Abstract
OBJECTIVE
A high amount of subcutaneous fat is suggested to explain the observation of lower obesity-associated metabolic risk among Inuit than among Europeans. We examined the association between measures of obesity (visceral adipose tissue [VAT], subcutaneous adipose tissue [SAT], BMI, waist circumference [WC], and percentage of body fat) and the indices of glucose metabolism (fasting and 2-h glucose levels, insulin resistance per homeostasis model assessment [HOMA-IR], and the insulin sensitivity index [ISI0,120]) among Greenland Inuit.
RESEARCH DESIGN AND METHODS
A total of 3,108 adult Inuit participated in a population-based study. The examination included a 75-g oral glucose tolerance test and anthropometric measurements. VAT and SAT were measured by ultrasound according to a validated protocol. Information on sociodemographic characteristics and health behaviors was obtained by interview.
RESULTS
Mean SATs were 1.8 and 3.5 cm in men and women, respectively. Mean VATs were 7.0 and 6.3 cm in men and women, respectively. The total prevalence of type 2 diabetes was 9%. Percentage of body fat generally was most strongly associated with all outcomes. Both SAT and VAT were significantly associated with glucose intolerance, fasting and 2-h plasma glucose levels, HOMA-IR, and ISI0,120. VAT was more strongly associated with all outcomes than was SAT. After further adjustment for BMI or WC, VAT was associated with glucose intolerance and insulin resistance, whereas there was a trend toward a negative or no association with SAT.
CONCLUSIONS
High mean values of SAT may to a large extent explain the high WC in Inuit populations, and this is suggested to contribute to the lower observed metabolic risk for a given level of obesity.
A growing body of evidence indicates that Inuit populations in the Arctic have high levels of obesity according to international guidelines for BMI and waist circumference (WC) (1–3). These populations are undergoing rapid social and health transitions, with the emergence of chronic diseases such as type 2 diabetes and ischemic heart disease (4,5). Among the Inuit, the mean levels of various cardiovascular risk factors increase with increasing levels of obesity, as they do in other populations. The metabolic impact of different levels of obesity, at least cross-sectionally, appears to be less pronounced among the Inuit than among Europeans, however, especially for indicators such as HDL cholesterol, triglycerides, blood pressure, and postprandial glucose and insulin levels (1,6). These population-specific differences in levels of cardiovascular risk factors presumably depend on interactions among environmental factors, lifestyle, and genetic factors that influence obesity and insulin sensitivity; however, an alternative explanation is that anthropometric measurements such as BMI, waist-to-hip ratio (WHR), and WC do not reflect the same amount of fat or the same pattern of fat distribution in different populations.
Evidence is now emerging that particular patterns of abdominal fat distribution may confer increased metabolic risk. Specifically, excess visceral adipose tissue (VAT) is thought to be a marker of the relative inability of subcutaneous adipose tissue (SAT) to store more energy during continued positive caloric balance. Individuals who cannot store their energy surplus in the SAT would be characterized by accumulation of fat at undesired sites such as the liver, the heart, the skeletal muscle, and the pancreas (7–10).
To elucidate the role of fat distribution in obesity-associated metabolic risk among Greenland Inuit, the overall aim of the current study was therefore to study the associations of ultrasound measures of fat distribution (visceral fat, subcutaneous fat, and the ratio of these two measures), anthropometric measurements (BMI, WC, and percentage of body fat [fat%]), and the following indices of glucose metabolism: type 2 diabetes, impaired fasting glycemia (IFG), impaired glucose tolerance (IGT), fasting and 2-h glucose values, insulin resistance per homeostasis model assessment (HOMA-IR), and the insulin sensitivity index (ISI0,120).
RESEARCH DESIGN AND METHODS
Participants
Data were collected during 2005–2010 in stratified random samples of adult Inuit in Greenland (11). The total population of Greenland is 57,000, of whom 90% are ethnic Greenlanders (Inuit). Genetically, Greenlanders are Inuit (Eskimos) with an admixture of European, mainly Scandinavian genes. They are genetically and culturally closely related to the Inuit (Iñupiat) in Canada and Alaska and are somewhat more distantly related to the Yupiit of Alaska and Siberia. Participants were selected as a stratified random sample. Greenland was divided into strata by geography (southwest coast, central west coast, northwest coast, east Greenland, and north Greenland) and community size (towns with ≥2000 inhabitants, towns with <2000 inhabitants, and villages with <500 inhabitants). From each of these strata, one or more towns and two or three villages were selected for the study as being representative of the stratum with regard to living conditions. A random sample was drawn from the central population register to obtain around 300 participants from each town; this number represents the practical limit for a research team during a visit of 4–6 weeks. Villages were chosen at random in the strata, and in the selected villages all adults were invited to participate. Information on adults aged ≥18 years who had been born in Greenland or Denmark was collected during 2005–2010 in 9 towns and 13 villages in Greenland. Ethnicity as a Greenlander was determined at enrolment on the basis of the primary language of the participant and self-identification. Only one ethnicity was allowed for each participant.
Interview and questionnaire
The survey questionnaires were developed in Danish and subsequently translated into Greenlandic. The translation procedure included translation by two or more interpreters, followed by an independent back translation into Danish and revision of the translation as needed.
Genetic heritage was estimated from questions on the ethnicities of the four grandparents, and if this information was missing, of the parents. It was subsequently recoded as full (all grandparents were Greenlanders) or partial Inuit heritage.
Information on physical activity was collected using a modified version of the long version of the 7-day International Physical Activity Questionnaire (12). Participants were classified as current smokers, past smokers, or nonsmokers. The frequency of alcohol consumption was reported. Education was recorded as educational attainment.
Obesity measurements
Height and weight were measured with the participants wearing underwear and socks. BMI was calculated as weight in kilograms divided by height in meters squared. WC was measured on the standing participant midway between the iliac crest and the costal margin. Bioimpedance and calculation of fat% were performed on a leg-to-leg Tanita TBF-300MA (Tanita Corporation, Tokyo, Japan). On the basis of a single reading, fat% was calculated by the internal algorithm of the device, which is based on height, weight, sex, impedance, and age; body type was set as standard. An ultrasound system (Pie Medical) with a 3C-RS curved transducer was used to determine VAT and subcutaneous abdominal fat thicknesses. According to a validated protocol, ultrasound VAT was defined as the depth in centimeters from the peritoneum to the lumbar spine, and ultrasound subcutaneous abdominal fat was defined as the depth in centimeters from the skin to the linea alba (13–15). Both measurements were obtained from where the xiphoid line and the WC met. Measurements were made at the end of a quiet expiration by applying minimal pressure to ensure no displacement of the abdominal cavity. Coefficients of variation for intraobserver and interobserver variation were in the range 1.9–5.6%.
Glucose homeostasis markers
At the baseline health examination, venous blood samples were drawn after a verified overnight fast (≥8.0 h). After this, participants received a standard 75-g oral glucose tolerance test, with blood samples drawn 2 h after the glucose intake. Participants with known type 2 diabetes did not have an oral glucose tolerance test, although fasting venous plasma glucose was measured. Plasma samples were immediately put on ice and spun at 4°C within 30 min of sampling. Samples were stored frozen at −20°C and shipped to Steno Diabetes Center, Gentofte, Denmark, for analyses. Plasma glucose values (0 and 2 h) were determined with the Hitachi 912 system (Roche Diagnostics, Mannheim, Germany). Serum insulin values (0 and 2 h) were determined by an immunoassay method (AutoDELFIA; Perkin Elmer, Waltham, MA). Type 2 diabetes and impaired glucose regulation were classified according to the World Health Organization criteria 1999 (16). The following categories of glucose tolerance status were included: normal glucose tolerance, IGT, IFG, and type 2 diabetes. Type 2 diabetes was defined as fasting plasma glucose ≥7.0 mmol/L, 2-h plasma glucose ≥11.1 mmol/L, or both; IFG was defined as fasting plasma glucose ≥6.1 and <7.0 mmol/L and 2-h plasma glucose <7.8 mmol/L; IGT was defined as fasting plasma glucose <6.1 and ≤7.8 mmol/L and 2-h plasma glucose <11.1 mmol/L (16).
Glycemic indices were derived from glucose and insulin measures from the glucose tolerance test. Measures of hepatic insulin resistance were derived from homeostasis model assessment: (HOMA-IR (mmol/L × mU/L) = [fasting plasma glucose (mmol/L)] × [fasting plasma insulin (pmol/L)]/6.945)/22.5 (17).
The insulin sensitivity index (ISI0,120) was calculated according to Gutt et al. (18) to give an estimate of insulin sensitivity in the peripheral tissues as follows:
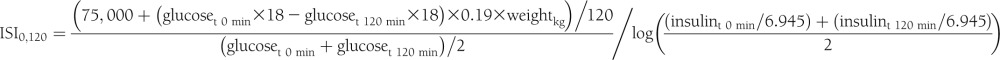 |
Statistical analysis
Analyses were performed with the SAS statistical software package (version 9.2; SAS Institute, Cary, NC). Obesity measures were standardized to a mean of 0 and SD of 1 to facilitate comparisons. Variables with a skewed distribution (HOMA-IR, ISI0,120, alcohol consumption, and energy expenditure) were log-transformed for analysis and presented as medians with interquartile ranges. The β-coefficients were calculated with linear regression models to assess the association between 1 SD change in obesity measure and continuous markers of glucose homeostasis. For outcomes that were not normally distributed, the β-coefficients were expressed as percentage change associated with 1 SD change in obesity measure.
To evaluate the associations between the obesity measures and glucose tolerance status, a multivariate logistic regression analysis was performed with diabetes, IFG, and IGT as dependent variable and with normal glucose tolerance as the reference category. Analyses that included plasma glucose and insulin concentrations were restricted to those with newly diagnosed diabetes.
All multivariate analyses were adjusted for age, sex, hormonal replacement therapy, Inuit heritage, physical activity, smoking status, and alcohol intake. The models for SAT and VAT were further adjusted for WC in separate models. Sex-interactions were tested by examining the significance of the cross-product between sex and obesity measures.
Ethics considerations
The studies were ethically approved by the Commission for Scientific Research in Greenland. Participants gave their written consent after being informed about the study orally and in writing.
RESULTS
With a participation rate of 66.1%, the total number of Inuit participants in the study was 3,108. The 169 participants without information on obesity or body composition were excluded, bringing the study base down to 2,939. The mean age of the participants was 44 years (range 18–95), and 56% were women. Compared with nonparticipants, participants were slightly older (44.4 vs. 43.9 years; P = 0.003), and there were fewer males (43.5% vs. 50.8%; P < 0.001).
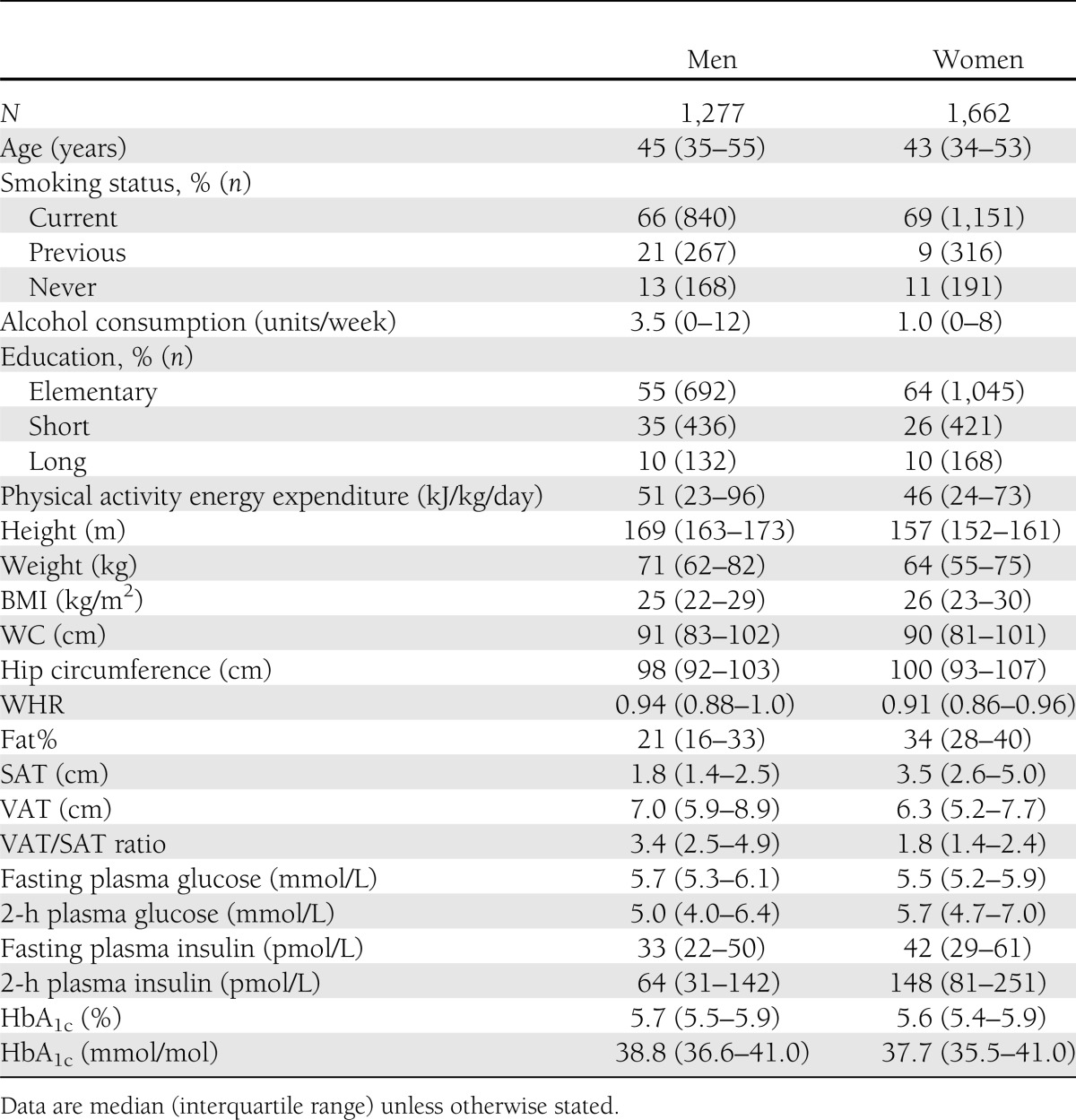
Characteristics of the study base are presented in Table 1. Median levels of WC were high and remarkably similar between men and women (91 and 90 cm, respectively) relative to a median BMI of 25 and 26 kg/m2 in men and women, respectively. Mean SATs measured by ultrasound were 1.8 cm among men and 3.5 cm among women. Mean VATs measured by ultrasound were 7.0 cm among men and 6.3 cm among women.
Table 1.
Demographic, behavioral, and clinical characteristics of the study population
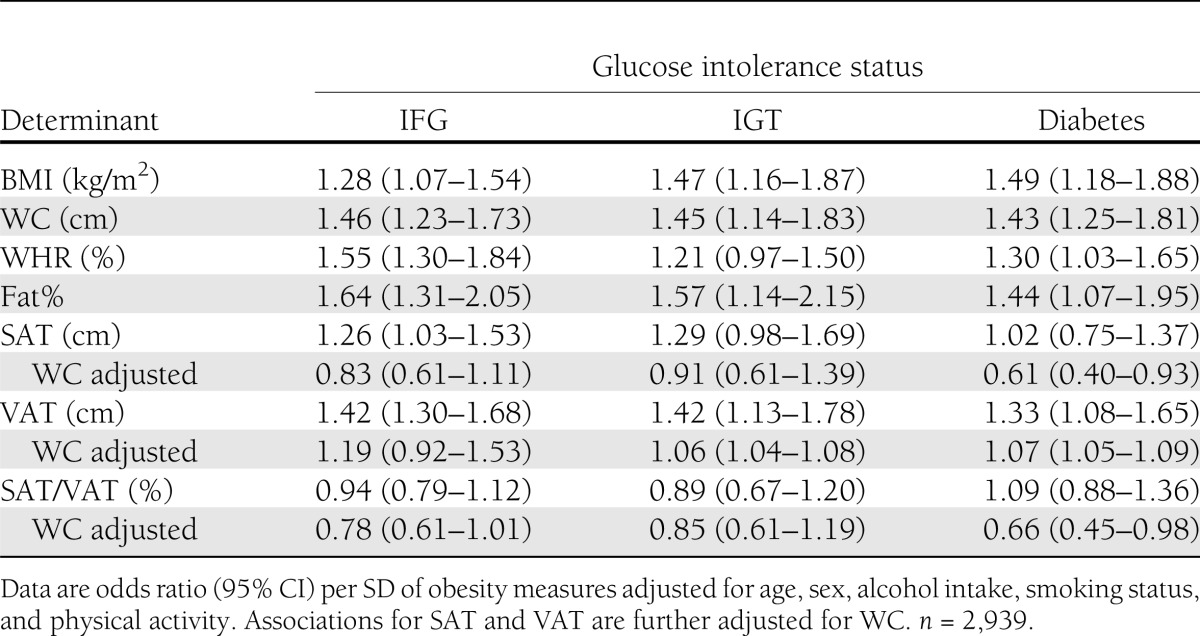
The total prevalence of type 2 diabetes was 9%, of which 79% of cases were previously unknown. More than half of the patients with type 2 diabetes (56%) were diagnosed on the basis of fasting values of plasma glucose. Of the patients without diabetes, 9% had IGT and 19% had IFG. Table 2 shows the results from the multivariable adjusted logistic regression of IFG, IGT, and type 2 diabetes on the continuous fat measures. The strongest associations were seen for fat% and WC, with all three binary outcomes having odds ratios ranging from 1.43 (1.25–1.81) for WC and type 2 diabetes to 1.64 (1.31–2.05) for fat% and IFG. While VAT was strongly associated with all glucose tolerance categories, the association with SAT was only modest and not statistically significant for IGT and diabetes. There was a trend toward a negative association between SAT/VAT ratio and all outcomes; however, this was not statistically significant. When the model for VAT was further adjusted for BMI or WC, the associations were attenuated; however, most of them remained significant, and the directions of the associations were unchanged. Adjustment of the model for SAT for WC or BMI revealed a negative association between SAT and all outcomes, although this was only statistically significant for the association between SAT and diabetes.
Table 2.
Associations of obesity measures with glucose intolerance
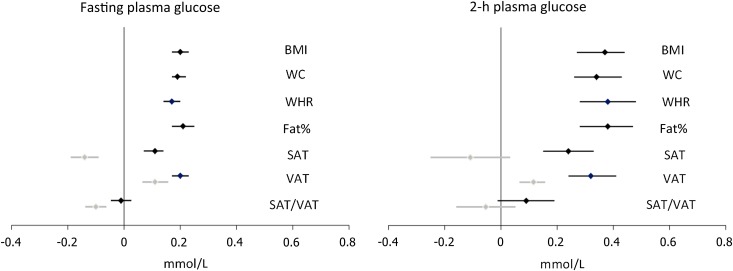
The multiple adjusted β-coefficients interpret the effect size of glucose homeostasis markers per 1 SD increase in obesity measures (Fig. 1). For fasting and 2-h plasma glucose, the magnitudes of the association were similar for BMI, WC, WHR, fat%, and VAT and stronger than for SAT (P < 0.001 for SAT vs. VAT comparison). No association was seen between SAT/VAT ratio and plasma glucose. After further adjustment for WC, VAT was still significantly positively associated with fasting glucose, whereas a negative association was observed for SAT and SAT/VAT ratio. The same trend, albeit not statistically significant, was seen for 2-h glucose.
Figure 1.
Linear associations between SDs of obesity measures and plasma glucose homeostasis markers adjusted for age, sex, alcohol intake, smoking status, and physical activity (black) and further adjusted for WC (gray).
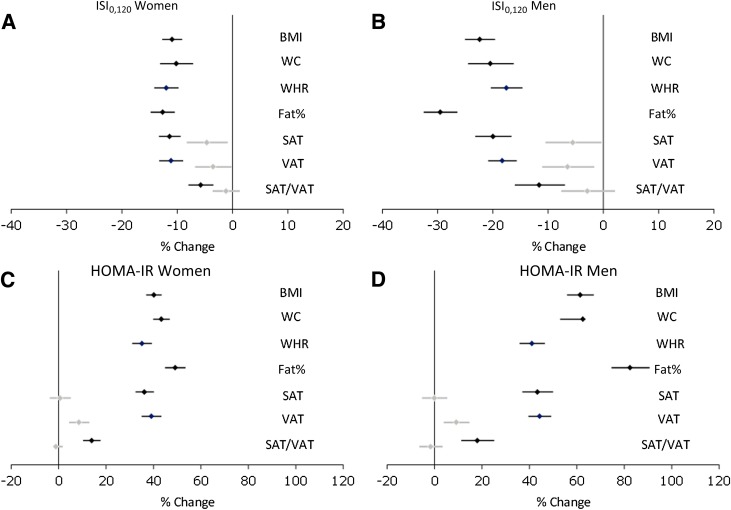
There was an interaction between sex and the association between all obesity measures and markers of insulin sensitivity, with stronger associations found among men (Fig. 2). Analyses were therefore performed separately for men and women. For markers of insulin sensitivity, 1 SD increase in BMI, WC, WHR, fat%, or VAT was roughly associated with a 30% increase in HOMA-IR and some 15% decrease in ISI0,120. Fat% was significantly stronger associated with HOMA-IR and the insulin sensitivity index than the other obesity measures, whereas the association between SAT/VAT ratio was less strong (P < 0.001 for SAT/VAT ratio vs. WC comparison). Models with VAT and WC demonstrated significant additional contribution of VAT for all variables.
Figure 2.
Linear associations between SDs of obesity measures and insulin resistance markers by sex adjusted for age, alcohol intake, smoking status, and physical activity (black) and further adjusted for WC (gray).
CONCLUSIONS
Summary
In this study, we examined the association between markers of fat distribution and overall obesity and glucose intolerance and glucose homeostasis markers. Our findings are threefold. First, fat% generally had the strongest association with all outcomes. Second, both SAT and VAT were significantly associated with glucose intolerance, fasting and 2-h plasma glucose, HOMA-IR, and ISI0,120. Third, VAT was more strongly associated with all outcomes than was SAT or the ratio between SAT and VAT. After further adjustment for BMI or WC, VAT was associated with glucose intolerance and insulin resistance, whereas there was a trend toward a negative or no association with SAT.
The mean SAT (3.5 and 1.8 cm among women and men, respectively) was higher than that of other populations measured by ultrasound (14,19), whereas VAT measures were similar to these populations. These findings suggest a large capacity among Inuit to store energy subcutaneously. Thus our study shows that the high WC in Inuit populations is to a large extent explained by a high amount of subcutaneously stored adipose tissue, and this may contribute to the apparent lower cardiometabolic risk for a given level of obesity compared with western populations. The VAT is similar to other populations, however, and mean levels of other obesity measurements are high and may explain why the absolute risk of type 2 diabetes is high among Greenland Inuit.
Comparison with other studies
Recent studies have focused on the magnitude of the association between directly measured VAT (by computed tomography [CT] imaging) and the risk of diabetes. The Framingham Heart Study examined cross-sectionally the association between abdominal fat distribution determined by CT and various metabolic risk factors (7,8). In a large community-based sample, both SAT and VAT were significantly associated with blood pressure, fasting plasma glucose, lipids, hypertension, impaired fasting glucose, type 2 diabetes, and the metabolic syndrome. For most risk factors, the association was stronger with VAT than with SAT. VAT but not SAT contributed significantly to risk factor variation after adjustment for BMI and WC. These results add weight to the hypothesized role of visceral fat as a pathogenic fat depot of particular importance.
The Insulin Resistance Atherosclerosis Study prospectively examined the association of VAT and SAT, as determined from CT imaging, and insulin sensitivity and β-cell dysfunction with incident diabetes in a Hispanic and African American population (20). The study showed that insulin resistance and β-cell dysfunction predicted diabetes independently of VAT and SAT. VAT and SAT were positively associated with incident diabetes, VAT more strongly so; however, the association was no longer significant when all four predictors were modeled together. This lack of an association of VAT with diabetes independent of insulin sensitivity and secretion may indicate that these mechanisms partially explain the link between VAT and diabetes. Similarly, other studies have demonstrated positive associations between VAT and glucose intolerance (21–24), but few have yielded significant associations for SAT. SAT has, however, been shown in some studies to be more strongly associated with insulin resistance than VAT (25). Two very small intervention studies have been conducted to examine the relation of SAT reduction with metabolic variables. In a small study of 15 women who underwent large-volume liposuction, improvements in cardiometabolic risk factors were not observed despite the loss of nearly 10 kg of subcutaneous fat (26). Another study of 30 obese women showed, 6 months after liposuction, lower insulin resistance; reduced concentrations of interleukin-6, interleukin-18, tumor necrosis factor-α, and C-reactive protein; and increased serum levels of adiponectin and HDL cholesterol (27). The small sample sizes, associated low power, and inclusion of morbidly obese study participants in these studies make it difficult, however, to rule out a beneficial effect.
Biological mechanisms
Beall and Goldstein (28) speculated that subcutaneous deposition of fat might be a useful genetic adaptation to a cold climate. A spherical body shape would reduce heat elimination, and when exposed to a cold environment, subcutaneous fat stores have the advantage that insulation does not fluctuate with nutritional status. The physiologists Rode and Shephard (29) have also suggested that long-term adaptation to the Arctic cold favors the deposition of subcutaneous fat, which stores quickly available fuel for heat production in response to cold-induced catecholamine stimulation. Although subcutaneous fat provides insulation, it is metabolically less active and has a limited blood supply and is thus relatively inflexible when responding to increases in heat flux generated by physical activity. In support of this view, the relationship of skin fold readings to body density among Inuit differed appreciably from that in a population of European ancestry (30).
Strengths and limitations
A major strength of this study is the large number of participants with fat distribution measured by imaging techniques. In epidemiology, WC or WHR ratio has thus far been used to quantify central obesity. Magnetic resonance imaging (MRI) and CT are the reference methods for estimating visceral and subcutaneous fat quantities and distribution; however, the use of these methods in an arctic setting as well as in large-scale studies is limited by their costs, accessibility, and, in the case of CT, constraints arising from radiation exposure. The ultrasound method used in the current study has been validated against MRI and CT as a way of estimating VAT and subcutaneous fat distributions in large-scale studies, where MRI and CT may not be feasible (13–15).
Unlike some studies of fat distribution and diabetes, participants with known diabetes were excluded from analyses that included plasma glucose and insulin concentrations because of the possibility that lifestyle changes or glucose-lowering agents might influence the concentration of glucose and insulin as a function of obesity. Although the population approach hindered the use of gold standard methods of glucose regulation (e.g., the use of a euglycemic clamp test to determine insulin sensitivity), we did obtain the most precise measures of glucose homeostasis achievable in an epidemiological setting.
Conclusion
Among Greenland Inuit, VAT is associated with glucose intolerance and insulin resistance independently of clinical anthropometric measurements, whereas there is a trend toward a negative or no association with SAT. High mean values of SAT in this population suggest that the high WC in Inuit populations is to a large extent explained by a high amount of subcutaneously stored adipose tissue, and this may contribute to a lower degree of insulin resistance for a given level of traditional adiposity measures such as WC.
Acknowledgments
The study was supported by Karen Elise Jensen’s Foundation, NunaFonden, Medical Research Council of Denmark, Medical Research Council of Greenland, and the Commission for Scientific Research in Greenland. Steno Diabetes Center receives part of its core funding from unrestricted grants from the Novo Foundation. None of the funding agencies had any role in study design or the collection or interpretation of data.
M.E.J. is employed by Steno Diabetes Center A/S, a research hospital working in the Danish National Health Service and owned by Novo Nordisk A/S. M.E.J. owns shares in Novo Nordisk A/S. Steno Diabetes Center receives part of its core funding from unrestricted grants from Novo Nordisk A/S. No other potential conflicts of interest relevant to this article were reported.
M.E.J. collected data and wrote the manuscript. K.B.J. and R.S. reviewed and edited the manuscript. P.B. collected data and reviewed and edited the manuscript. M.E.J., K.B.J., and P.B. contributed to the study design and planning. M.E.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Young TK, Bjerregaard P, Dewailly E, Risica PM, Jørgensen ME, Ebbesson SE. Prevalence of obesity and its metabolic correlates among the circumpolar Inuit in 3 countries. Am J Public Health 2007;97:691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jørgensen ME, Young TK. Cardiovascular diseases, diabetes and obesity. In Health Transitions in Arctic Populations. Young TK, Bjerregaard P, Eds. Toronto, University of Toronto Press, 2008, p. 291–307 [Google Scholar]
- 3.Bjerregaard P, Young T. The Circumpolar Inuit. Health of a Population in Transition. Copenhagen, Munksgaard, 1998 [Google Scholar]
- 4.Jørgensen ME, Bjerregaard P, Kjaergaard JJ, Borch-Johnsen K. High prevalence of markers of coronary heart disease among Greenland Inuit. Atherosclerosis 2008;196:772–778 [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen ME, Borch-Johnsen K, Witte DR, Bjerregaard P. Diabetes in Greenland and its relationship with urbanization. Diabet Med 2012;29:755–760 [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen ME, Glümer C, Bjerregaard P, Gyntelberg F, Jørgensen T, Borch-Johnsen K, Greenland Population Study Obesity and central fat pattern among Greenland Inuit and a general population of Denmark (Inter99): relationship to metabolic risk factors. Int J Obes Relat Metab Disord 2003;27:1507–1515 [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48 [DOI] [PubMed] [Google Scholar]
- 8.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays HE, Fox KM, Grandy S, SHIELD Study Group Anthropometric measurements and diabetes mellitus: clues to the “pathogenic” and “protective” potential of adipose tissue. Metab Syndr Relat Disord 2010;8:307–315 [DOI] [PubMed] [Google Scholar]
- 10.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci 2002;967:363–378 [DOI] [PubMed] [Google Scholar]
- 11.Bjerregaard P. Inuit health in transition—Greenland survey 2005-2010: population sample and survey methods [Internet], 2nd revised edition, 2011. Copenhagen, National Institute of Public Health. Available from http://si-folkesundhed.dk/upload/inuit_health_in_transition_greenland_methods_5_2nd_revision.pdf Accessed 28 November 2012
- 12.IPAQ core group. IPAQ: International Physical Activity Questionnaire [Internet], 2013. Available from https://sites.google.com/site/theipaq/ Accessed 28 November 2012
- 13.Stolk RP, Wink O, Zelissen PM, Meijer R, van Gils AP, Grobbee DE. Validity and reproducibility of ultrasonography for the measurement of intra-abdominal adipose tissue. Int J Obes Relat Metab Disord 2001;25:1346–1351 [DOI] [PubMed] [Google Scholar]
- 14.De Lucia Rolfe E, Sleigh A, Finucane FM, et al. Ultrasound measurements of visceral and subcutaneous abdominal thickness to predict abdominal adiposity among older men and women. Obesity (Silver Spring) 2010;18:625–631 [DOI] [PubMed] [Google Scholar]
- 15.De Lucia Rolfe E, Norris SA, Sleigh A, et al. Validation of ultrasound estimates of visceral fat in black South African adolescents. Obesity (Silver Spring) 2011;19:1892–1897 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation, Part 1: Diagnosis and Classification of Diabetes Mellitus Geneva, World Health Org., 1999 (WHO/NCD/NCS/99.2)
- 17.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 18.Gutt M, Davis CL, Spitzer SB, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract 2000;47:177–184 [DOI] [PubMed] [Google Scholar]
- 19.Christensen DL, Eis J, Hansen AW, et al. Obesity and regional fat distribution in Kenyan populations: impact of ethnicity and urbanization. Ann Hum Biol 2008;35:232–249 [DOI] [PubMed] [Google Scholar]
- 20.Hanley AJ, Wagenknecht LE, Norris JM, et al. Insulin resistance, beta cell dysfunction and visceral adiposity as predictors of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Family study. Diabetologia 2009;52:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–379 [DOI] [PubMed] [Google Scholar]
- 22.Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR, Health, Aging, and Body Composition (ABC) Study Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care 2004;27:1375–1380 [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care 2003;26:650–655 [DOI] [PubMed] [Google Scholar]
- 24.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000;23:465–471 [DOI] [PubMed] [Google Scholar]
- 25.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab 2004;89:4206–4210 [DOI] [PubMed] [Google Scholar]
- 26.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350:2549–2557 [DOI] [PubMed] [Google Scholar]
- 27.Giugliano G, Nicoletti G, Grella E, et al. Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women. Br J Plast Surg 2004;57:190–194 [DOI] [PubMed] [Google Scholar]
- 28.Beall CM, Goldstein MC. High prevalence of excess fat and central fat patterning among Mongolian pastoral nomads. Am J Hum Biol 1992;4:747–756 [DOI] [PubMed] [Google Scholar]
- 29.Rode A, Shephard RJ. Prediction of body fat content in an Inuit community. Am J Hum Biol 1994;6:249–254 [DOI] [PubMed] [Google Scholar]
- 30.Rode A, Shephard RJ. Modernization of lifestyle, body fat content and body fat distribution: a comparison of Igloolik Inuit and Volochanka nGanasan. Int J Obes Relat Metab Disord 1995;19:709–716 [PubMed] [Google Scholar]