Abstract
OBJECTIVE
Sleep restriction has been associated with deteriorated insulin sensitivity. The effects of short sleep duration have been explored little in patients with type 1 diabetes. This study addresses the question of whether sleep curtailment affects HbA1c levels in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Seventy-nine adult patients with type 1 diabetes (median age 40 years [IQR 23–49]; 47% men) were recruited to wear a wrist actimetry sensor during 3 consecutive days to assess mean sleep duration during normal daily life. A subsample of 37 patients also performed 24-h ambulatory blood pressure monitoring (ABPM). Medical history, sleep questionnaires, and diabetes-related quality of life (DQOL) were assessed.
RESULTS
Patients having shorter sleep duration—less than 6.5 h (n = 21)—had higher levels of HbA1c (P = 0.01) than patients with longer sleep duration, above 6.5 h (n = 58). In a multivariable regression model including shorter versus longer sleep duration, diabetes duration, DQOL score, and daily activity, sleep duration was the only variable independently associated with HbA1c (R2 = 10%). In patients who performed 24-h ABPM, patients with a nondipping pattern of blood pressure exhibited shorter sleep duration than patients with a dipping pattern of blood pressure.
CONCLUSIONS
Shorter sleep duration is associated with higher HbA1c levels in patients with type 1 diabetes, as well as with a nondipping pattern of blood pressure, anticipating a long-term deleterious impact on the risk of microvascular complications. Further studies should test whether extending the duration of sleep may improve both HbA1c and blood pressure in type 1 diabetes.
Voluntary sleep curtailment is spreading among our societies, particularly in children and adolescents (1). Such chronic sleep deprivation is also frequent among patients suffering from type 1 diabetes as well as their families (2). Several prospective cohorts in the general population demonstrate a link between short sleep duration and incident obesity (3), type 2 diabetes (4–6), and hypertension (7,8). The main weakness of these studies, which were conducted with large samples, is that sleep duration was not objectively assessed but only estimated subjectively by sleep questionnaires. Mechanisms underlying such associations seem to involve down-regulation of the satiety hormone leptin and up-regulation of the appetite-stimulating hormone ghrelin, increasing hunger and food intake (9). Sleep restriction has been associated with deterioration of insulin sensitivity (10).
The effects of short sleep duration have been poorly explored in patients with type 1 diabetes. Short sleep duration has been associated with a nondipping pattern of blood pressure in patients with type 1 diabetes (11), which means that nocturnal blood pressure did not demonstrate the normal 10% decrease compared with daytime values. No study has yet addressed the question of the potential deleterious effect of sleep curtailment on HbA1c in type 1 diabetes. Priou et al. (12) recently have shown that sleep apnea syndrome (obstructive sleep apnea [OSA]) was associated with increased levels of HbA1c in subjects with OSA but without any diabetes, suggesting that sleep respiratory disorders could alter plasma glucose levels; however, this work did not evaluate the potential association of total sleep time with HbA1c.
Strict control of HbA1c and blood pressure levels, including the normal dip of nocturnal blood pressure, have been clearly associated with lower incidence of microvascular complications in type 1 diabetes (13–15). Thus, this study looked for the effects of sleep duration on glycemic control and nocturnal blood pressure patterns in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Adults with type 1 diabetes (81 men and women) (16) were recruited prospectively and at random from an outpatient clinic during the period ranging from December 2009 until April 2012. Participants were asked to wear a wrist actigraphy sensor for 3 consecutive days to objectively measure sleep duration in “real-life” conditions. One patient dropped out. One patient had invalid wrist actimetry values. Thus, 79 patients were included in the analyses. The exclusion criterion was unstable type 1 diabetes, that is, severe hypoglycemic events or acidoketosis during the previous 30 days. A subsample of 37 patients also performed a 24-h ambulatory blood pressure monitoring (ABPM) on a separate day, within 1 month after the actimetric measurement. All patients provided informed consent and the study was approved by institutional ethics committee (Sud-Est V, France). The protocol was registered on www.clinicaltrials.gov (NCT01017965).
Wrist actimetry
The Actiwatch AW7 (Cambridge Neurotechnology Ltd., Cambridge, U.K.) is an actigraph that measures activity by means of a piezoelectric accelerometer that records the combination of intensity, amount, and duration of movement, and the corresponding voltage produced is converted and stored as an activity count. Activity counts were summed over 1-min intervals, called epochs. The software (Actiwatch activity and sleep analysis 7.31, Cambridge Neurotechnology Ltd.) was set to detect activity with “medium” sensitivity, that is, 40 counts per epoch. Determination of sleep and wakefulness by the software relies on an algorithm that looks at each data point and calculates a total score based on the activity counts from each epoch and those surrounding it. The event marker button on top of the Actiwatch had to be pressed when the participants went to bed and when they got up. The sleep analysis software determines the start of sleep by searching for a period of at least 10 min of consecutively recorded immobile data after bed time. To determine the end of sleep, the software analyzed the end of immobility corresponding to the time when patients got up. In addition, a self-reported sleep diary was completed by participants to determine bedtime, subjective sleep latency, wake-up and get-up times, sleep duration, as well as number of awakenings during the night, as recommended (17). The Actiwatch-AW7 was worn to the wrist during 3 consecutive days. This small device does not affect the subject’s movements during normal activity. The ability of the device to measure sleep duration accurately and objectively has been reported previously (18). In addition to sleep duration, sleep latency, sleep fragmentation, and sleep efficiency as well as daily activity were analyzed automatically by the actimetry software. These measurements have been validated to be similar to those measured by polysomnography, the gold standard (19,20).
Sleep questionnaires
Pittsburgh Sleep Quality Index.
The Pittsburgh Sleep Quality Index (PSQI) examined seven components (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction). A global score was fulfilled by all participants. With a total of 19 questions, participants rated the components on a scale of 0 to 3, with a possible total range from 0 to 21 and with higher scores indicating worse sleep quality (>5 = poor sleepers) (21).
Berlin questionnaire.
The Berlin questionnaire evaluated the risk of having a sleep apnea syndrome (22). It consists of 10 questions on risk factors for OSA, subdivided into three symptom categories: 1) snoring behavior and apnea (six points); 2) wake-time sleepiness or fatigue (three points); and 3) the presence of obesity or hypertension (two points). The patient marks only one response per question and the ultimate interpretation of the answers suggests whether he or she has high risk of OSA (positive) or not (negative). If two or more categories are positive (categories 1 and 2: two or more points; category 3: one or more points), a person is considered at high risk of having OSA. Those who denied having persistent symptoms or who qualified for only one symptom category were placed in the lower-risk group.
International restless leg syndrome severity score.
For patients self-reporting a restless leg syndrome, its severity was assessed with the international restless leg syndrome (IRLS) severity score. The IRLS severity score rating scale comprises 10 questions that assess symptom severity and frequency on a 40-point scale (0 = no symptoms; 40 = very severe symptoms) (23).
Diabetes quality of life.
Quality of life was determined using the Diabetes Quality of Life (DQOL) questionnaire, as implemented during the Diabetes Control and Complications Trial and validated in French (24). The DQOL questionnaire was analyzed in five different subscales: satisfaction with life, impact of diabetes, social/vocational worry, diabetes-related worry, and health perception. It is a multiple-choice tool, with higher scores indicating higher quality of life.
Biological measurements
HbA1c was measured by high-pressure liquid chromatography (normal values 4–6%) (Varian II; Bio-Rad, Hercules, CA). All HbA1c values also were presented in millimoles per mole according to the NGSP’s HbA1c converter (available at http://www.ngsp.org/convert1.asp). Microalbuminuria was measured by immunoturbidimetry (Nephelometer Analyser II; Berhing, Marburg, Germany). The level of microalbuminuria (milligrams per liter) was assessed using a urine sample collected at least 6 weeks after any urinary tract infections or acute hyperglycemic events and after exclusion of all other causes of albuminuria.
24-h ABPM.
The Enregistreur MAPA 90207-3Q (Spacelabs Healthcare, Issaquah, WA) was used to record 24-h ABPM with an adapted-sized cuff. Blood pressure measurements were performed every 15 min. Blood pressure was considered nocturnal between 10:00 p.m. and 6:00 a.m. Men and women were considered to be nondippers if they had a day/night systolic blood pressure decrease of less than 10%. We included in the nondipper group four patients who were “reverse dippers,” that is, those whose daytime blood pressure was below the nocturnal blood pressure. They were no “extreme dippers” (nocturnal blood pressure dip >20%) among our population.
Statistical analysis
Results are expressed as mean (SD) for normally distributed variables and as median (interquartile range) for nonnormally distributed variables. Categorical variables were presented as number (percentage). The normal distribution of residuals was verified by stem and leaf plots and by Shapiro-Wilk tests. Parameters with skewed distributions (daily insulin dose, diabetes duration, sleep latency) were transformed by log or square root. Age, BMI, and microalbuminuria remained nonnormally distributed.
Patients presenting with sleep duration less than 6.5 h (first quartile) were compared with patients with sleep duration longer than 6.5 h by ANOVA for normally distributed variables and by the Wilcoxon test for nonnormally distributed variables. Categorical variables were compared by χ2 test or Fischer test. Similar comparison tests were used to compare dippers with nondippers in the subsample of participants who performed a 24-h ABPM.
Univariate Pearson correlations were made to identify factors associated with levels of HbA1c. All variables tending to be associated with HbA1c (P < 0.2), that is, short versus long sleep duration, diabetes duration, DQOL score, and daily level of activity, were included in a multivariable linear regression model to assess the relative contribution of each factor to HbA1c levels.
The significance level was set at P < 0.05. All analyses were performed using the SAS statistical package version 9.2 (SAS Institute, Cary, NC).
RESULTS
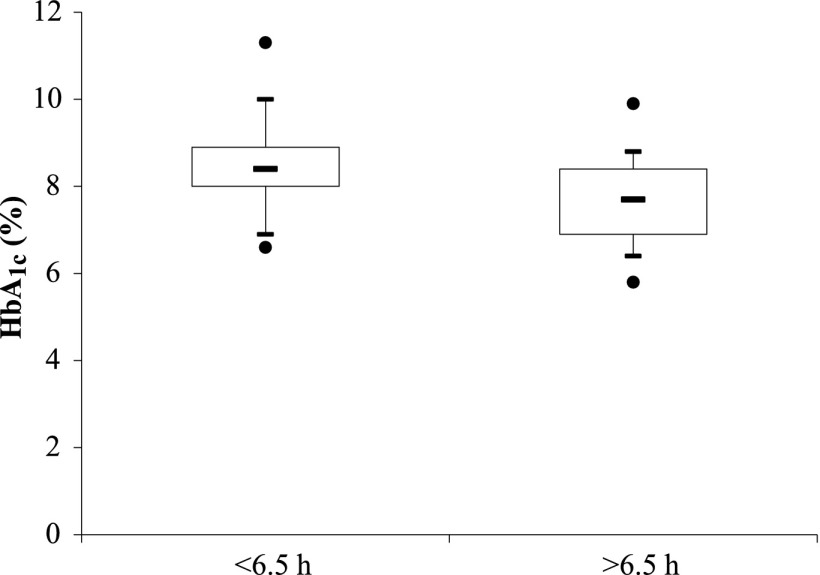
Characteristics of the whole sample of patients and differences between patients presenting with a shorter versus longer sleep duration are reported in Table 1. Thirty-eight patients (49%) were full-time workers; none were shift workers. Seventeen patients (22%) were students, 11 (14%) were retired, and 12 (15%) did not work (1 missing data for work activity). The distribution of working status was not different between those who slept for shorter (<6.5 h) and longer (>6.5 h) durations (P = 0.411). Patients with shorter sleep duration presented with a higher prevalence of known sleep apnea syndrome (19 vs. 5%; P = 0.040) and restless leg syndrome (30 vs. 9%; P = 0.056) in their medical history than patients with longer sleep duration. The severity of restless leg syndrome was also higher in patients with shorter sleep duration. Beside known sleep apnea syndrome (8% of the patients), 27% of all patients presented with a positive score to the Berlin questionnaire, suggesting a high risk of undiagnosed sleep apnea syndrome. This risk tended to be higher in patients with shorter than with longer sleep duration (P = 0.060). Among patients with hypertension (n = 26), the use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, calcium channel blockers, thiazide, or antihypertensive therapy with central effect was not different between the two groups. Finally, HbA1c was higher in patients with shorter sleep duration than in patients with longer sleep duration (8.5 [1.2] vs. 7.7 [1.0]% or 70 [13] vs. 60 [10] mmol/mol; P = 0.001) (Fig. 1).
Table 1.
Characteristics of patients
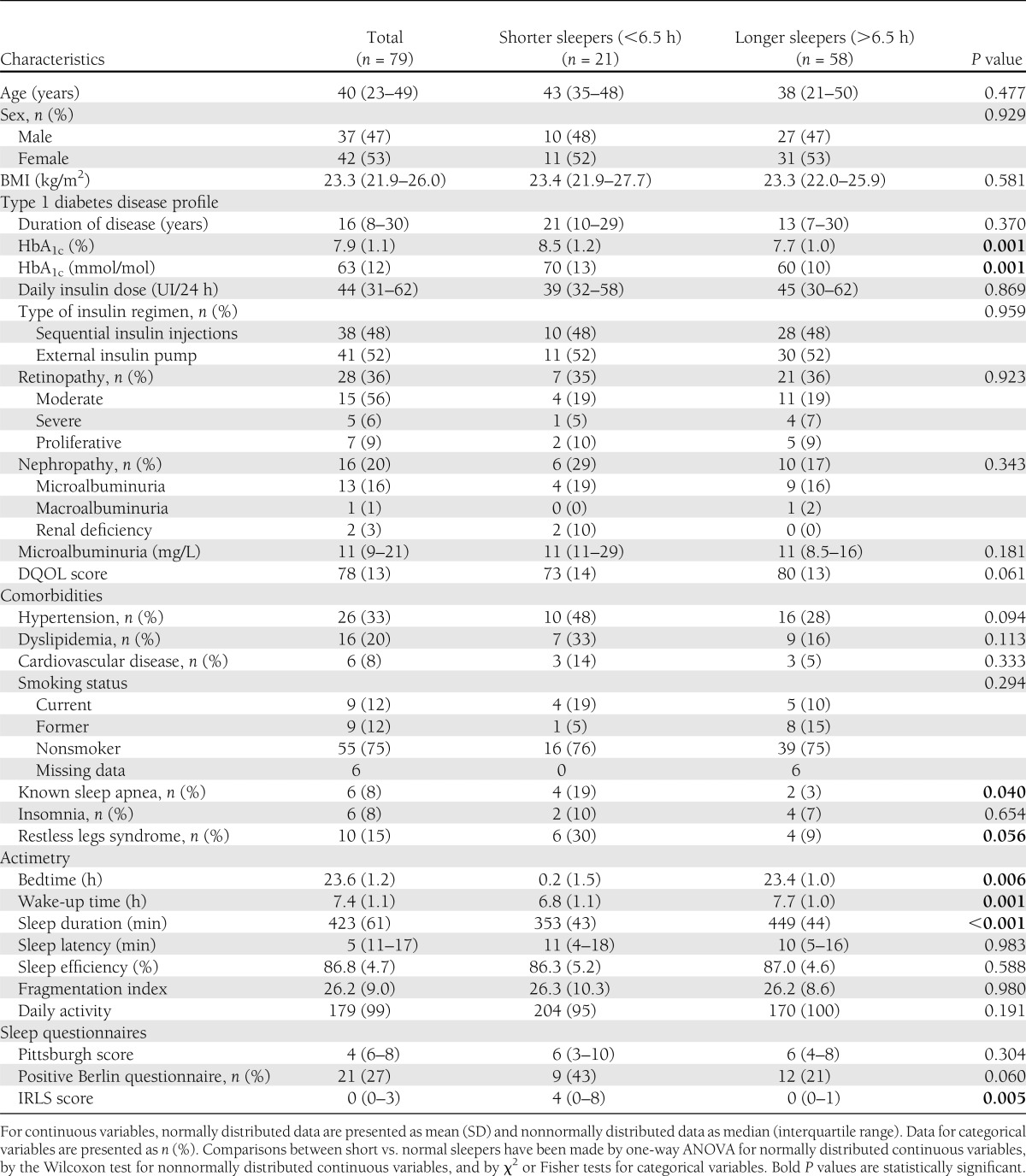
Figure 1.
Distribution of HbA1c levels in patients with shorter (<6.5 h) vs. longer (>6.5 h) sleep duration. Comparisons between short vs. normal sleepers were determined using one-way ANOVA and were statistically significant (P = 0.001).
Regression analyses
Univariate Pearson correlations were computed to determine which variables were associated with the level of HbA1c. Only the sleep duration dichotomized (threshold 6.5 h) between shorter versus longer sleep duration was significantly associated with HbA1c. Shorter sleep duration increased the mean level of HbA1c by 0.87% (10 mmol/mol). Diabetes duration, DQOL score, and daily level of activity tended to be associated with HbA1c levels (P < 0.2). Therefore, these variables were included in a multivariable model along with sleep duration dichotomized between shorter versus longer sleep duration to assess the relative contribution of each variable to the level of HbA1c. Sleep duration was the only variable independently associated with the level of HbA1c, and the model explained 10% of the HbA1c variance. In this adjusted model, a 0.64% (7 mmol/mol) increase in HbA1c level was associated with shorter sleep duration.
24-h ABPM
In the subsample of patients who performed a 24h-ABPM (n = 37), 12 patients presented with a dipping blood pressure pattern, 21 were nondippers, and 4 were reverse dippers. The four patients with a reverse dipping pattern were associated with nondipper patients for the analyses. The nondipping status of nocturnal blood pressure was found in 11 of 13 patients (85%) with shorter sleep duration and in 14 of 24 patients (58%) with longer sleep duration (P = 0.149, Fischer test). Of note, in this subsample of patients, those with shorter sleep duration also had higher levels of HbA1c than those with longer sleep duration (8.9 [1.2] vs. 7.8 [0.8]% or 74 [14] vs. 61 [9] mmol/mol, respectively; P = 0.002).
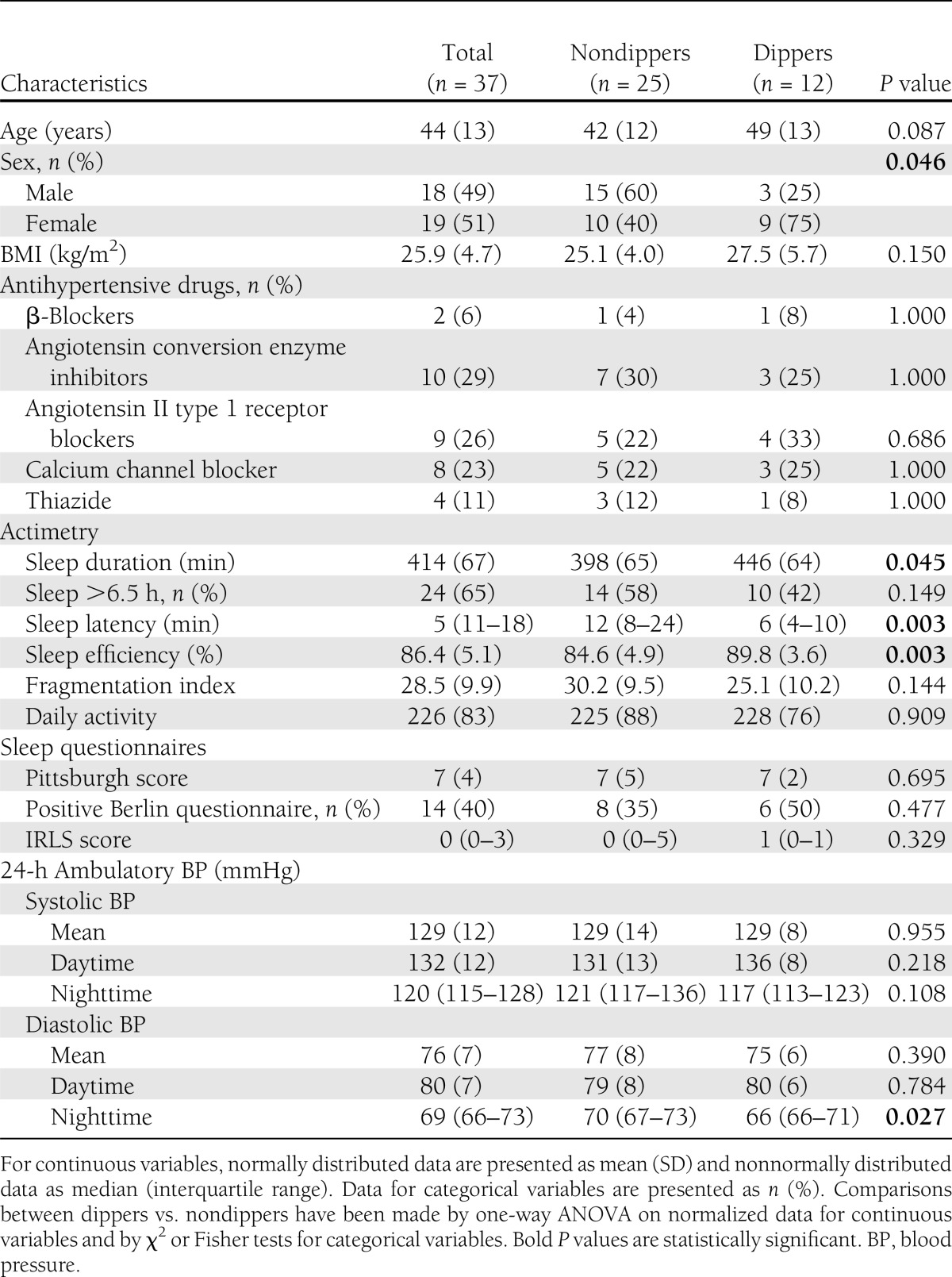
Compared with dipping patients, nondipping patients were more often men and had shorter sleep duration, higher sleep latency, and lower sleep efficiency than dipping patients (Fig. 2). Diabetes duration, daily insulin dose, HbA1c levels, and the presence of diabetes complications did not differ among dippers and nondippers (Table 2).
Figure 2.
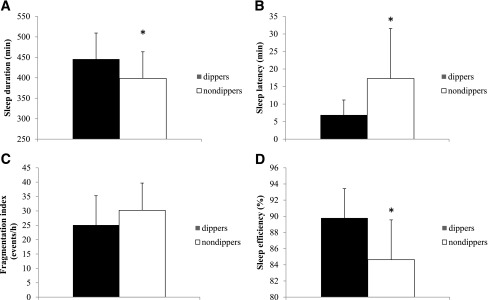
Sleep characteristics of patients with a dipping vs. nondipping pattern of nocturnal blood pressure. A: Sleep duration. B: Sleep latency. C: Fragmentation index. D: Sleep efficiency derived from actimetric measurement. Results are mean (SD). For each patient, the data were the mean of the 3 consecutive days of measurements. *P < 0.05.
Table 2.
Characteristics of patients with dipping vs. nondipping blood pressure patterns
CONCLUSIONS
This study assessed the potential role of sleep duration on glycemic control in patients with type 1 diabetes. Sleep curtailment to less than 6.5 h was independently associated with a higher level of HbA1c. Sleep duration was assessed objectively by wrist actigraphy when the majority of studies in the field used only sleep questionnaires. In addition, patients presenting with a nondipping pattern of nocturnal blood pressure also had shorter sleep duration as well as longer sleep latency and lower sleep efficiency than patients with a normal dipping pattern of nocturnal blood pressure.
No study to date has specifically explored the effects of chronic sleep deprivation on glycemic control in patients with type 1 diabetes. However, several observations suggest that sleep deprivation may impact plasma glucose/insulin homeostasis. First, epidemiological studies (25–27) (see Spiegel et al. [28] for a review) suggest that short sleep duration is associated with an increased incidence of type 2 diabetes. Second, in patients without diabetes, experimental sleep deprivation was shown to alter plasma glucose/insulin homeostasis. Indeed, Spiegel et al. (29) performed a study of 6 nights of sleep deprivation (<4 h of sleep) in 11 healthy young men, followed by 6 nights of sleep recovery. An intravenous glucose tolerance test was performed at the end of each period. Sleep deprivation was associated with 40% decrease in glucose clearance and a 30% decrease in acute insulin response compared with results in the same subjects after sleep recovery. As a possible explanation for the deterioration in plasma glucose/insulin homeostasis during sleep restriction, these subjects also showed higher sympathetic activity and higher late-afternoon and evening cortisol levels after sleep restriction than after sleep recovery. These alterations were reproduced in a second study by Spiegel et al. (30) in which sleep deprivation also was associated with increased levels of the orexigenic hormone ghrelin and with reduced levels of leptin. Thus, sleep deprivation seems to affect plasma glucose/insulin homeostasis through several mechanisms: 1) sympathetic overactivity, 2) changes in food intake–related hormones that are likely to increase appetite, and 3) increases in hormones that up-regulate glucose. These mechanisms are likely to alter plasma glucose/insulin homeostasis in patients with type 1 diabetes, and therefore their glycemic control. Sympathetic overactivity may also participate to poor blood pressure control.
In a cohort including patients with type 1 diabetes and their first- and second-degree relatives, Estrada et al. (2) showed that 40.9% of these subjects slept insufficiently according to the U.S. age-specific recommendations for sleep duration. However, this study did not provide an additional analysis of patients with type 1 diabetes separate from their relatives without any diabetes. Our group has conducted a pilot study suggesting that patients with type 1 diabetes might have an elevated prevalence of sleep apnea syndrome (31). Accordingly, the risk of having undiagnosed sleep apnea syndrome was high (27%) in the current study. This risk tended to be higher in shorter than in longer sleepers, meaning that unknown sleep apnea syndrome may have participated in the reduced sleep duration of these patients. By contrast, the frequency of insomnia was not different between shorter and longer sleepers. Insomnia is defined by the difficulty to initiate and/or maintain sleep but is not necessarily associated with a reduction in objectively measured sleep time. Interestingly, Vgontzas et al. (32) have shown that the risk of incident diabetes among insomniac patients was increased only in patients with an objective sleep duration of less than 5 h per night. Van Dijk et al. (33) used questionnaires to assess subjective sleep characteristics of patients with type 1 diabetes compared with control subjects without diabetes. They found that subjective sleep quality was impaired in patients with type 1 diabetes in relation to the sleep disturbance and daytime dysfunction items of the PSQI. They also presented higher levels of anxiety and depression in the Hospital Anxiety and Depression Scale than control subjects.
However, sleep duration, number of short sleepers, and number of long sleepers were not different in patients with type 1 diabetes compared with control subjects. The mean sleep duration in the study by Van Dijk et al. was 7.2 h/night in patients and controls, which is comparable to the mean sleep duration found in the whole sample of patients in our study. Thus, symptoms associated with diabetes, such as thirst, nocturia, extreme glucose excursions, and mood alterations, may interfere with sleep quality and may contribute to sleep fragmentation but do not seem to alter sleep duration. In the present study, patients with shorter or longer sleep durations did not present any difference in sleep quality as assessed by the PSQI. However, patients with shorter sleep duration had delayed bedtime and earlier wake-up time compared with longer sleepers. This trend was observed at a population level using the Munich ChronoType Questionnaire, a simple, Internet-based questionnaire (34). Indeed, sleep duration has been reduced over the past decade by delaying bedtime, whereas get-up time on workdays has not changed. The sleep debt is often compensated for by oversleeping on free days, which induces a “social jetlag,” which is a chronic change in circadian rhythm every several days (35). These new paradigms may participate in glucose variability in type 1 diabetes. Further studies inquiring about the reason(s) for shorter sleep duration (e.g., voluntary sleep curtailment, constraints linked to professional status) or other factors and their specific effect on glucose control in patient with diabetes are warranted.
Nocturnal blood pressure has a normal decline of more than 10% compared with daytime blood pressure, corresponding to the so-called dipping status. In type 1 diabetes, nondipping status is more prevalent (36) and is associated with increased risks for sustained hypertension, retinopathy, and nephropathy (15,36,37). Our group has previously shown that shorter sleep duration, assessed by a one-night polysomnography in a sleep laboratory, was associated with a nondipping pattern of blood pressure in patients with type 1 diabetes (11). In this previous study, patients spent the night in a sleep laboratory while polysomnography, 24-h ABPM, and continuous glucose monitoring were performed simultaneously. The current study assessed sleep duration using wrist actimetry at home and over 3 consecutive days to be closer to the routine sleep habits of patients. Results were concordant since patients with a nondipping blood pressure pattern had shorter sleep duration but longer sleep latency and lower sleep efficiency than patients with a dipping blood pressure pattern.
Thus the present results show that short sleep duration is associated with deteriorated glycemic control and nocturnal blood pressure pattern in patients with type 1 diabetes. Both conditions are associated with an increased risk of developing microvascular complications (15,37,38). Thus, short sleep duration could affect the development of retinopathy and nephropathy by deteriorating synergistic glycemic levels and blood pressure in patients with type 1 diabetes. Further studies should address the efficiency of educational interventions aimed at enhancing sleep duration on glycemic levels and nocturnal blood pressure.
Acknowledgments
This study was supported by a grant from the Direction de la Recherche Clinique, University Hospital, Grenoble, France.
No potential conflicts of interest relevant to this article were reported.
A.-L.B. analyzed data and wrote the manuscript. J.-L.P. contributed to the discussion and reviewed/edited the manuscript. L.N. researched data and reviewed/edited the manuscript. J.-P.B. and S.N. researched data. P.-Y.B. researched data, contributed to the discussion, and reviewed/edited the manuscript. P.-Y.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01017965, www.clinicaltrials.gov.
References
- 1.Research IoMUCoSMa Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC). US, National Academies Press, 2006 [PubMed] [Google Scholar]
- 2.Estrada CL, Danielson KK, Drum ML, Lipton RB. Insufficient sleep in young patients with diabetes and their families. Biol Res Nurs 2012;14:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008;31:517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 2007;30:1667–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol 2009;19:351–357 [DOI] [PubMed] [Google Scholar]
- 6.Chaput JP, Després JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med 2009;10:919–924 [DOI] [PubMed] [Google Scholar]
- 7.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 2007;50:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med 2009;169:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–850 [DOI] [PubMed] [Google Scholar]
- 10.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab 2010;24:687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borel AL, Benhamou PY, Baguet JP, et al. Short sleep duration is associated with a blood pressure nondipping pattern in type 1 diabetes: the DIAPASOM study. Diabetes Care 2009;32:1713–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priou P, Le Vaillant M, Meslier N, et al. IRSR Sleep Cohort Group Independent association between obstructive sleep apnea severity and glycated hemoglobin in adults without diabetes. Diabetes Care 2012;35:1902–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Aroca P, Mendez-Marin I, Baget-Bernaldiz M, Fernéndez-Ballart J, Santos-Blanco E. Review of the relationship between renal and retinal microangiopathy in diabetes mellitus patients. Curr Diabetes Rev 2010;6:88–101 [DOI] [PubMed] [Google Scholar]
- 15.Benhamou PY, Halimi S, De Gaudemaris R, et al. Early disturbances of ambulatory blood pressure load in normotensive type I diabetic patients with microalbuminuria. Diabetes Care 1992;15:1614–1619 [DOI] [PubMed] [Google Scholar]
- 16.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 17.Littner M, Kushida CA, Anderson WM, et al. Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 2003;26:337–341 [DOI] [PubMed] [Google Scholar]
- 18.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep 2004;27:158–165 [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Wong KK, Dungan GC, 2nd, Buchanan PR, Yee BJ, Grunstein RR. The validity of wrist actimetry assessment of sleep with and without sleep apnea. J Clin Sleep Med 2008;4:450–455 [PMC free article] [PubMed] [Google Scholar]
- 20.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 2011;15:259–267 [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491 [DOI] [PubMed] [Google Scholar]
- 23.Walters AS, LeBrocq C, Dhar A, et al. International Restless Legs Syndrome Study Group Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 2003;4:121–132 [DOI] [PubMed] [Google Scholar]
- 24.Renard E, Schaepelynck-Bélicar P, EVADIAC Group Implantable insulin pumps. A position statement about their clinical use. Diabetes Metab 2007;33:158–166 [DOI] [PubMed] [Google Scholar]
- 25.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003;26:380–384 [DOI] [PubMed] [Google Scholar]
- 26.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care 2005;28:2762–2767 [DOI] [PubMed] [Google Scholar]
- 27.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care 2006;29:657–661 [DOI] [PubMed] [Google Scholar]
- 28.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 2009;5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–1439 [DOI] [PubMed] [Google Scholar]
- 30.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol 2005;99:2008–2019 [DOI] [PubMed] [Google Scholar]
- 31.Borel AL, Benhamou PY, Baguet JP, et al. High prevalence of obstructive sleep apnoea syndrome in a Type 1 diabetic adult population: a pilot study. Diabet Med 2010;27:1328–1329 [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009;32:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dijk M, Donga E, van Dijk JG, et al. Disturbed subjective sleep characteristics in adult patients with long-standing type 1 diabetes mellitus. Diabetologia 2011;54:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003;18:80–90 [DOI] [PubMed] [Google Scholar]
- 35.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 2012;22:939–943 [DOI] [PubMed] [Google Scholar]
- 36.Dost A, Klinkert C, Kapellen T, et al. DPV Science Initiative Arterial hypertension determined by ambulatory blood pressure profiles: contribution to microalbuminuria risk in a multicenter investigation in 2,105 children and adolescents with type 1 diabetes. Diabetes Care 2008;31:720–725 [DOI] [PubMed] [Google Scholar]
- 37.Stella P, Tabak AG, Zgibor JC, Orchard TJ. Late diabetes complications and non-dipping phenomenon in patients with Type 1 diabetes. Diabetes Res Clin Pract 2006;71:14–20 [DOI] [PubMed] [Google Scholar]
- 38.Nordwall M, Arnqvist HJ, Bojestig M, Ludvigsson J. Good glycemic control remains crucial in prevention of late diabetic complications—the Linköping Diabetes Complications Study. Pediatr Diabetes 2009;10:168–176 [DOI] [PubMed] [Google Scholar]