Abstract
OBJECTIVE
Blood pressure control can reduce the risk of coronary heart disease (CHD) among diabetic patients; however, it is not known whether the lowest risk of CHD is among diabetic patients with the lowest blood pressure level.
RESEARCH DESIGN AND METHODS
We performed a prospective cohort study (2000–2009) on diabetic patients including 17,536 African Americans and 12,618 whites. Cox proportional hazards regression models were used to estimate the association of blood pressure with CHD risk.
RESULTS
During a mean follow-up of 6.0 years, 7,260 CHD incident cases were identified. The multivariable-adjusted hazard ratios of CHD associated with different levels of systolic/diastolic blood pressure at baseline (<110/65, 110–119/65–69, 120–129/70–80, and 130–139/80–90 mmHg [reference group]; 140–159/90–100; and ≥160/100 mmHg) were 1.73, 1.16, 1.04, 1.00, 1.06, and 1.11 (P trend <0.001), respectively, for African American diabetic patients, and 1.60, 1.27, 1.08, 1.00, 0.95, and 0.99 (P trend<0.001) for white diabetic patients, respectively. A U-shaped association of isolated systolic and diastolic blood pressure at baseline as well as blood pressure during follow-up with CHD risk was observed among both African American and white diabetic patients (all Ptrend <0.001). The U-shaped association was present in the younger age-group (30–49 years), and this U-shaped association changed to an inverse association in the older age-group (≥60 years).
CONCLUSIONS
Our study suggests that there is a U-shaped or inverse association between blood pressure and the risk of CHD, and aggressive blood pressure control (blood pressure <120/70 mmHg) is associated with an increased risk of CHD among both African American and white patients with diabetes.
Hypertension and diabetes are two important public health problems in the U.S. Hypertension is a prevalent condition affecting ~65 million Americans (1), and diabetes is considered ‘‘the epidemic of the 21st century,’’ affecting ~24 million Americans (2). As many as 70% of people aged >40 years who have diabetes are also affected by hypertension, with black and Hispanic individuals affected disproportionately compared with the rest of the population (3,4). All guidelines recommend that blood pressure goals should be more aggressive (<130/80 mmHg) in diabetic patients than in people without diabetes (<140/90 mmHg) (5–7). In the aggressive control of hypertension, the mantra of “lower is better” was mostly based on the landmark randomized clinical trials (RCTs) published in the 1990s (8–10) that established clear benefit with regard to cardiovascular risk reduction when blood pressure was lowered intensively in patients with diabetes.
Aggressive targets for blood pressure treatment in type 2 diabetes guidelines have recently been questioned. Data from more contemporary populations with hypertension and diabetes do not confirm the benefit to coronary heart disease (CHD) of intensive blood pressure control (11,12). Specifically, a relationship between adverse cardiovascular outcomes and low blood pressure has been observed in some studies (13,14). However, most studies only use a single baseline measurement of blood pressure to predict CHD risk, which may produce potential bias. Moreover, very few studies have assessed the race-specific association of blood pressure with CHD risk. The aim of the current study is to examine the race-specific association between blood pressure and the risk of CHD among African American and white diabetic patients in the Louisiana State University Hospital–Based Longitudinal Study (LSUHLS).
RESEARCH DESIGN AND METHODS
LSU Health Care Services Division (LSUHCSD) operates seven public hospitals and affiliated clinics in Louisiana, which provide quality medical care to the residents of Louisiana regardless of their income or insurance coverage (15–20). Overall, LSUHCSD facilities have served ~1.6 million patients (35% of the Louisiana population) since 1997. Administrative (name, address, date of birth, sex, race/ethnicity, types of insurance, family income, and smoking status), anthropometric (date of examination, measurements of body weight, height, and blood pressure for each visit), laboratory (test code, test collection date, test result values, and abnormal flag), clinical diagnosis (date of diagnosis, diagnosis code, priority assigned to diagnosis and ICD-9 and Current Procedural Terminology procedure codes), and medication (medication generic name, pharmacopeia dispensable drug ID, medication strength-dose form, medication strength units, medication rote code and description, medication form, etc.) data collected at these facilities are available in electronic form for both inpatients and outpatients from 1997. Using these data, we have established the LSUHLS (15). A cohort of diabetic patients was set up by using the ICD-9 (code 250) through the LSUHLS database between 1 January 1999 and 31 December 2009. Both inpatients and outpatients were included, and all patients were under primary care. LSUHCSD’s internal diabetes disease-management guidelines call for physician confirmation of diabetes diagnoses by applying the American Diabetes Association criteria: a fasting plasma glucose level ≥126 mg/ dL, 2-h glucose level ≥200 mg/dL after a 75-g 2-h oral glucose tolerance test, and one or more classic symptoms plus a random plasma glucose level ≥200 mg/dL (21). The first record of diabetes diagnosis was used to establish the baseline for each patient in the present analyses due to the design of the cohort study. These newly diagnosed diabetic participants had benefited from LSUHCSD hospitals for 3.68 ± 4.35 years prior to the baseline. The current study included 30,154 diabetic patients (12,618 whites and 17,536 African Americans) who were 30–94 years of age without a history of CHD or stroke and with complete repeated data on major risk factor variables. In these diabetic patients, ~78.9% of patients qualify for free care (by virtue of being low income and uninsured—any individual or family unit whose income is ≤200% of federal poverty level), ~5.1% of patients are self-pay (uninsured, but incomes not low enough to qualify for free care), ~5.1% of patients are covered by Medicaid, ~8.9% of patients have Medicare, and ~2.2% of patients are covered by commercial insurance. The study and analysis plan were approved by both Pennington Biomedical Research Center and LSU Health Sciences Center institutional review boards, LSU System. We did not obtain informed consent from participants involved in our study because we used anonymized data compiled from electronic medical records.
Baseline and follow-up measurements
The patient’s characteristics, including age of diabetes diagnosis, sex, race/ethnicity, family income, smoking status, types of insurance, body weight, height, BMI, blood pressure, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, HbA1c, estimated glomerular filtration rate (eGFR), and medication (antihypertensive drug, cholesterol-lowing drug and antidiabetes drug), within a half year after the diabetes diagnosis (baseline) and during follow-up after the diabetes diagnosis (follow-up) were extracted from the computerized hospitalization records. Blood pressure was measured from the right arm of the participant after 5 min of sitting using a mercury sphygmomanometer or electronic blood pressure meter in each visit (19). An updated mean value of blood pressure over time was calculated for each individual from diabetes diagnosis to the final clinical measure of follow-up or to the last value before the occurrence of CHD. The average number of blood pressure measurements during the follow-up period was 14.6 times.
Prospective follow-up
Follow-up information was obtained from the LSUHLS inpatient and outpatient database by using the unique number assigned to every patient who visits the LSUHCSD hospitals each time. The diagnosis of CHD was the primary end point of interest of the study and was defined according to the ICD-9: CHD (ICD-9 codes 410–414). Since 1997, diagnoses of CHD were made by the treating physicians based on a clinical assessment and examinations as considered relevant by the clinician in charge of treatments. Follow-up of each cohort member continued until the date of the diagnosis of CHD, the date of the last visit if the subject stopped use of LSUHCSD hospitals, death, or 31 May 2012 (18).
Statistical analyses
The association between blood pressure and the risk of CHD was analyzed by using Cox proportional hazards models. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were evaluated in the following two ways 1) as six categories (SBP <110, 110–119, 120–129, 130–139 [reference group], 140–159, and ≥160 mmHg; DBP <65, 65–69, 70–79, 80–89 [reference group], 90–100, and ≥100 mmHg; and SBP/DBP <110/65, 110–119/65–69, 120–129/70–79, 130–139/80–89 [reference group], 140–159/90–99, and ≥160/100 mmHg), and 2) as a continuous variable (SBP and DBP). Different levels of blood pressure were included in the models as dummy and categorical variables, and the significance of the trend over different categories of blood pressure was tested in the same models by giving an ordinal numeric value for each dummy variable. The proportional hazards assumption in the Cox model was assessed with graphical methods. All analyses were adjusted for age and sex and further for smoking, income, types of insurance, HbA1c, LDL cholesterol, eGFR, use of antihypertensive drugs, use of diabetes medications, use of cholesterol-lowering agents, SBP/DBP during follow-up in baseline analysis, or baseline SBP/DBP in follow-up analysis. Since the interactions between sex and blood pressure and CHD risk were not statistically significant among both whites and African Americans, data for men and women were combined in the analyses. To avoid the potential bias due to severe diseases at baseline, additional analyses were carried out excluding the subjects who were diagnosed CHD during the first 2 years of follow-up. We used restricted cubic splines in Cox models to test whether there is a dose-response or nonlinear association of blood pressure as a continuous variable with CVD risk because a J- or U-shaped association of blood pressure with CVD risk has been found in prospective studies and RCTs (11,12,22). Statistical significance was considered to be P < 0.05. All statistical analyses were performed with PASW for Windows, version 20.0 (IBM SPSS, Chicago, IL).
RESULTS
General characteristics of the study population are presented by race in Supplementary Table 1. During a mean follow-up period of 6.0 years, 7,260 subjects (3,580 whites and 3,680 African Americans) developed incident CHD. The age- and sex-adjusted hazard ratios (HRs) for incident CHD at different levels of SBP at baseline (<110, 110–119, 120–129, 130–139 [reference group], 140–159, and ≥160 mmHg) were 1.27 (95% CI 1.07–1.51), 1.10 (0.95–1.27), 1.03 (0.91–1.16), 1.00, 1.05 (0.95–1.16), and 1.12 (1.01–1.24) (P trend = 0.058) for African American diabetic patients and 1.57 (1.35–1.82), 1.14 (1.00–1.30), 1.05 (0.93–1.18), 1.00, 0.98 (0.89–1.08), and 1.03 (0.93–1.15) (P trend<0.001) for white diabetic patients, respectively (Table 1). A significantly increased risk of CHD was observed among both African American and white diabetic patients with SBP <120 mmHg and a borderline significantly increased risk with SBP ≥160 mmHg. After further adjustment for other confounding factors (smoking, income, type of insurance, BMI, A1C, LDL cholesterol, eGFR, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents), this U-shaped association did not change among white (P trend <0.001) or African American (P trend = 0.057) diabetic patients. The multivariable-adjusted HRs of CHD associated with DBP at baseline (<65, 65–69, 70–80, 80–90 [reference group], 90–100, and ≥100 mmHg) were 1.25, 1.07, 0.96, 1.00, 1.03, and 1.12 (P trend <0.001) for African American diabetic patients and 1.38, 1.29, 1.03, 1.00, 0.97, and 1.13 (P trend <0.001) for white diabetic patients, respectively (Table 1). When SBP or DBP was considered as a continuous variable by using restricted cubic splines, a nadir of the U-shaped association of blood pressure with CHD risk was observed at 130–140 mmHg SBP and 80–90 mmHg DBP (Supplementary Fig. 1).
Table1.
HR (95% CI) of CHD according to different levels of SBP and DBP at baseline and during follow-up among African American and white patients with diabetes
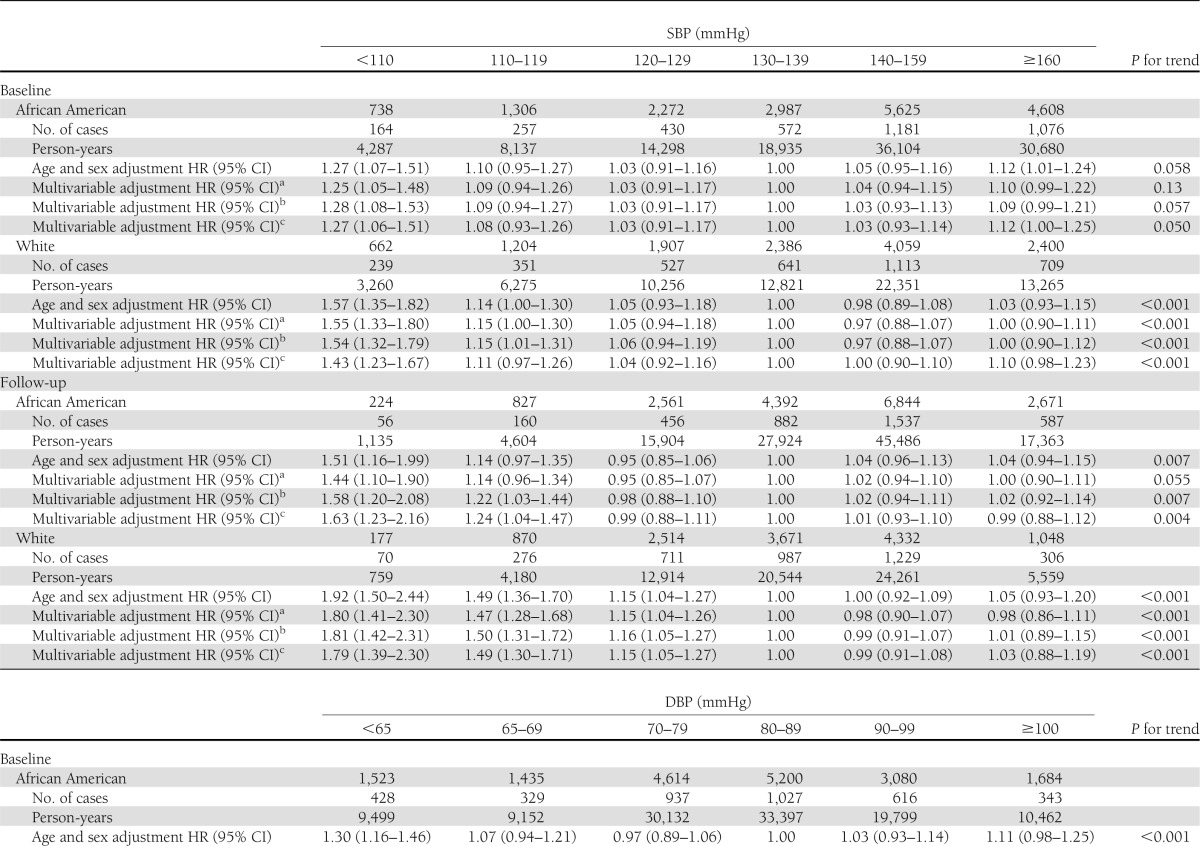
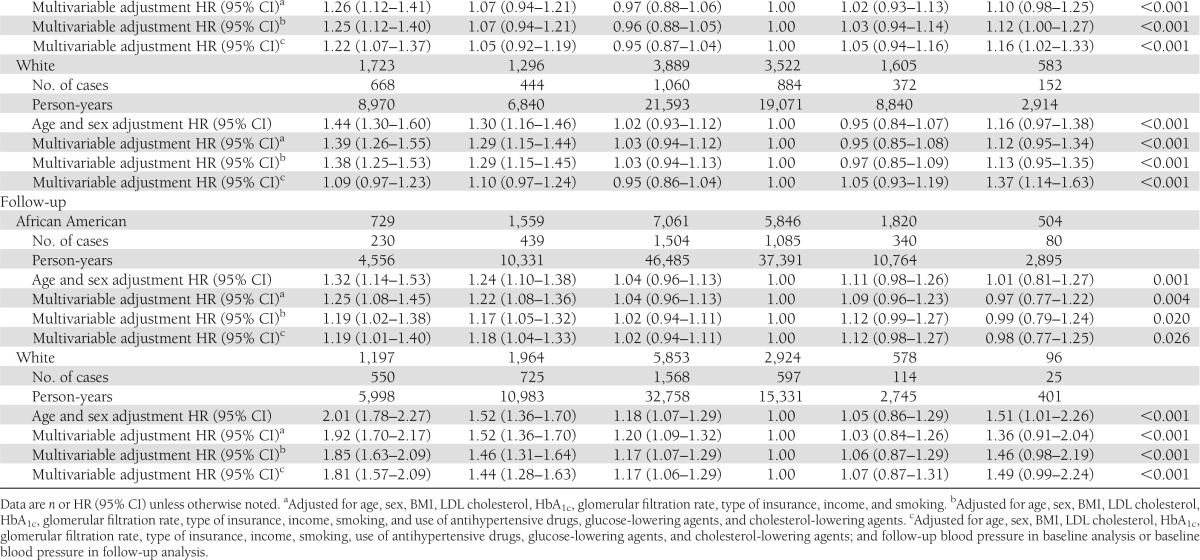
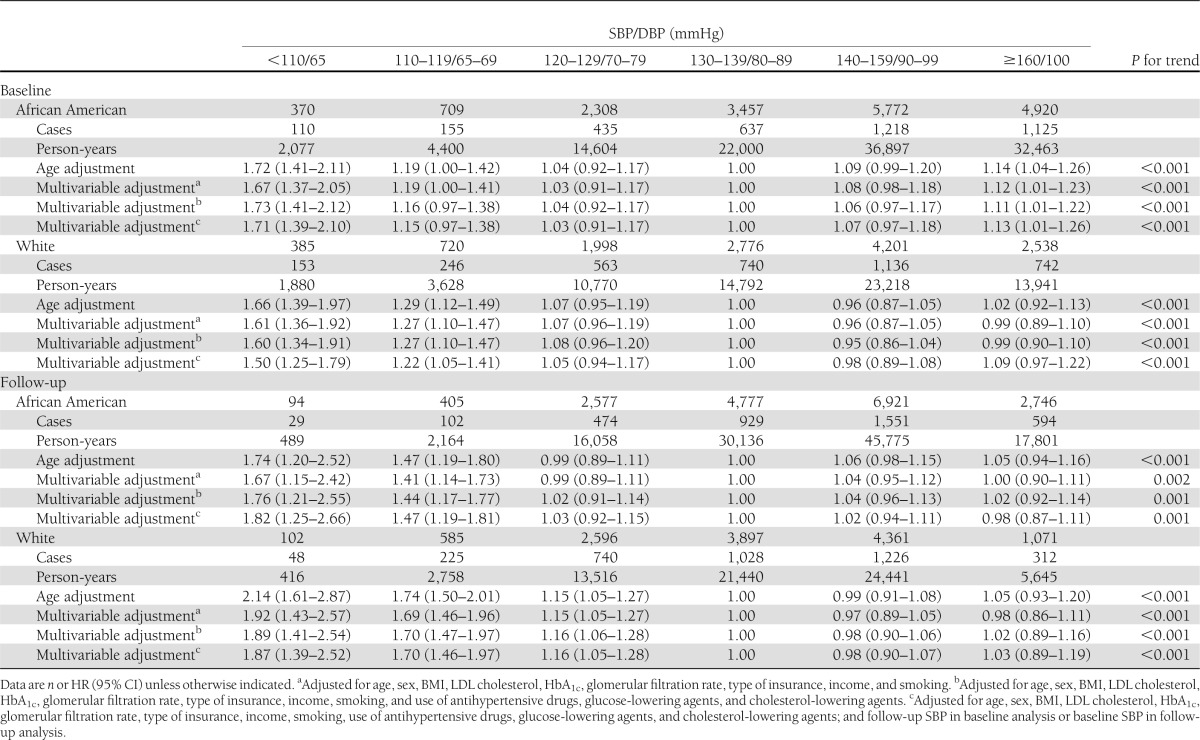
The multivariable-adjusted HRs of CHD associated with different levels of joint SBP/DBP at baseline (<110/65, 110–119/65–69, 120–129/70–80, 130–139/80–90 [reference group], 140–159/90–100, and ≥160/100 mmHg) were 1.73, 1.16, 1.04, 1.00, 1.06, and 1.11 (P trend <0.001) for African American diabetic patients and 1.60, 1.27, 1.08, 1.00, 0.95, and 0.99 (P trend <0.001) for white diabetic patients, respectively (Table 2). After further adjustment for SBP/DBP during follow-up in baseline analysis or baseline SBP/DBP blood pressure in follow-up analysis, this U-shaped association did not change (Tables 1 and 2).
Table 2.
HR (95% CI) of CHD according to different levels of SBP/DBP at baseline and during follow-up among African American and white patients with diabetes
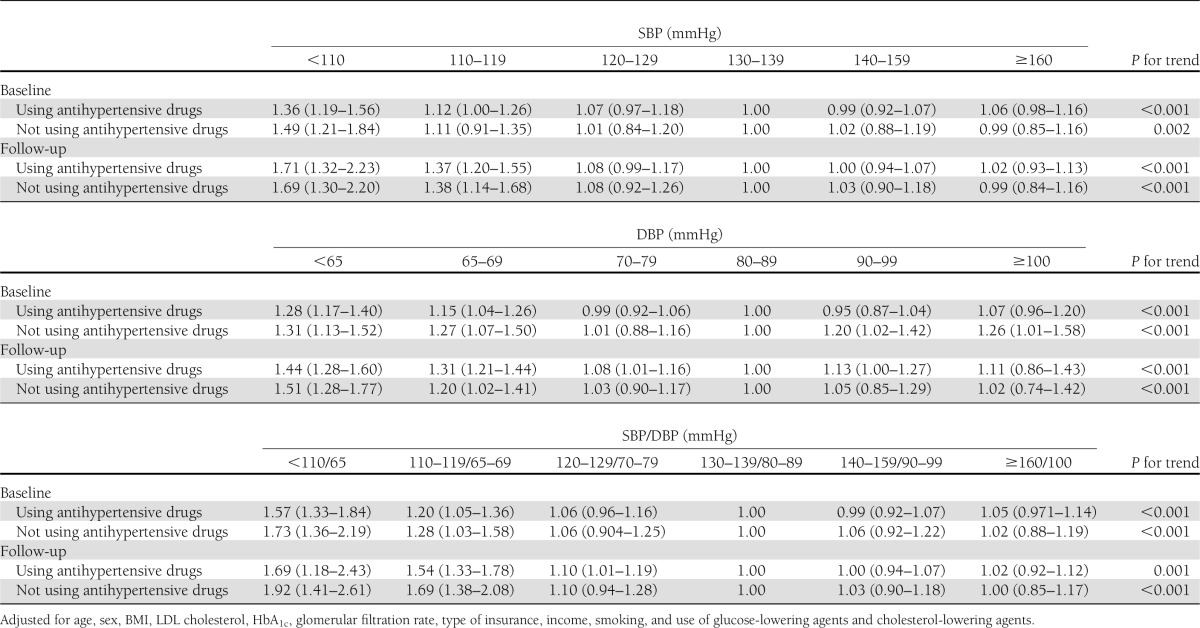
The U-shaped association of blood pressure with CHD risk was confirmed among African American and white diabetic patients with or without antihypertensive drugs (all P trend <0.01) (Table 3). After exclusion of the subjects who were diagnosed with CHD during the first 2 years of follow-up (n = 589), the multivariable-adjusted HRs for different levels of blood pressure did not change (data not shown).
Table 3.
HR (95% CI) of CHD according to different levels of blood pressure at baseline and during follow-up among diabetic patients using (n = 25,061) and not using (n = 5,093) antihypertensive drugs
There was a significant interaction between age and blood pressure on CHD risk (Table 4). When stratified by age, the U-shaped association of blood pressure at baseline with CHD was present in diabetic patients aged 30–49 and 50–59 years; however, this U shape changed to an inverse association in diabetic patients aged ≥60 years.
Table 4.
HR (95% CI) of CHD according to different levels of blood pressure at baseline and during follow-up among diabetic patients stratified by age
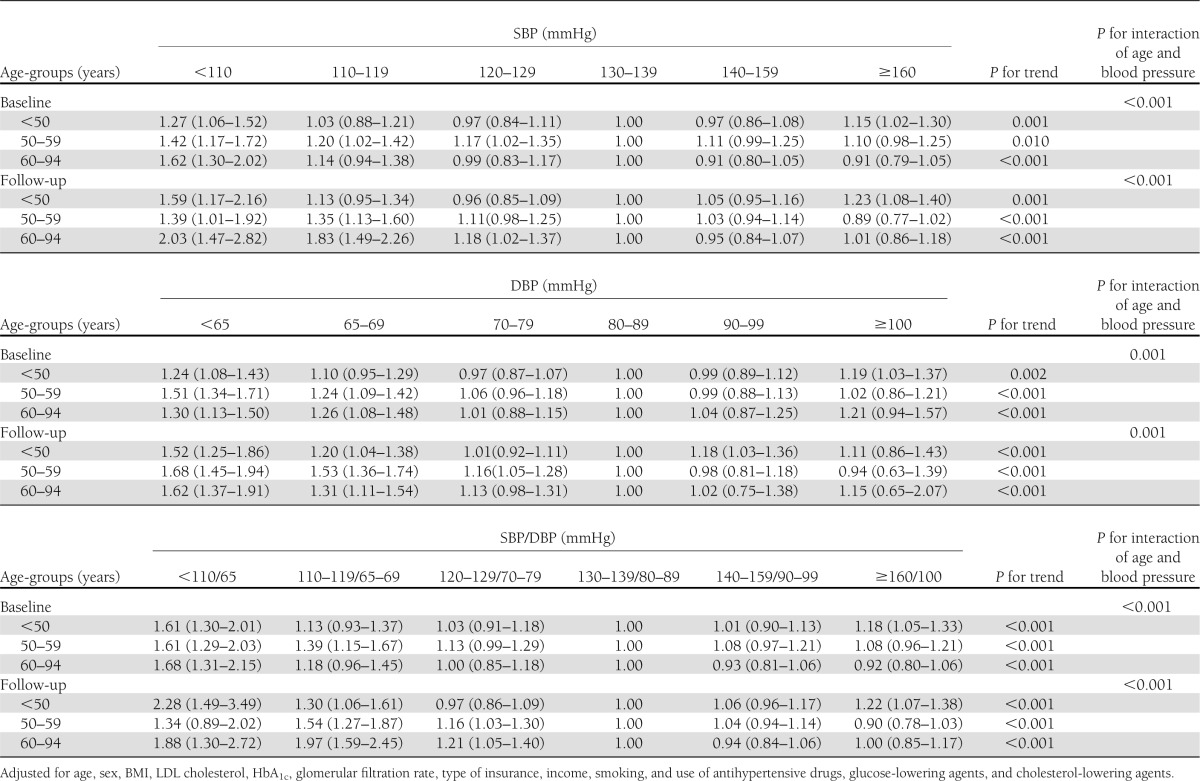
When we did an additional analysis by using an updated mean of blood pressure, we found almost the same U-shaped associations between baseline blood pressure levels and an updated mean of blood pressure levels with CHD risk among both African American and white diabetic patients (Table 1–4).
CONCLUSIONS
Our study found a U-shaped association between blood pressure at baseline and during follow-up with the risk of CHD among both African American and white diabetic patients. In addition, we found that this U-shaped association was present in different age-groups, especially among diabetic patients aged <50 years. For the oldest group (age ≥60 years), the U-shaped association changed to an inverse association.
The benefits of treating hypertension in diabetes patients are well documented in several early RCTs (8,9). However, there is a lack of supporting evidence for an aggressive blood pressure goal (<130/80 mmHg) (5–7). One recent analysis from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study did not confirm the beneficial effect of intensive blood pressure treatment (SBP <120 mmHg) among diabetic patients compared with standard therapy (SBP <140 mmHg) (11). The International Verapamil SR-Trandolapril (INVEST) study also indicated that tight control of SBP (<130 mmHg) among diabetic patients was not associated with improved CVD outcomes compared with usual control (SBP 130–139 mmHg) (11,12). The limitations of these RCTs include the low incident CHD events, short follow-up time, high loss to follow-up rates, and strict inclusion and exclusion criteria that limit their applicability to diabetic patients in clinical practice. It has been indicated that observational studies and RCTs overall produced similar results (23,24), and observational studies, especially from hospital-based cohorts, may reflect everyday clinical practice.
One recent U.K. observational study has indicated a U-shaped association of SBP or DBP with all-cause mortality among type 2 diabetic patients (22). Other studies have demonstrated an inverse association between blood pressure levels and all-cause mortality among elderly diabetic patients (14,25). Several reasons or limitations for the inconsistency of these studies can be considered. First, small sample sizes, short follow-up, and few CVD cases in some studies may limit the statistical power. Second, most epidemiological studies only assess a single baseline measurement of blood pressure with the CVD risk, which may produce potential bias. In the current study, our data showed that a significantly increased risk of CHD was observed among both African American and white diabetic patients in the high blood pressure group and the low blood pressure group as well. The most benefit for CHD risk was achieved with blood pressure as 130–140/80–90 mmHg. This U-shaped association of CVD risk was confirmed by blood pressure at baseline or during follow-up, which may suggest that a single blood pressure determination was sufficient where addition of subsequent values did not change the result very much. In addition, this U-shaped association was present in different age-groups, but in the oldest group (age ≥60 years) the U-shaped association changed to an inverse association.
Potential explanations of this association among diabetic patients are unclear. Some studies have suggested that tight control of blood pressure might increase cardiovascular risk by the underperfusion of vital organs (26). An impaired coronary circulation may be particularly sensitive to decreases in DBP (27,28). Avoidance of reducing DBP below a critical level is especially important to ensure coronary flow during diastole. Lower blood pressure has been shown to be more common with comorbidities at older ages and is often reflective of poor health. Elderly patients with type 2 diabetes represent a population that is highly enriched with underlying coronary artery disease and may be more prone than others to display the U-shaped or inversed association. This may contribute to the inverse association between blood pressure and CHD risk in the old diabetic patients. We carried out sensitivity analyses excluding participants who were diagnosed with CHD during the first 2 years of follow-up (n = 589), which can reduce the possibility of potential bias due to poor health during the subclinical stage prior to the diagnosis of CHD. The association of lower blood pressure control (<120/70 mmHg) with the increased CHD risk in the elder group did not change; on the other hand, the harm of uncontrolled blood pressure (≥160/100 mmHg) seemed to decrease compared with other two younger groups. This might suggest that lower blood pressure control is more harmful than uncontrolled blood pressure and that an inverse association exists in elderly patients. The possible explanations why the inverse relationship was only found for elderly patients are unclear, but a recent analysis of 13 cohort studies including 180,000 Japanese participants showed that the effect of hypertension on the risk of mortality gradually weakened with advancing age (29).
There are several strengths of our study, including the large sample size, high proportion of African Americans, long follow-up time, and use of administrative databases to avoid differential recall bias. We have used both baseline blood pressure levels and updated mean values of blood pressure during follow-up in the analyses, which can avoid potential bias from a single baseline measurement. In addition, participants in this study use the same public health care system, which minimizes the influence from the accessibility of health care, particularly in comparing African Americans and whites. One limitation of our study is that our analysis was not performed on a representative sample of the population, which limits the generalizability of this study; however, LSUHCSD hospitals are public hospitals and cover >1.6 million patients, most of whom are middle- or low-income persons in Louisiana. The results of the current study will have wide applicability for the population with low income and without health insurance in the U.S. Second, the validity of myocardial infarction diagnoses in our study has not been confirmed by specialists. But the method we used is hospital discharge register to diagnose major nonfatal CHD, which has been widely used in American and European cohort studies, such as the Kaiser Permanente Medical Care Program (30,31), the Atherosclerosis Risk in Communities (ARIC) study (32), the Framingham Study (33,34), the National FINRISK Survey (35), and the Whitehall II study (36). The validity of the diagnoses of myocardial infarction by using hospital discharge register in these cohort studies is available (agreement 83–98%) (31,37). Third, even though our analyses adjusted for an extensive set of confounding factors, residual confounding due to the measurement error in the assessment of confounding factors and unmeasured factors such as heart rate, physical activity, education, dietary factors, cognitive function for all patients cannot be excluded. Based on the limitation above, our findings may need to be further confirmed by RCTs or meta-analysis concerning this specific issue.
In summary, in this large hospital-based cohort study, we found that aggressive blood pressure control (i.e., SBP <120 mmHg or DBP <70 mmHg) is associated with an increased risk of CHD among both African American and white diabetic patients with type 2 diabetes. Furthermore, for the elder group, the harm is even higher. Since there is currently no robust evidence available for lowering the blood pressure <130/80 mmHg in people with diabetes, it might be advisable to maintain blood pressure between 130–139 and 80–89 mmHg and to recommend less intense goals to elderly patients than to younger ones.
Acknowledgments
This work was supported by LSU’s Improving Clinical Outcomes Network.
No potential conflicts of interest relevant to this article were reported.
W.Z. wrote the manuscript and researched data. R.H., Y.W., and W.L. researched data. P.T.K., J.J., S.B.H., W.T.C., and D.H.R. reviewed and edited the manuscript. G.H. reviewed and edited the manuscript and researched data. G.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the Epidemiology and Prevention (EPI) and Nutrition, Physical Activity and Metabolism (NPAM) Spring Scientific Sessions, New Orleans, Louisiana, 19–22 March 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0189/-/DC1.
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 2010;303:2043–2050 [DOI] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fryar CD, Hirsch R, Eberhardt MS, Yoon SS, Wright JD. Hypertension, high serum total cholesterol, and diabetes: racial and ethnic prevalence differences in U.S. adults, 1999-2006. NCHS Data Brief, 2010:1–8 [PubMed] [Google Scholar]
- 4.Mancia G. The association of hypertension and diabetes: prevalence, cardiovascular risk and protection by blood pressure reduction. Acta Diabetol 2005;42(Suppl. 1):S17–S25 [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, De Backer G, Dominiczak A, et al. The task force for the management of arterial hypertension of the European Society of Hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462–1536 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson L, Zanchetti A, Carruthers SG, et al. HOT Study Group Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet 1998;351:1755–1762 [DOI] [PubMed] [Google Scholar]
- 10.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berl T, Hunsicker LG, Lewis JB, et al. Collaborative Study Group Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005;16:2170–2179 [DOI] [PubMed] [Google Scholar]
- 14.van Hateren KJ, Landman GW, Kleefstra N, et al. Lower blood pressure associated with higher mortality in elderly diabetic patients (ZODIAC-12). Age Ageing 2010;39:603–609 [DOI] [PubMed] [Google Scholar]
- 15.Li W, Wang Y, Chen L, et al. Increasing prevalence of diabetes in middle or low income residents in Louisiana from 2000 to 2009. Diabetes Res Clin Pract 2011;94:262–268 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen L, Xiao K, et al. Increasing incidence of gestational diabetes mellitus in Louisiana, 1997-2009. J Womens Health (Larchmt) 2012;21:319–325 [DOI] [PubMed] [Google Scholar]
- 17.Hu G, Horswell R, Wang Y, et al. Body mass index and the risk of dementia among Louisiana low income diabetic patients. PLoS One 2012;7:e44537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Katzmarzyk PT, Horswell R, et al. Racial disparities in diabetic complications in an underinsured population. J Clin Endocrinol Metab 2012;97:4446–4453 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Li W, Wang Y, et al. Increasing prevalence of hypertension in low income residents within Louisiana State University Health Care Services Division Hospital System. Eur J Intern Med 2012;23:e179–e184. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Chen L, Horswell R, et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. J Womens Health (Larchmt) 2012;21:628–633 [DOI] [PubMed] [Google Scholar]
- 21.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 22.Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ 2012;345:e5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342:1878–1886 [DOI] [PubMed] [Google Scholar]
- 24.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rönnback M, Isomaa B, Fagerudd J, et al. Botnia Study Group Complex relationship between blood pressure and mortality in type 2 diabetic patients: a follow-up of the Botnia Study. Hypertension 2006;47:168–173 [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, Laurent S, Agabiti-Rosei E, et al. European Society of Hypertension Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009;27:2121–2158 [DOI] [PubMed] [Google Scholar]
- 27.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581–584 [DOI] [PubMed] [Google Scholar]
- 28.Cruickshank JM. Coronary flow reserve and the J curve relation between diastolic blood pressure and myocardial infarction. BMJ 1988;297:1227–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami Y, Hozawa A, Okamura T, Ueshima H, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group (EPOCH-JAPAN) Relation of blood pressure and all-cause mortality in 180,000 Japanese participants: pooled analysis of 13 cohort studies. Hypertension 2008;51:1483–1491 [DOI] [PubMed] [Google Scholar]
- 30.Kanaya AM, Adler N, Moffet HH, et al. Heterogeneity of diabetes outcomes among Asians and Pacific Islanders in the US: the diabetes study of Northern California (DISTANCE). Diabetes Care 2011;34:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–3072 [DOI] [PubMed] [Google Scholar]
- 34.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–115 [DOI] [PubMed] [Google Scholar]
- 35.Hu G, Tuomilehto J, Silventoinen K, Barengo N, Jousilahti P. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J 2004;25:2212–2219 [DOI] [PubMed] [Google Scholar]
- 36.Kivimäki M, Batty GD, Hamer M, et al. Using additional information on working hours to predict coronary heart disease: a cohort study. Ann Intern Med 2011;154:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pajunen P, Koukkunen H, Ketonen M, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil 2005;12:132–137 [DOI] [PubMed] [Google Scholar]


