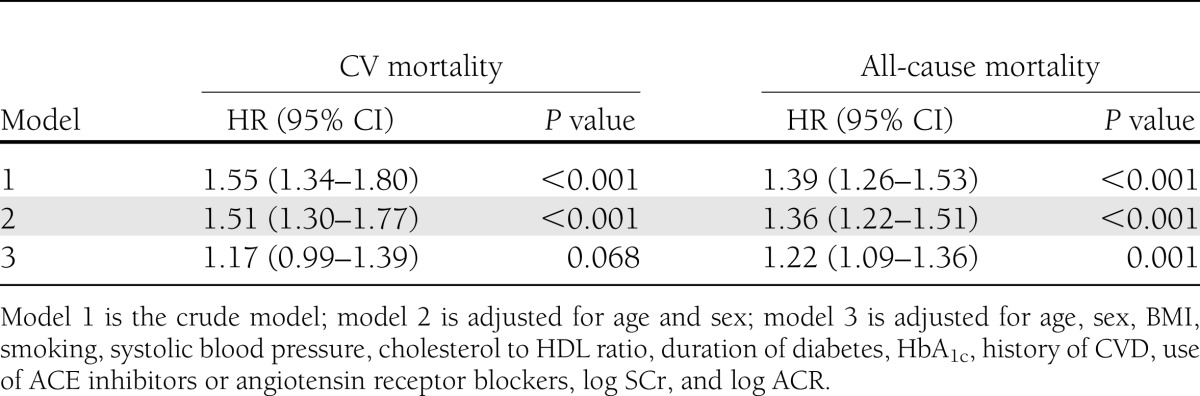
As sensitivity analyses, we repeated the Cox regression analyses with follow-up time as time scale (Table 3). The HRs and 95% CIs of the adjusted models in the sensitivity analyses were not materially different from the analyses with age as time scale. In the Cox regression models with follow-up time as time scale, the associations of copeptin with CV and all-cause mortality remained significant after adjustment for the different confounders (Table 3).
Table 3.
Association of baseline log2 copeptin concentrations with CV and all-cause mortality in Cox regression analyses with follow-up time as time scale
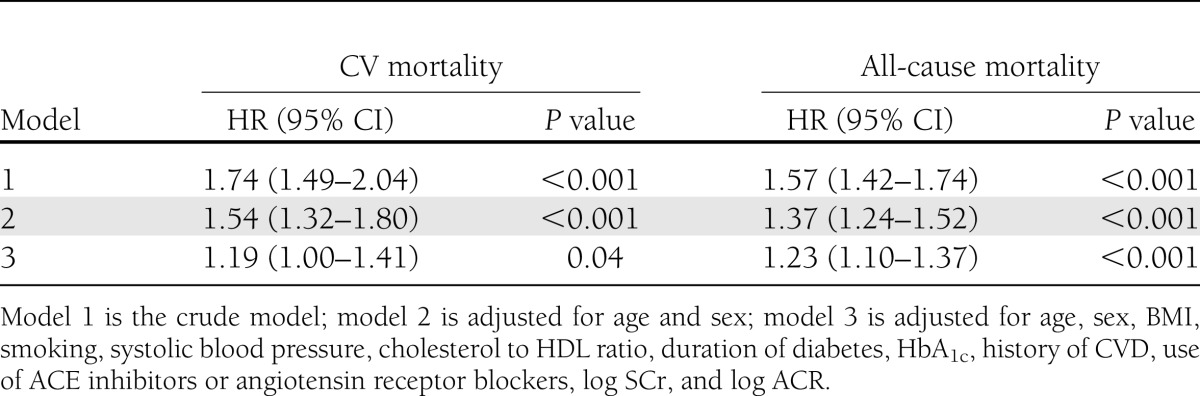
We also investigated the influence of time-dependent covariates in Cox regression analyses (Supplementary Table 2). In the fully adjusted models, log2 copeptin was significantly associated with CV mortality (HR 1.21 [95% CI 1.03–1.42]; P = 0.02) and all-cause mortality (1.23 [1.10–1.36]; P < 0.001).
Furthermore, we tested whether an association existed between the presence or absence of a copeptin measurement and CV and all-cause mortality in the combined cohort of 1,689 patients. In univariable and multivariable Cox regression analyses we found no association of the presence or absence of a copeptin measurement with CV and all-cause mortality.
Predictive value of copeptin
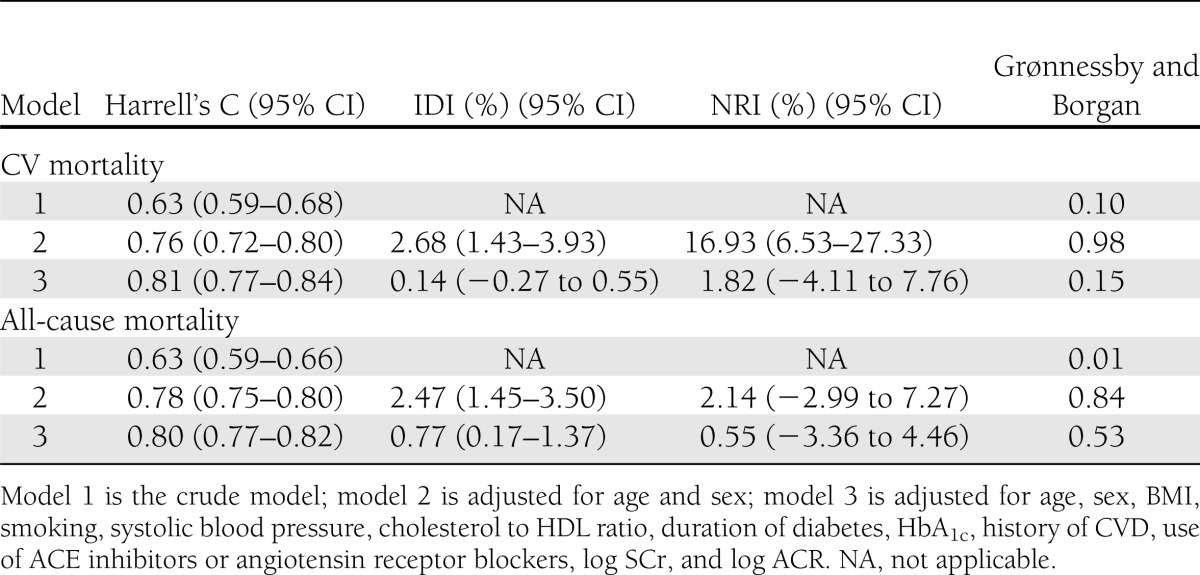
The additional value of copeptin for risk prediction of CV and all-cause mortality was assessed in terms of discrimination (Harrell's C statistic), NRI, and IDI (Table 4). Harrell's C statistics for models 2 and 3 without copeptin predicting CV mortality were 0.75 (0.71–0.78) and 0.80 (0.77–0.84), respectively. Harrell's C statistics for models 2 and 3 without copeptin predicting all-cause mortality were 0.77 (0.75–0.80) and 0.79 (0.77–0.82), respectively. Harrell's C statistics in Table 4 show that the more confounders we adjusted for, the better the model predicted CV and all-cause mortality. The Grønessby and Borgan P values in Table 4 indicate that predicted probabilities correspond well with observed risks (except for model 1 predicting all-cause mortality), so the models were well calibrated. The IDI and NRI values for model 2 predicting CV mortality were 2.68% and 16.93%, respectively, indicating that copeptin had additional value on top of age and sex for risk prediction of CV mortality. In the fully adjusted models, however, the IDI and NRI values appeared to be <2%, indicating that copeptin did not substantially improve risk prediction for CV and all-cause mortality beyond currently used clinical markers.
Table 4.
Additional value of baseline log2 copeptin concentrations in risk prediction compared with established CV risk markers
CONCLUSIONS
In this prospective cohort of 1,195 patients with type 2 diabetes treated in primary care, we found copeptin to be associated with CV and all-cause mortality. After adjustment for established CV risk factors, we observed only a trend between copeptin and CV mortality, whereas the association of baseline plasma copeptin with all-cause mortality remained significant. Our findings are of particular interest because the AVP system is potentially modifiable through pharmacological and nonpharmacological interventions and could provide a possible target for treatment and prevention of CV events and mortality in type 2 diabetes.
Several studies have reported that plasma AVP levels are elevated in animals and patients with diabetes (4–7). AVP promotes water reabsorption through stimulation of V2 receptors, and it is suggested that increased levels of AVP limit glucose-induced water loss in patients with diabetes (23).
Increased levels of AVP may, however, have long-term deleterious renal and CV effects. In experimental animal studies, as well as in humans, it has been shown that AVP contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes (24,25). This notion is supported by the renal protective effects of AVP inhibition by drinking water or chronic treatment with a V2 receptor antagonist in rats with renal failure (26,27) and diabetes (28).
High concentrations of plasma AVP are known to stimulate V1a receptors preferentially (8), which results in coronary vasoconstriction (29), increasing afterload, ventricular stress, and cardiac hypertrophy (8,30,31). Several studies have reported that copeptin, a surrogate for AVP, is associated with CV events and mortality in patients with CVD (acute myocardial infarction, heart failure, and stroke) (3). A recent study of Fenske et al. (19) showed that copeptin is associated with CV events, sudden death, and all-cause mortality in patients with type 2 diabetes and end-stage renal disease.
Stimulation of V3 (V1b) receptors through AVP results in the release of ACTH, which stimulates cortisol release from the adrenal gland (3). AVP-induced ACTH release is reported to be less sensitive to feedback inhibition by glucocorticoids than ACTH induced by corticotropin-releasing hormone (32), which might worsen multiple aspects of the metabolic syndrome.
Median copeptin concentration of this study group was 5.4 (range 0.9–85.7) pmol/L, which is higher than the median value of 4.2 (range 1–13.8 pmol/L) pmol/L measured in healthy subjects (33). In line with previous studies, we found baseline plasma copeptin to be associated with renal function and albuminuria (34,35).
In addition, we found that baseline copeptin concentration was associated with CV and all-cause mortality; however, we found no differences in the number of deaths, number of CV deaths, or follow-up time between male and female subjects. This lack of difference is consistent with the literature on CV risk in diabetes, which indicates that women with diabetes have a higher relative risk for CV events than men with diabetes (36,37). Furthermore, copeptin values have consistently been shown to be higher in males than in females, even in healthy subjects (12,19). Risk categories that are based on copeptin level or reference values for copeptin concentrations should therefore be sex specific.
Several studies have shown that copeptin measurement has diagnostic and prognostic value in patients with acute CVD (3). Because copeptin was found to be associated with CV and all-cause mortality in patients with type 2 diabetes, we investigated whether copeptin had additional value for risk prediction of CV and all-cause mortality. In this study population, copeptin did not substantially improve risk prediction for CV and all-cause mortality beyond currently used clinical markers; however, copeptin was found to be associated with several CV risk factors (BMI, HbA1c, systolic blood pressure, SCr, and ACR), and copeptin substantially improved risk prediction for CV mortality beyond age and sex. Thus copeptin might be a unified marker for these known causes of CVD and therefore useful to discriminate patients who could benefit from intensification of therapy (38).
We acknowledge that this study has several limitations. First, given the observational nature of this study, it is impossible to draw a definite conclusion about the causality of the association of copeptin with CV and all-cause mortality. Second, selection bias may have occurred because patients whose copeptin had not been measured were excluded from statistical analysis. In additional Cox regression analyses, however, we found no significant association of the presence or absence of a copeptin measurement with CV and all-cause mortality. Third, blood samples were taken without restriction on food or water intake, which could have influenced plasma osmolarity and consequently copeptin concentration. Furthermore, measured plasma osmolarity, data required to calculate plasma osmolarity, and data on the use of diuretics were not available in this study group, which is a limitation of the current study because plasma osmolality and plasma volume are determinants of AVP secretion. In addition, no data on plasma albumin levels and total plasma protein were available in the current study. It could have been interesting to include these measures, because plasma albumin and protein may influence plasma osmolality and subsequently levels of AVP. Finally, the number of CV deaths in this study population was relatively small, which limits the number of covariates used in Cox regression analyses. It has been suggested that for each variable included in the model at least 10 events are required (20). With inclusion of 13 variables in the final Cox regression models, we are approaching the maximum number of variables allowed by the number of 148 CV deaths.
A strength of this study is that it is the first to investigate the association of copeptin with CV and all-cause mortality in patients with type 2 diabetes. In addition, this study included a relatively large observational cohort of patients with type 2 diabetes with a relatively long follow-up period (10 years) and a reasonable number of events (all-cause mortality). We therefore could prospectively investigate the association of baseline plasma copeptin levels with CV and all-cause mortality.
In conclusion, in this cohort of patients with type 2 diabetes, plasma copeptin, a surrogate marker for AVP, was associated with CV and all-cause mortality. These findings suggest that AVP may play a role in CV complications of type 2 diabetes and that interventions intended to lower AVP (e.g., by limiting sodium intake, improving glycemic control, and improving or preventing nephropathy-associated albuminuria) may be beneficial for the prevention of CV complications in type 2 diabetes. Water supplementation in patients without low serum albumin, edema, or risk for hyponatremia would also be a possible means of lowering AVP secretion. Furthermore, copeptin might be a unified marker for known causes of CVD and might be useful to discriminate patients who would benefit from intensification of therapy.
Acknowledgments
I.J.R. and S.J.L.B. received support from the Netherlands Heart Foundation, the Dutch Diabetes Research Foundation, and the Dutch Kidney Foundation, together participating in the framework of the Center for Translational Molecular Medicine (www.ctmm.nl), project PREDICCt (grant 01C-104).
J.S. is employed by B.R.A.H.M.S. GmbH, a company that manufactures and holds patent rights on the CT-pro-AVP (copeptin) assay. No other potential conflicts of interest relevant to this article were reported.
I.J.R. researched data, contributed to the discussion, and wrote the manuscript. W.E.B. and K.H.G. researched data and reviewed and edited the manuscript. A.A., K.J.J.v.H., J.S., and H.J.G.B. reviewed and edited the manuscript. N.K., R.T.G., G.N., and S.J.L.B. reviewed and edited the manuscript and contributed to the discussion. I.J.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 48th Annual Meeting of the European Association for the Study of Diabetes, 1–5 October 2012, Berlin, Germany, and at the Kidney Week of the American Society of Nephrology, San Diego, California, 30 October–4 November 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2165/-/DC1.
References
- 1.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes 2012;19:93–96 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgenthaler NG. Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail 2010;16(Suppl. 1):S37–S44 [DOI] [PubMed] [Google Scholar]
- 4.Bankir L, Bardoux P, Ahloulay M. Vasopressin and diabetes mellitus. Nephron 2001;87:8–18 [DOI] [PubMed] [Google Scholar]
- 5.Zerbe RL, Vinicor F, Robertson GL. Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes 1979;28:503–508 [DOI] [PubMed] [Google Scholar]
- 6.Kamoi K, Ishibashi M, Yamaji T. Thirst and plasma levels of vasopressin, angiotensin II and atrial natriuretic peptide in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 1991;11:195–202 [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki Y, Kondo K, Murase T, Hasegawa H, Oiso Y. Osmoregulation of plasma vasopressin in diabetes mellitus with sustained hyperglycemia. J Neuroendocrinol 1996;8:755–760 [DOI] [PubMed] [Google Scholar]
- 8.Fenske W, Wanner C, Allolio B, et al. German Diabetes, Dialysis Study Investigators Copeptin levels associate with cardiovascular events in patients with ESRD and type 2 diabetes mellitus. J Am Soc Nephrol 2011;22:782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voors AA, von Haehling S, Anker SD, et al. OPTIMAAL Investigators C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J 2009;30:1187–1194 [DOI] [PubMed] [Google Scholar]
- 10.Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 1983;5:I129–I138 [DOI] [PubMed] [Google Scholar]
- 11.Bolignano D, Zoccali C. Vasopressin beyond water: implications for renal diseases. Curr Opin Nephrol Hypertens 2010;19:499–504 [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–119 [DOI] [PubMed] [Google Scholar]
- 13.Mellbin LG, Rydén L, Brismar K, Morgenthaler NG, Ohrvik J, Catrina SB. Copeptin, IGFBP-1, and cardiovascular prognosis in patients with type 2 diabetes and acute myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care 2010;33:1604–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enhorning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, Morgenthaler NG, Nilsson PM, Melander O. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: the prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes (Lond) 2012;37;598–603 [DOI] [PubMed]
- 15.Ubink-Veltmaat LJ, Bilo HJ, Groenier KH, Rischen RO, Meyboom-de Jong B. Shared care with task delegation to nurses for type 2 diabetes: prospective observational study. Neth J Med 2005;63:103–110 [PubMed] [Google Scholar]
- 16.Drion I, Kleefstra N, Landman GW, et al. Plasma COOH-terminal proendothelin-1: a marker of fatal cardiovascular events, all-cause mortality, and new-onset albuminuria in type 2 diabetes? (ZODIAC-29). Diabetes Care 2012;35:2354–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutgers HL, Gerrits EG, Graaff R, et al. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia 2009;52:789–797 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenske W, Störk S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 2009;94:123–129 [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387 [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal 1998;4:109–120 [DOI] [PubMed] [Google Scholar]
- 23.Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol 2005;16:1920–1928 [DOI] [PubMed] [Google Scholar]
- 24.Bardoux P, Martin H, Ahloulay M, et al. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressin-deficient Brattleboro rats. Proc Natl Acad Sci USA 1999;96:10397–10402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardoux P, Bichet DG, Martin H, et al. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 2003;18:497–506 [DOI] [PubMed] [Google Scholar]
- 26.Bouby N, Bachmann S, Bichet D, Bankir L. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol 1990;258:F973–F979 [DOI] [PubMed] [Google Scholar]
- 27.Sugiura T, Yamauchi A, Kitamura H, et al. High water intake ameliorates tubulointerstitial injury in rats with subtotal nephrectomy: possible role of TGF-beta. Kidney Int 1999;55:1800–1810 [DOI] [PubMed] [Google Scholar]
- 28.Bardoux P, Bruneval P, Heudes D, Bouby N, Bankir L. Diabetes-induced albuminuria: role of antidiuretic hormone as revealed by chronic V2 receptor antagonism in rats. Nephrol Dial Transplant 2003;18:1755–1763 [DOI] [PubMed] [Google Scholar]
- 29.Maturi MF, Martin SE, Markle D, et al. Coronary vasoconstriction induced by vasopressin. Production of myocardial ischemia in dogs by constriction of nondiseased small vessels. Circulation 1991;83:2111–2121 [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith SR. Vasopressin as vasopressor. Am J Med 1987;82:1213–1219 [DOI] [PubMed] [Google Scholar]
- 31.Fukuzawa J, Haneda T, Kikuchi K. Arginine vasopressin increases the rate of protein synthesis in isolated perfused adult rat heart via the V1 receptor. Mol Cell Biochem 1999;195:93–98 [DOI] [PubMed] [Google Scholar]
- 32.Rabadan-Diehl C, Aguilera G. Glucocorticoids increase vasopressin V1b receptor coupling to phospholipase C. Endocrinology 1998;139:3220–3226 [DOI] [PubMed] [Google Scholar]
- 33.Morgenthaler NG, Struck J, Jochberger S, Dünser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab 2008;19:43–49 [DOI] [PubMed] [Google Scholar]
- 34.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int 2010;77:29–36 [DOI] [PubMed] [Google Scholar]
- 35.Meijer E, Bakker SJ, de Jong PE, et al. Copeptin, a surrogate marker of vasopressin, is associated with accelerated renal function decline in renal transplant recipients. Transplantation 2009;88:561–567 [DOI] [PubMed] [Google Scholar]
- 36.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002;162:1737–1745 [DOI] [PubMed] [Google Scholar]
- 37.Rivellese AA, Riccardi G, Vaccaro O. Cardiovascular risk in women with diabetes. Nutr Metab Cardiovasc Dis 2010;20:474–480 [DOI] [PubMed] [Google Scholar]
- 38.Enhörning S, Struck J, Wirfält E, Hedblad B, Morgenthaler NG, Melander O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab 2011;96:E1065–E1072 [DOI] [PubMed] [Google Scholar]


