Abstract
OBJECTIVE
Sexual dysfunction is a prevalent problem in obese women with type 2 diabetes. This study examined the effects of intensive lifestyle intervention (ILI) in these women.
RESEARCH DESIGN AND METHODS
Look AHEAD is a 16-center, randomized, controlled trial evaluating the health effects of ILI compared with a control group (diabetes support and education [DSE]). The Look AHEAD Sexual Function Ancillary study included 375 female participants at five Look AHEAD sites. Participants completed the Female Sexual Function Inventory (FSFI) and Beck Depression Inventory (BDI), and assessments of weight and cardiovascular risk factors at baseline and 1 year were made.
RESULTS
At baseline, 50% of the 229 participants who reported being sexually active met criteria for female sexual dysfunction (FSD); only BDI score was related to FSD. One-year weight losses were greater in the ILI group than in the DSE group (7.6 vs. 0.45 kg; P < 0.001). Among women with FSD at baseline, those in the ILI group (N = 60) compared with those in the DSE group (N = 53) were significantly more likely to remain sexually active (83 vs. 64%; P < 0.008), reported greater improvement in total FSFI scores and in most FSFI domains (P < 0.05), and were more likely to experience remission of FSD (28 vs. 11%; P < 0.04) at 1 year. No significant differences between ILI and DSE were seen in women who did not have FSD at baseline.
CONCLUSIONS
Participation in ILI appeared to have beneficial effects on sexual functioning among obese women with diabetes, particularly in those who had FSD at baseline.
Sexual dysfunction is associated with chronic diseases in women, including cancer, heart disease, and diabetes (1,2). Recent studies have reported high rates of sexual problems in women with type 1 and type 2 diabetes, including loss of sexual desire, difficulties with arousal and orgasm, and dyspareunia (painful intercourse) (3–6). Despite these reports, it is unclear to what extent sexual dysfunction in women with diabetes is related to the effects of the disease on hormonal or vascular mechanisms involved in sexual response or to indirect effects via weight gain, alterations in body image, other comorbidities, or psychosocial concomitants of the disease. In a large study of women with type 1 diabetes, Enzlin et al. (4) reported loss of lubrication and desire problems in >50% of women in the study, which was more strongly related to the presence of comorbid depression rather than degree of diabetes control or other factors more directly related to the disease. Similarly, sexual dysfunction among type 2 diabetic women has been associated with comorbid depression in a large population-representative study (7).
Obesity also has been strongly associated with increased risk of sexual dysfunction in women (8,9), with more obese women reporting increased frequency and severity of sexual problems (10). Higher rates of sexual problems have been reported in women seeking bariatric surgery compared with nonobese women or women in a residential obesity treatment program (11).
Improvements in female sexual function have been reported after bariatric surgery (12) and in participants randomized to a Mediterranean diet (13). However, to date, there have been no studies examining whether a lifestyle intervention focused on producing modest weight losses will improve sexual function in overweight and obese women with type 2 diabetes. In the current study, we assessed sexual function using a validated self-report measure (Female Sexual Function Index [FSFI]) (14) in a subgroup of female participants who were participating in the Look AHEAD trial. Look AHEAD is a randomized trial evaluating the long-term effects on cardiovascular morbidity and mortality of an intensive lifestyle intervention (ILI) designed to produce weight loss and increases in physical activity in overweight and obese participants with type 2 diabetes compared with a control group (15). This ancillary study assessed the baseline prevalence and risk factors for sexual dysfunction among 375 women participating in Look AHEAD and examined the changes in sexual function of participants in the ILI group compared with the control group over the course of 1 year of intervention.
RESEARCH DESIGN AND METHODS
Participants
The main Look AHEAD trial included 5,145 participants. To be eligible for the trial, participants were required to have type 2 diabetes, to be 45–76 years of age, and to have a BMI ≥25 kg/m2 (≥27 kg/m2 for individuals using insulin). Subjects with uncontrolled hyperglycemia (HbA1c >11% [97 mmol/mol]), hypertension (blood pressure >160/100 mmHg), or hyperlipidemia (fasting triglycerides ≥600 mg/dL) were excluded. A detailed description of other eligibility criteria has been published previously (15,16).
The 375 women who participated in the sexual dysfunction ancillary study were recruited from 5 of the 16 Look AHEAD sites (University of Pennsylvania, The Miriam Hospital/Brown University, Johns Hopkins University, University of Alabama–Birmingham, and University of Tennessee–Memphis). These sites were selected on the basis of geographic and ethnic diversity of the study participants and the willingness of the project staff to participate in the ancillary study. Participants were required to complete a separate informed consent statement for the sexual dysfunction ancillary study and received a $25 gift card for participation. Separate Institutional Review Board approval for the ancillary study was obtained for each of the participating sites.
Interventions
Participants in Look AHEAD were randomly assigned to ILI or diabetes support and education (DSE; the control condition). Those in the ILI group were offered group and individual sessions with weekly meeting for the first 6 months, followed by 3 meetings per month between months 7 and 12. The goal for participants in ILI was to lose 10% of their body weight, which was accomplished by following a calorie-restricted and fat-restricted diet and by increasing physical activity (17). The DSE group attended three meetings focused on education about diet and activity and social support but was given no behavioral strategies.
Measurements
All measures were completed at baseline before randomization and at 1 year by staff who were trained and certified and masked to treatment assignment.
Sexual function assessment.
Sexual function in women was assessed using the FSFI, a widely used self-report measure of sexual function in females (14). This questionnaire was designed as an assessment tool for use in clinical trials and recognizes the multidimensional nature of female sexual dysfunction (FSD). The questionnaire has been validated in several studies by comparison with clinical interviews (14,18,19). The questionnaire assesses sexual function over the past 4 weeks; it includes 19 items and provides scale scores in six separate domains (sexual desire, arousal, lubrication, orgasm, satisfaction, and pain), as well as an overall index of sexual function. Participants must complete all questions within a specific domain to analyze that domain, resulting in inconsistent sample sizes across domains, and must answer all 19 questions to calculate the total FSFI score. FSFI total scores range from 2 to 36, with higher scores indicating better sexual function. The validated FSFI total score of ≤26.55 was used to classify participants as having FSD at baseline and 1 year (18). The questionnaire asks women whether they are sexually active, and an additional question was added to determine whether the participant had a partner. Because of the sensitive nature of the questions, staff did not review questionnaires with participants or ask about missing information.
Physical measures.
Body weight was recorded to the nearest 0.1 kg. Height was recorded to the nearest 0.5 cm. BMI was calculated as weight (kg) divided by height (m2). Systolic blood pressure and diastolic blood pressure readings were measured in duplicate using a Dinamap Monitor Pro 100 automated blood pressure device (GE Medical Systems, Tampa, FL) and averaged. Glucose, HbA1c, cholesterol, HDL, LDL, and triglycerides were measured via standard, chemistry, hematology, and lipid blood testing (16).
Questionnaires.
Depressive symptoms were assessed using the Beck Depression Inventory (BDI) (20). Total scores on the BDI range from 0 to 63, with higher scores indicating more symptoms of depression. Self-reported data also were collected regarding medical history, employment, education, family income, smoking, prescription medications, alcohol use, and family medical history. Race/ethnicity was self-reported using questions from the 2000 United States Census questionnaire.
Statistical analyses
Descriptive statistics were used to characterize the ancillary study sample and to compare our sample with the main Look AHEAD trial with respect to relevant demographic and medical history variables. Group differences for continuous variables were examined using Student unpaired t tests and ANOVA models. ANCOVAs were conducted to examine differences between the ILI and DSE groups regarding changes in FSFI domain and total scores from baseline to 1 year, adjusting for baseline scores. Logistic regression analyses were used to examine potential factors associated with having FSD at baseline and differences between the ILI and DSE groups regarding odds of experiencing remission or development of FSD from baseline to 1 year. Analyses were performed using SPSS (version 20) software.
RESULTS
Characteristics of the sample
A total of 375 women (193 in ILI and 182 in DSE) were enrolled in the sexual dysfunction ancillary study of Look AHEAD. These participants averaged 61.4 ± 6.1 (mean ± SD) years of age and had a mean BMI of 36.4 ± 6.0. They reported having had diabetes for 6.7 ± 6.5 years on average. There were no significant differences at baseline between those women who were randomized to ILI versus those in the DSE group (all P > 0.14). Participants in the substudy were comparable with women in the main trial regarding baseline variables, such as BMI and duration of diabetes (Supplementary Table 1). However, women in the substudy were older (61.4 ± 6.1 years vs. 58.0 ± 6.8 years) and more likely to be married (63 vs. 54%), and the substudy included a greater percentage of African Americans (40 vs. 20%) and a smaller percentage of Hispanics (1.6 vs. 15.8%) than the main trial. Eighty-six percent of women in Look AHEAD were postmenopausal.
Sexual function at baseline
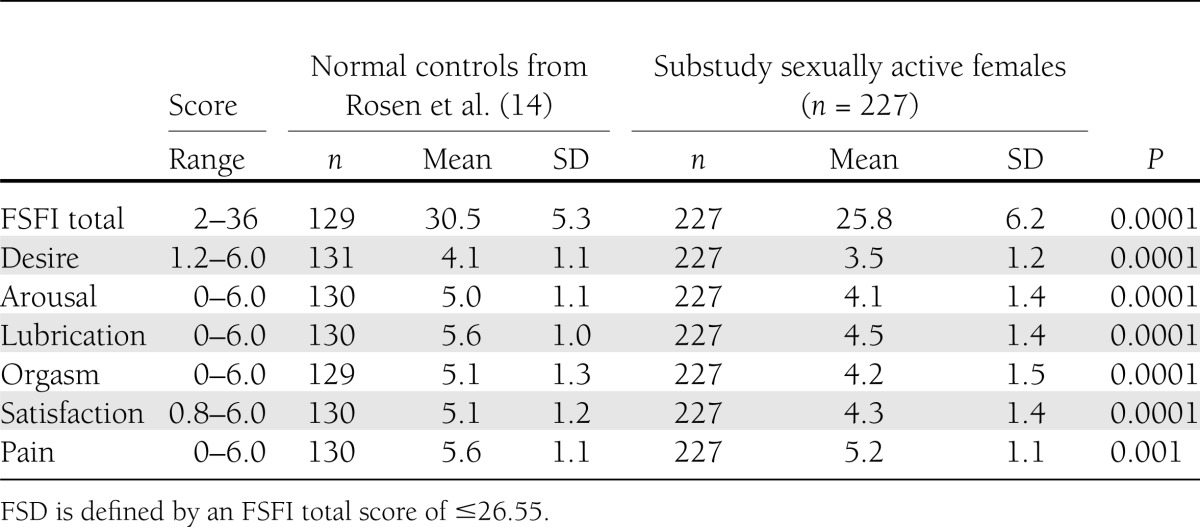
Of the 375 participants, 37 (9.9%) did not indicate whether they were sexually active and 227 (67% of the 338 who responded) indicated that they were sexually active, whereas the remainder (111 [33%]) were inactive. The proportion of women who were inactive at baseline was comparable in the DSE group (53 of 164 [32%]) and ILI group (58 of 174 [33%]). Fifty-five percent of the sexually inactive women indicated that they had no partner. Table 1 presents the baseline FSFI scores of the 227 sexually active women in this study and compares their scores with those of a control group of healthy United States women of similar age (14). The control group, drawn from a previous study (14), is shown here to provide a context for considering the scores of women in the current study. The sample of women in our study had significantly reduced scores for both overall sexual function and in each of the domains assessed (all P < 0.005). Using the previously established cutoff of 26.55 to define FSD, 50% of the sexually active women met criteria for FSD at baseline (48% in DSE and 52% in ILI).
Table 1.
FSFI total and domain scores at baseline
Logistic regression analyses were conducted to determine whether any of the physical, demographic, or self-reported measures were associated with having FSD at baseline. The only significant predictor was baseline score on the BDI (odds ratio, 1.101; 95% CI, 1.045–1.160; P = 0.0003). A 1-point increase in BDI score was associated with a 10% increase in the odds of having FSD. None of the other baseline variables, including age, race/ethnicity, duration of diabetes, BMI, cardiovascular risk factor levels, or concomitant drug use, were significantly related to having FSD.
Effect of sexual function on treatment outcome
Baseline sexual function was not related to subsequent weight loss or changes in depression, with similar changes in those who reported being inactive at baseline and those who were active, with or without FSD. However, there were highly significant differences in weight loss between the ILI and DSE participants. Women in the ILI group in this ancillary study lost, on average, 7.6 ± 6.7 kg, whereas those in the DSE group lost 0.45 ± 4.8 kg (P < 0.0001), and these results were similar to those seen in women in the full trial (7.7 ± 6.9 kg in ILI and 0.75 ± 4.9 kg in DSE). Depression and cardiovascular risk factors also improved more in ILI than in DSE over the course of 1 year. For example, BDI scores decreased more in ILI than in DSE (−1.12 vs. 0.49; P < 0.02), as did systolic blood pressure (−6.8 vs. −2.1 mmHg; P = 0.03), diastolic blood pressure (−2.2 vs. −1. 1 mmHg; P = 0.003), and HbA1c (−0.57 vs. 0.09%; P < 0.001).
Effect of intervention on remaining sexually active and on sexual dysfunction
A total of 340 women (90.6%) completed the FSFI again at 1 year. The proportion of women who were sexually active at baseline but reported that they were no longer sexually active at 1 year follow-up differed significantly by treatment condition; 28 of the 100 women (28%) in the DSE group who were active at baseline reported no longer being sexually active at 1 year compared with 14 out of 103 (13.5%) in the ILI group (P = 0.01). This difference did not reflect more frequent loss of a partner in the DSE group; in fact, there was a trend (P = 0.08) in the opposite direction, with loss of the partner occurring in a larger proportion of women in the ILI group than in the DSE group. There was also a trend for more women in the ILI group than in the DSE group who reported being sexually inactive at baseline to report that they were sexually active at 1 year (8 out of 58 [14%] in ILI vs. 2 out of 50 [4%] in DSE; P = 0.08).
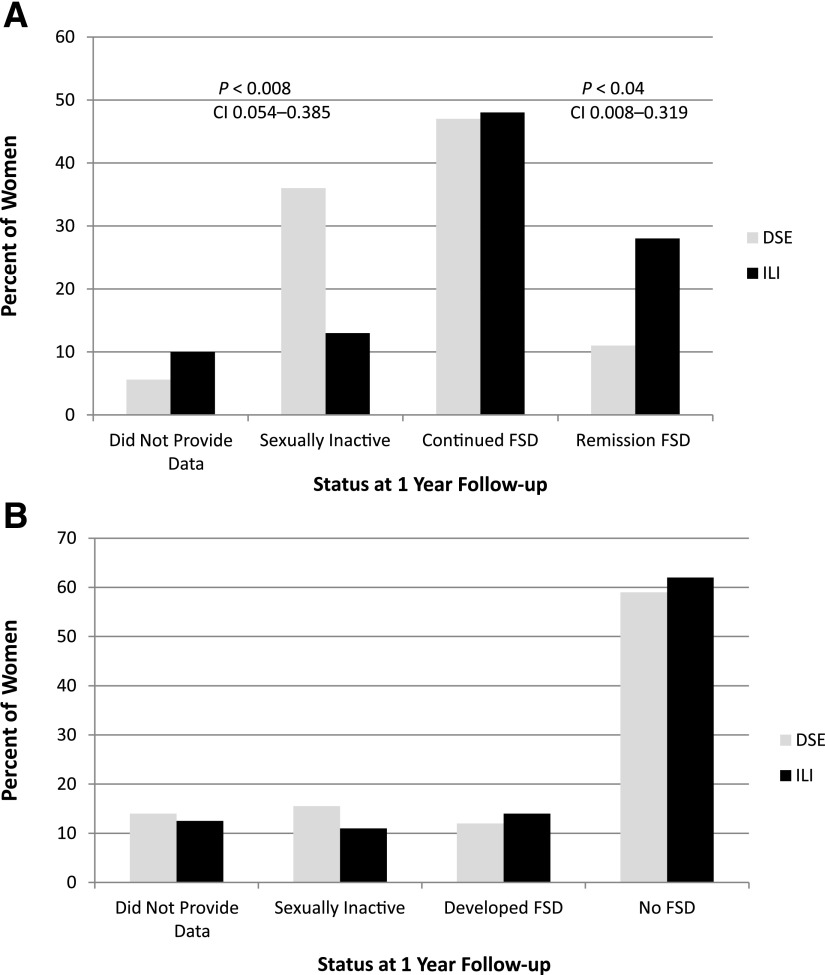
Subsequent analyses were performed separately for sexually active women with FSD at baseline and for those who did not have FSD at baseline. Among sexually active women with FSD at baseline (N = 60 in ILI and 53 in DSE), changes from baseline to 1 year differed significantly between women in the ILI group and those in DSE (Fig. 1A). First, 36% of the women in the DSE group who had FSD at baseline reported that they were no longer sexually active at 1 year, compared with only 13% of ILI group participants (P < 0.008). Of those who remained active, a significantly greater proportion of ILI participants experienced remission of their FSD compared with those in the DSE group (28% in ILI vs. 11% in DSE; P < 0.04).
Figure 1.
Percent of women in each category at 1 year. A: Status at 1 year of women who were sexually active and had FSD at baseline in the ILI group (N = 60) and in the DSE group (N = 53). B: Status at 1 year of women who were sexually active and did not have FSD at baseline in the ILI group (N = 56) and in the DSE group (N = 58).
In contrast, among women who were sexually active at baseline but did not have FSD (N = 56 in ILI and 58 in DSE) (Fig. 1B), there were no differences between groups in the proportion who became sexually inactive (15.5% in DSE vs. 11% in ILI), who developed FSD (12% in DSE and 14% in ILI), or who continued to be active with no FSD (59% in DSE and 62% in ILI).
Effect of intervention on FSFI total and domain scores
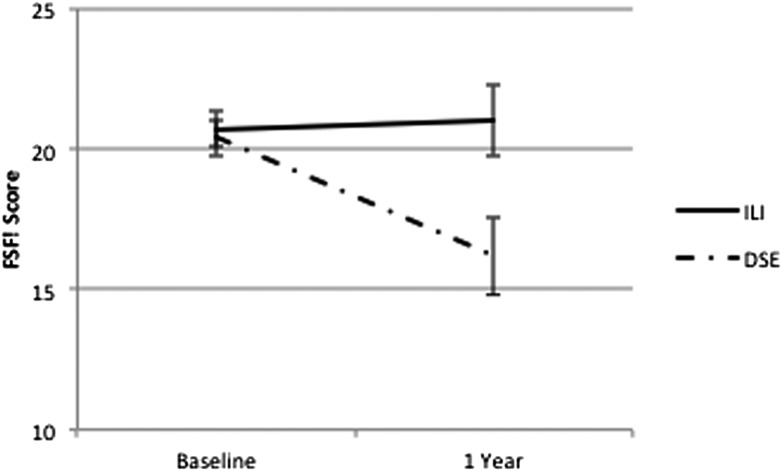
Table 2 shows changes in FSFI total and domain scores from baseline to 1 year, retaining women who became sexually inactive at 1 year (and providing scores of zero on the relevant FSFI items). When all women are included, regardless of whether they had FSD at baseline (Table 2, top panel), the changes in FSFI scores for ILI and DSE are quite similar. However, if we focus only on women who reported FSD at baseline and remained sexually active (Table 2, second panel), almost all sexual function domains show significantly greater improvements in ILI group women than in DSE group women. Figure 2 shows the marked differences in total FSFI scores for women in the ILI and DSE groups who had FSD at baseline. In contrast, no significant differences between ILI and DSE were seen among those who did not have FSD at baseline (Table 2, third panel).
Table 2.
Changes in weight, depressive symptoms, and sexual domain scores from baseline to 1 year
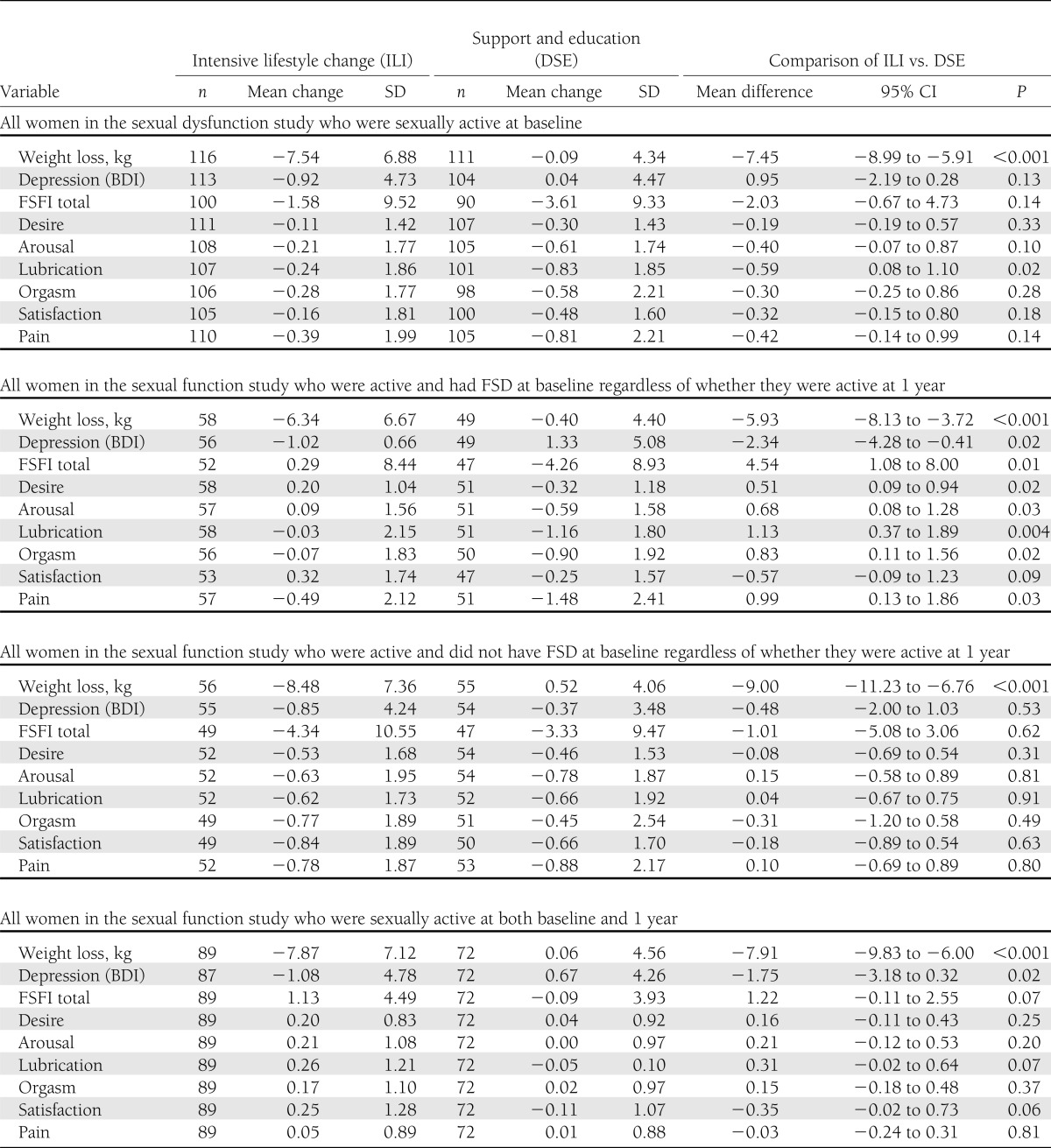
Figure 2.
FSFI scores at baseline and 1 year for women in the ILI group (N = 52) and in the DSE group (N = 47) who were sexually active and had FSD at baseline (including women who became sexually inactive at 1 year).
As noted, because becoming sexually inactive appeared to occur disproportionately in women in the DSE group who reported FSD at baseline, we also analyzed the changes in FSFI for women in the ILI (N = 89) and DSE (N = 72) groups who reported being sexually active at both baseline and 1 year (Table 2, bottom panel). On average, women in the ILI group reported slight improvements in total FSFI score, whereas DSE participants reported slight declines (+1.1 in ILI and −0.09 in DSE; P = 0.073). Similar trends were noted for satisfaction (P = 0.06) and lubrication (P = 0.07), for which small positive changes were observed among ILI group participants and declines were observed among DSE group participants.
Associations between changes in weight, depression, and sexual function
Finally, we examined the association between changes in weight and depression and changes in sexual function from baseline to 1 year within the subgroup of women who were sexually active and had FSD at baseline (n = 107). These analyses controlled for randomization arm, race/ethnicity, and baseline levels of weight, BDI, systolic blood pressure, diastolic blood pressure, and HbA1c and examined the associations with changes in these parameters. After controlling for the baseline variables, we found that greater weight loss from baseline to 1 year was the only variable associated with increased odds (odds ratio, 0.1.149; 95% CI, 1.018–1.297; P = 0.025) of remaining sexually active (vs. becoming inactive) at 1 year. However, among those women who remained sexually active, decreases in depression were related to greater odds of remission of FSD (odds ratio, 1.546; 95% CI, 1.138–2.099; P = 0.005), whereas changes in weight and other metabolic parameters were not.
CONCLUSIONS
The current study is the first to prospectively examine the effect of randomization to ILI on changes in female sexual function and in FSD in overweight/obese women with type 2 diabetes. The primary finding in our study was that among women with FSD at baseline, those in the ILI group were more likely to remain sexually active over the 1 year of follow-up (87% in ILI vs. 64% of DSE) and were more than twice as likely to experience remission of FSD (28% experienced remission vs. 11% in DSE). These findings suggest that weight loss intervention may have beneficial effects for overweight/obese women with type 2 diabetes and FSD. In contrast, there was little evidence that participation in the ILI group helped to prevent the development of FSD in those who did not have such problems at baseline.
At entry into the trial, sexually active women in this ancillary study had significant impairments across all major domains of sexual function compared with normative controls (14). Moreover, 50% met criteria for FSD (i.e., total FSFI score of ≤26.55). Previous studies have shown that obesity and diabetes are both associated with increased risk of FSD (4,8,9). In a previous study with the FSFI, for example, Bond et al. (10) found that 60% of severely obese women seeking bariatric surgery met criteria for FSD. Thus, prevalence of FSD may increase with severity of obesity. The age of the participants and menopause also may have contributed to their FSD.
In addition, we found that one-third of the women in our trial reported not being sexually active at baseline. For half of these women, this was likely related to their lack of a sexual partner; however, for the other half, it may reflect either sexual problems in the partner or a response to their own sexual problems. Excluding women who reported being sexually inactive from the estimates of the number with FSD may underestimate the rates of FSD in this population. Moreover, the FSFI does not define sexual activity, so women may differ on whether they include masturbation as sexual activity.
This study also found that scores on the BDI were the only significant correlate of FSD at baseline; none of the other baseline variables, including those related to diabetes duration and glycemic control, were related to sexual function in these diabetic women. This finding, which confirms previous studies of women with type 1 and type 2 diabetes (4,7), suggests that sexual dysfunction in women with diabetes is more closely related to psychosocial variables than to the physiological consequences of diabetes. However, given that our baseline data are cross-sectional, it is not clear whether sexual dysfunction predisposes diabetic women to depression or vice versa.
These findings are in contrast to those we reported previously for male Look AHEAD participants (21). Although male subjects showed similarly high rates of erectile dysfunction (ED) at baseline, loss of sexual function in Look AHEAD men was related strongly to diminished exercise capacity and increased cardiovascular risk factors. Although men with ED were more likely to report symptoms of depression at baseline than men with normal sexual function, mood was a less significant predictor of ED in these obese diabetic men than exercise capacity and cardiovascular risk. Based on similar findings from multiple studies of diabetic and nondiabetic men, ED has been proposed as an early sign or harbinger of coronary artery disease in men (22,23). In contrast, psychosocial factors and depressed mood are more frequently associated with sexual dysfunction in diabetic (4) and nondiabetic women (24,25).
This study also suggests that becoming sexually inactive may be one response to worsening in sexual function in women with diabetes. In this study, among women in the ILI group, only 11–15% of women who did not have problems with sexual function at baseline and 13% of those with FSD became inactive at 1 year. In contrast, 36% of women with FSD in the DSE group reported becoming sexually inactive. This difference was not attributable to loss of partner being more common in DSE group women. Consequently, becoming sexually inactive may be an important outcome in this study, and excluding women who become inactive may result in misleading conclusions. Therefore, we chose not to remove women who reported becoming sexually inactive at 1 year from certain analyses of the FSFI, and we used their zero scores (indicating no sexual activity) as actual data. With this approach, we found that changes in total FSFI scores and in most subscales also were significantly different between ILI and DSE for women with FSD at baseline, with greater worsening in the DSE group. For example, among women in the ILI group with FSD at baseline, mean FSFI scores improved modestly (from 20.7 to 21.0), whereas those in the DSE group showed a marked decline (20.7 to 16.1) during the 1-year period of study. In contrast, no differences between the ILI and DSE groups were seen for changes in total or domain scores among those who did not have FSD at baseline. For these women, FSFI scores tended to decrease over time, perhaps reflecting that FSD is an age-related progressive problem (25).
Although the current study suggests benefits of weight loss on sexual dysfunction in obese diabetic women, the magnitude of these benefits was modest in comparison with those reported previously. For example, Esposito et al. (13) studied 59 women with metabolic syndrome who had sexual dysfunction at baseline; those who were randomly assigned to a Mediterranean diet had significant improvements in total FSFI scores (from 19.7 to 26.1; P = 0.01) over the course of the 2-year trial, whereas scores remained stable in the control group. Bond et al. (12) studied sexual function before and 6 months after bariatric surgery and found significant increases in FSFI total scores (from 24.0 to 29.4) over the course of this interval. Moreover, FSD resolved in 23 out of 34 (68%) women with FSD at baseline. In contrast, FSD resolved in 17 out of 60 women (28%) in the ILI arm in the present trial. Unlike these other studies, the current study was conducted exclusively in overweight/obese women with type 2 diabetes, which may have contributed to the reduced benefits on sexual function, and it is unclear how other studies addressed those women who became sexually inactive.
This study also found no evidence that baseline sexual function affected subsequent weight loss or changes in depression. However, decreases in depression were associated with no longer having FSD at 1 year. Weight loss was associated with less risk of becoming sexually inactive but was not related to resolution of FSD. The fact that both depression and sexual function were assessed at the same time point makes it impossible to determine if the improvements in depression led to the improvements in sexual function or vice versa. However, this finding does reemphasize the close association between mood and sexual function in women (4,7).
Other potential limitations of our study include the possibility of selection biases in our ancillary study participants and potential unreliability of self-reported data on sexual function. Female participants in the sexual function ancillary study were similar to the main trial population in terms of major demographic characteristics, although they were slightly older. This substudy also included more African Americans and fewer Hispanics because of the characteristics of the population studied at the five participating clinical sites. We reported previously on the similarities between male participants in the sexual function ancillary study and overall male population in the main Look AHEAD trial (21). Taken together, these findings support the representativeness of our sexual function study participants in comparison with the main Look AHEAD trial. In addition, the FSFI scale has been widely used in both observational and clinical studies of sexual function in women and it has been shown to consistently correlate with other interview-based or clinical assessments of FSD (18,19).
In conclusion, participation in a lifestyle intervention program has beneficial effects on sexual function for overweight or obese women with type 2 diabetes, particularly in those women with sexual dysfunction at baseline.
Acknowledgments
The Sexual Dysfunction substudy of Look AHEAD was funded by a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK060438 to R.C.R.). The Look AHEAD study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support was received from the following: The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056 44) and National Institutes of Health grant (DK 046204); and the University of Washington/VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs, and Frederic C. Bartter General Clinical Research Center (M01RR01346).
This study was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (Barbara Harrison, MS; Van S. Hubbard, MD, PhD; and Susan Z. Yanovski, MD), National Heart, Lung, and Blood Institute (Lawton S. Cooper, MD, MPH; Jeffrey Cutler, MD, MPH; and Eva Obarzanek, PhD, MPH, RD), and Centers for Disease Control and Prevention (Edward W. Gregg, PhD; David F. Williamson, PhD; and Ping Zhang, PhD).
The following organizations have committed to make major contributions to Look AHEAD: Federal Express; Health Management Resources; Johnson & Johnson, LifeScan Inc.; Optifast-Novartis Nutrition; Roche Pharmaceuticals; Ross Product Division of Abbott Laboratories; Slim-Fast Foods Company; and Unilever.
R.C.R. is a paid consultant for Palatin and Sprout Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
R.R.W. wrote the manuscript and led the ancillary study at the Look AHEAD site. D.S.B. completed statistical analysis and edited the manuscript. I.N.G. managed data and completed the initial statistical analysis. T.W. led the ancillary study at the Look AHEAD site and edited the manuscript. J.B. was the coordinating center representative for data coordination of the ancillary study and edited the manuscript. C.E.L. led the ancillary study at the Look AHEAD site and edited the manuscript. F.B. led the ancillary study at the Look AHEAD site and edited manuscript. S.S. assisted in grant submission and edited the manuscript. A.E.K. led the ancillary study at the Look AHEAD site and edited the manuscript. B.V.D. edited the manuscript. R.C.R. was the principal investigator of the ancillary study, was responsible for overseeing all aspects of the study, and edited the manuscript. R.R.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all investigators and staff involved in the baseline and 1-year results of Look AHEAD. The authors acknowledge the specific clinical sites and investigators involved in the sexual dysfunction ancillary study. Sexual Dysfunction Research Group, New England Research Institutes (NERI): Raymond C. Rosen, PhD. Robert Wood Johnson Medical School: Isaias Noel C. Gendrano III, MPH, and Stephen H. Schneider, MD. The Johns Hopkins Medical Institutions: Frederick L. Brancati, MD, MHS; Jeff Honas, MS; Lawrence Cheskin, MD; Jeanne M. Clark, MD, MPH; Kerry Stewart, EdD; Richard Rubin, PhD; Jeanne Charleston, RN; and Kathy Horak, RD. The University of Tennessee Health Science Center: Karen C. Johnson, MD, MPH; Abbas E. Kitabchi, PhD, MD; Carolyn Gresham, RN; Stephanie Connelly, MD, MPH; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; and J. Lee Taylor, MEd, MBA (the staff at the University of Tennessee East). Helen Lambeth, RN, BSN; Debra Clark, LPN; Andrea Crisler, MT; Gracie Cunningham; Donna Green, RN; Debra Force, MS, RD, LDN; Robert Kores, PhD; Renate Rosenthal, PhD; Elizabeth Smith, MS, RD, LDN; Maria Sun, MS, RD, LDN; and Judith Soberman, MD (the staff at the University of Tennessee Downtown). The University of Pennsylvania: Thomas A. Wadden, PhD; Barbara J. Maschak-Carey, MSN, CDE; Stanley Schwartz, MD; Gary D. Foster, PhD; Robert I. Berkowitz, MD; Henry Glick, PhD; Shiriki K. Kumanyika, PhD, RD, MPH; Johanna Brock; Helen Chomentowski; Vicki Clark; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Louise Hesson, MSN; Stephanie Krauthamer-Ewing, MPH; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, MS, RD; Leslie Womble, PhD, MS; and Nayyar Iqbal, MD. The Miriam Hospital/Brown Medical School: Rena R. Wing, PhD; Renee Bright, MS; Vincent Pera, MD; John Jakicic, PhD; Deborah Tate, PhD; Amy Gorin, PhD; Kara Gallagher, PhD; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; J.P. Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; and Jane Tavares, BS. The University of Alabama at Birmingham: Cora E. Lewis, MD, MSPH; Sheikilya Thomas, MPH; Monika Safford, MD; Vicki DiLillo, PhD; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Stacey Gilbert, MPH; Stephen Glasser, MD; Sara Hannum; Anne Hubbell, MS; Jennifer Jones, PhD, MA; DeLavallade Lee; Ruth Luketic, MA, MBA, MPH; Karen Marshall; L. Christie Oden; Janet Raines, MS; Cathy Roche, PhD, RN, BSN; Janet Truman; Nita Webb, MA; and Audrey Wrenn, MAEd.
Footnotes
Clinical trial reg. no. NCT00017953, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0315/-/DC1.
The clinical sites and investigators involved in the sexual dysfunction ancillary study are listed in the acknowledgments.
References
- 1.Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol 2008;112:970–978 [DOI] [PubMed] [Google Scholar]
- 2.Piak A, Laumann EO. Prevalence of women's sexual problems in the USA. In: Goldstein I, Meston C, Traish AM, Eds. Women's Sexual Function and Dysfunction London, Taylor & Francis, 2006, p. 23–33 [Google Scholar]
- 3.Bitzer J, Alder J. Diabetes and female sexual health. Womens Health (Lond Engl) 2009;5:629–636 [DOI] [PubMed] [Google Scholar]
- 4.Enzlin P, Rosen R, Wiegel M, et al. DCCT/EDIC Research Group Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/EDIC study cohort. Diabetes Care 2009;32:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito K, Maiorino MI, Bellastella G, Giugliano F, Romano M, Giugliano D. Determinants of female sexual dysfunction in type 2 diabetes. Int J Impot Res 2010;22:179–184 [DOI] [PubMed] [Google Scholar]
- 6.Copeland KL, Brown JS, Creasman JM, et al. Diabetes mellitus and sexual function in middle-aged and older women. Obstet Gynecol 2012;120:331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallner LP, Sarma AV, Kim C. Sexual functioning among women with and without diabetes in the Boston Area Community Health Study. J Sex Med 2010;7:881–887 [DOI] [PubMed] [Google Scholar]
- 8.Esposito K, Ciotola M, Giugliano F, et al. Association of body weight with sexual function in women. Int J Impot Res 2007;19:353–357 [DOI] [PubMed] [Google Scholar]
- 9.Melin I, Falconer C, Rössner S, Altman D. Sexual function in obese women: impact of lower urinary tract dysfunction. Int J Obes (Lond) 2008;32:1312–1318 [DOI] [PubMed] [Google Scholar]
- 10.Bond DS, Vithiananthan S, Leahey TM, et al. Prevalence and degree of sexual dysfunction in a sample of women seeking bariatric surgery. Surg Obes Relat Dis 2009;5:698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolotkin RL, Crosby RD, Gress RE, Hunt SC, Engel SG, Adams TD. Health and health-related quality of life: differences between men and women who seek gastric bypass surgery. Surg Obes Relat Dis 2008;4:651–658; discussion 658–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond DS, Wing RR, Vithiananthan S, et al. Significant resolution of female sexual dysfunction after bariatric surgery. Surg Obes Relat Dis 2011;7:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito K, Ciotola M, Giugliano F, et al. Mediterranean diet improves sexual function in women with the metabolic syndrome. Int J Impot Res 2007;19:486–491 [DOI] [PubMed] [Google Scholar]
- 14.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208 [DOI] [PubMed] [Google Scholar]
- 15.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 16.Wadden TA, West DS, Neiberg RH, et al. Look AHEAD Research Group One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadden TA, West DS, Delahanty L, et al. Look AHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it [corrected in Obesity (Silver Spring) 2007;15:1339]. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther 2005;31:1–20 [DOI] [PubMed] [Google Scholar]
- 19.Meston CM, Derogatis LR. Validated instruments for assessing female sexual function. J Sex Marital Ther 2002;28(Suppl. 1):155–164 [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571 [DOI] [PubMed] [Google Scholar]
- 21.Rosen RC, Wing RR, Schneider S, et al. Erectile dysfunction in type 2 diabetic men: relationship to exercise fitness and cardiovascular risk factors in the Look AHEAD trial. J Sex Med 2009;6:1414–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carson CC, Burnett AL, Levine LA, Nehra A. The efficacy of sildenafil citrate (Viagra) in clinical populations: an update. Urology 2002;60(Suppl. 2):12–27 [DOI] [PubMed] [Google Scholar]
- 23.Jackson G, Rosen RC, Kloner RA, Kostis JB. The second Princeton consensus on sexual dysfunction and cardiac risk: new guidelines for sexual medicine. J Sex Med 2006;3:28–36; discussion 36 [DOI] [PubMed] [Google Scholar]
- 24.Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health (Larchmt) 2009;18:461–468 [DOI] [PubMed] [Google Scholar]
- 25.Lutfey KE, Link CL, Rosen RC, Wiegel M, McKinlay JB. Prevalence and correlates of sexual activity and function in women: results from the Boston Area Community Health (BACH) Survey. Arch Sex Behav 2009;38:514–527 [DOI] [PubMed] [Google Scholar]