Abstract
OBJECTIVE
To identify if there is a dose-dependent risk of diabetes complications in patients treated with corticosteroids who have both diabetes and chronic obstructive pulmonary disorder (COPD).
RESEARCH DESIGN AND METHODS
A retrospective study of administrative claims data from the Australian Government Department of Veterans’ Affairs, from 1 July 2001 to 30 June 2008, of diabetes patients newly initiated on metformin or sulfonylurea. COPD was identified by dispensings of tiotropium or ipratropium in the 6 months preceding study entry. Total corticosteroid use (inhaled and systemic) in the 12 months after study entry was determined. The outcome was time to hospitalization for a diabetes-related complication. Competing risks and Cox proportional hazard regression analyses were conducted with adjustment for a number of covariates.
RESULTS
A total of 18,226 subjects with diabetes were identified, of which 5.9% had COPD. Of those with COPD, 67.2% were dispensed corticosteroids in the 12 months from study entry. Stratification by dose of corticosteroids demonstrated a 94% increased likelihood of hospitalization for a diabetes complication for those who received a total defined daily dose (DDD) of corticosteroids ≥0.83/day (subhazard ratio 1.94 [95% CI 1.14–3.28], P = 0.014), by comparison with those who did not receive a corticosteroid. Lower doses of corticosteroid (<0.83 DDD/day) were not associated with an increased risk of diabetes-related hospitalization.
CONCLUSIONS
In patients with diabetes and COPD, an increased risk of diabetes-related hospitalizations was only evident with use of high doses of corticosteroids. This highlights the need for constant revision of corticosteroid dose in those with diabetes and COPD, to ensure that the minimally effective dose is used, together with review of appropriate response to therapy.
The presence of comorbidity in older people with diabetes is common, at least 50% will have three or more comorbid chronic conditions (1,2). Comorbidity adds considerable complexity to therapeutic management (2) and is a major determinant of functional capabilities and health outcomes (3,4). Chronic obstructive pulmonary disorder (COPD) is a common comorbidity; ∼10% of patients with diabetes will have COPD (1,2). COPD is a progressive, largely nonreversible pulmonary disease that is a major cause of morbidity and mortality worldwide (5). COPD is associated with increased complexity of care in those with diabetes, potentially the result of a reduced ability to perform self-management activities necessary for optimal diabetes control and the associated therapeutic challenges (6).
Current guideline treatment recommendations for COPD include the use of inhaled corticosteroids to reduce exacerbations and improve health status in patients with moderate to severe COPD who have frequent exacerbations (7,8). Oral corticosteroids are also recommended for the short-term treatment of exacerbations to improve lung function and hypoxemia and to reduce recovery time, but are not recommended chronically due to their unfavorable risk/benefit profile (7,8). Corticosteroids are not recommended in patients with diabetes (9) due to a dose-dependent increase in blood glucose levels with corticosteroid use and increased risk of diabetes progression (10,11). There is little evidence-based information to facilitate the treatment of patients with both diabetes and COPD, where optimal treatment of COPD with corticosteroids may result in a potentially increased risk of diabetes complications and poorer health outcomes. This study aimed to examine the dose-dependent risk of diabetes-related complications associated with corticosteroid use in older patients with diabetes and COPD.
RESEARCH DESIGN AND METHODS
Data source and study design
This study was approved by the University of South Australia and the Australian Government Department of Veterans’ Affairs (DVA) Human Research Ethics Committees. A retrospective cohort study was undertaken from 1 July 2001 to 30 June 2008 using data from the DVA health administrative claims database. The DVA database contains details of all prescription medicines and medical and allied health services and hospitalizations subsidized by DVA for a treatment population of 290,000 veterans, war widows, and widowers. Over 70% of the population are 70 years of age or older; 54% are male and 9.8% live in residential aged care, and they are dispensed an average of 11 unique medicines annually (12). Within the dataset, medicines are coded according to the World Health Organization (WHO) anatomical and therapeutic chemical (ATC) classification (13) and the Schedule of Pharmaceutical Benefits item codes (14). Hospitalizations are coded according to the WHO Australian modification of the ICD-10 (ICD-10-AM) classification (15).
Cohort selection
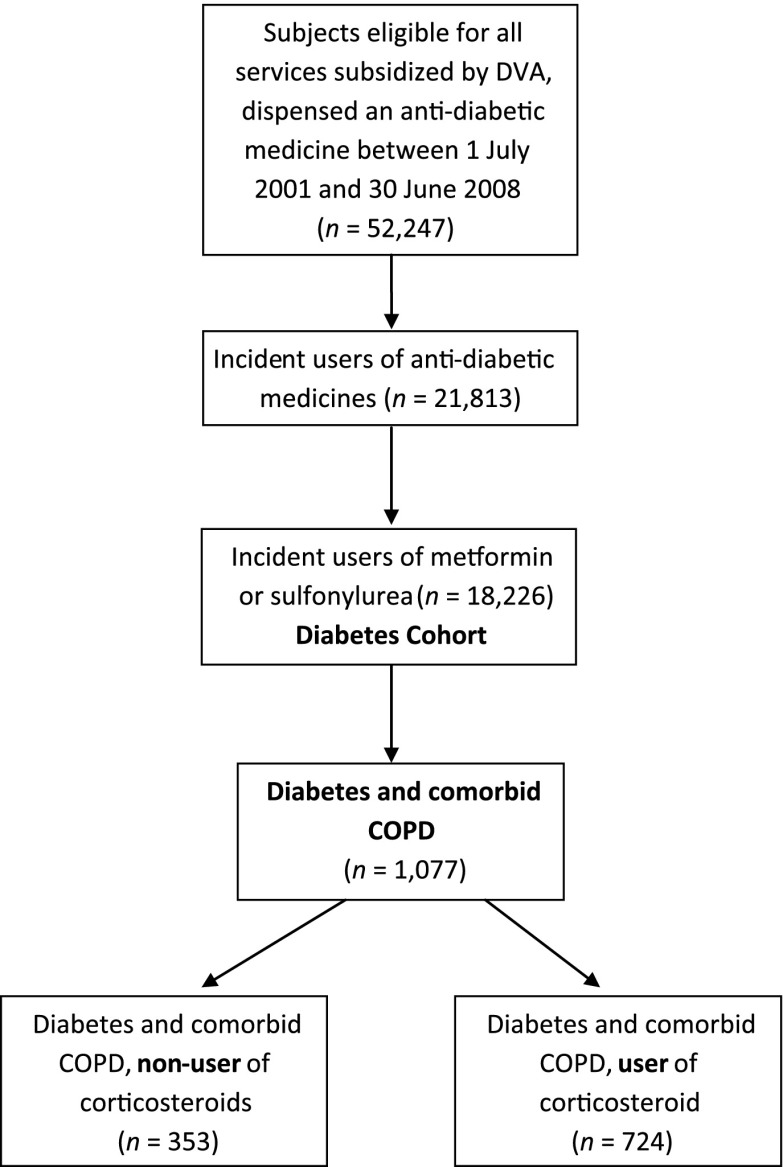
Subjects were included if they were eligible for all health services subsidized by DVA in the 12 months prior to the date of their first (index) dispensing of an oral antidiabetic medicine. The study included new users of either metformin or sulfonylurea medicines, defined by those who did not have a dispensing of these medicines in the 12 months prior. Users of other classes of diabetes medicines (insulin, other oral medications, and combination of metformin) were excluded, as prescription of these medicines at the initiation of diabetes treatment is not recommended as first-line pharmacotherapy and may relate to unusual clinical circumstances (16). Newly initiated medicine users were selected for study as these patients are less likely to have existing complications than those who are on second- and third-line agents (insulin or other oral medications) (16). Comorbid COPD was identified from medicine use, defined as two dispensings of tiotropium or ipratropium (ATC codes R03BB04 and R03BB01, respectively) in the 6 months preceding the first dispensing of the oral antidiabetic medicine. Figure 1 shows the flowchart of the study cohort selection criteria.
Figure 1.
Study flowchart of diabetes and COPD cohort selection.
Calculation of corticosteroid dose
The total cumulative corticosteroid dose (both inhaled and systemic [oral], ATC codes R01AD-R03BA and H02AB01-AB10, respectively) was calculated in the 12 months from study entry and converted to a defined daily dose (DDD) per day (17). In order to account for the systemic bioavailability of inhaled corticosteroids by comparison with that of oral corticosteroids, the WHO-defined dose of inhaled corticosteroids was reduced by a factor of 10 (18). Users of corticosteroids were stratified into three groups: >0 to <0.25, ≥0.25 to <0.83, and ≥0.83 DDD/day.
Outcome definition
The study end point was time until first hospitalization for a diabetes-related complication, defined by the ICD-10-AM codes E11 and E13–E14 (15). These include diabetic hyperosmolarity with or without coma, diabetic ketoacidosis with or without coma, diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, diabetic angiopathy, and diabetic arthropathy. Follow-up commenced 12 months from index dispensing of antidiabetes medicine. Participants were censored at death or study end.
Statistical analyses
We used an intention-to-treat approach to compare risk of diabetes complications between users and nonusers of corticosteroids in our diabetes and COPD cohort. These analyses assessed the effect of treatment in the first 12 months from incident antidiabetes medicine use. Characteristics of nonusers and users of corticosteroids were compared using a Mann-Whitney U test for continuous variables and χ2 test for categorical variables. As death is a competing risk event in this elderly study population that may preclude the onset of the outcome of interest (time to hospitalization for a diabetes-related complication), we calculated the cumulative incidence of outcome of interest in the presence of competing risk events. Competing risk regression analyses were conducted using the Fine and Gray approach, which extends the Cox model to competing risks data by considering the subdistribution hazard (19). The strength of the association between each variable and the outcome was assessed using the subhazard ratio (SHR), which is the ratio of hazards associated with the cumulative incidence function under different values of the covariates. Covariates included age, sex, socioeconomic status (20), residential status (community dwelling or aged-care resident), number of comorbid conditions (identified using the pharmaceutical-based comorbidity index, Rx-Risk-V [21], excluding those conditions used to identify patients), number of prescribers, number of unique medicines (excluding those medicines used to identify patients), number of visits to an endocrinologist, number of visits to a pulmonary specialist, number of hospitalizations for conditions other than diabetes, exposure to corticosteroids in the 12 months prior to study entry, and glycated hemoglobin (HbA1c) testing in the 12 months prior to study entry. All covariates were time varying, adjusted at study entry and then annually, apart from age, socioeconomic status, prior corticosteroid exposure, and HbA1c testing, which were adjusted at baseline only. The subproportional hazards assumption was confirmed by examining log[−log(survival)] curves, and no violation was detected. We also examined cause-specific Cox proportional hazard models, with death censored and adjusting for the same covariates as included in the competing risks regression analyses, to provide a more complete understanding of the association between corticosteroid use and time until diabetes complication (22). Statistical analyses were performed using SAS 9.2 and Stata 11.0.
RESULTS
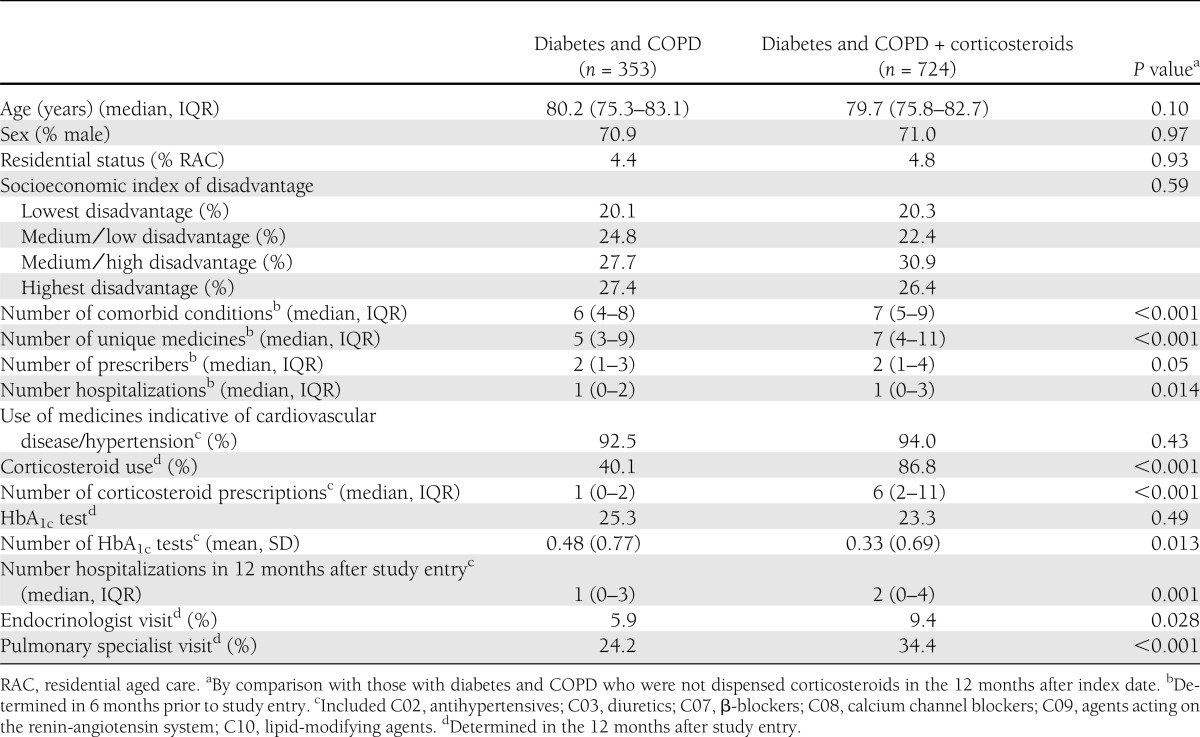
A total of 1,077 (5.9%) of the 18,226 patients with diabetes had comorbid COPD. In the 12 months after study entry, corticosteroids were used by 724 (67.2%) of those with diabetes and COPD. For those who received a corticosteroid, the median corticosteroid dose over the 12-month period was 0.34 DDD/day (IQR 0.11–0.83). The characteristics of the study cohort at study entry are described in Table 1. Those who were users of corticosteroids had a statistically significant greater number of comorbid conditions and hospitalizations and were more likely to visit an endocrinologist or pulmonary specialist.
Table 1.
Demographic and clinical characteristics of study cohort (n = 1,077)
After 1 year, there was little difference between the proportion of those who had a hospitalization for a diabetes complication between nonusers (6.3% [95% CI 4.01–9.63]) and users (7.1% [5.29–9.16]) of corticosteroids (P = 0.37; data not shown). After 5 years of follow-up, 19.8% (18.72–23.45) of corticosteroid users had a diabetes-related hospitalization, by comparison with 16.2% (16.91–22.84) for nonusers of corticosteroids (P = 0.18).
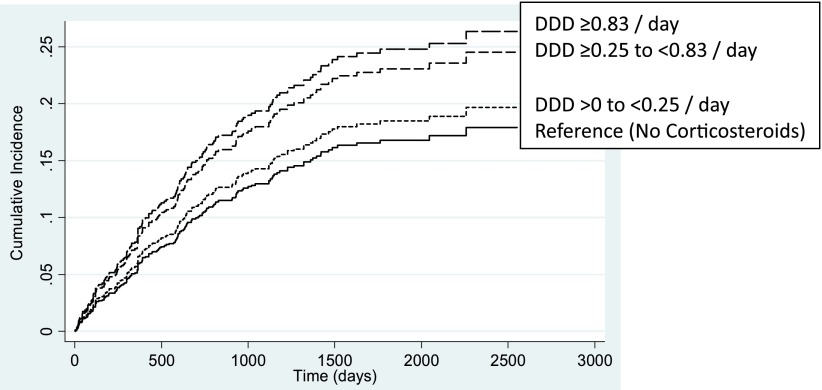
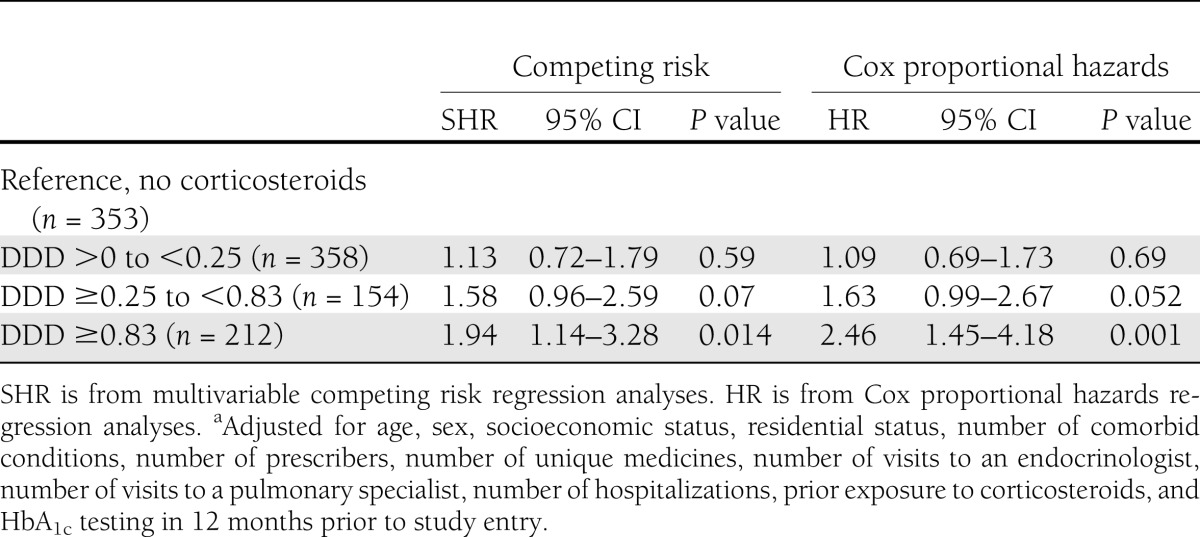
Shown in Fig. 2 is the dose-dependent analysis of corticosteroid use and time to hospitalization for a diabetes complication. Stratification of corticosteroid dose demonstrated that a total dose of ≥0.83 DDD/day, over the 12 months from study entry, resulted in a 94% increased risk of diabetes complication (SHR 1.94 [95% CI 1.14–3.28], P = 0.014) by comparison with those who did not receive a corticosteroid (Table 2). Despite observing a dose-dependent increase in risk, this was not statistically significant for the lower two corticosteroid dose treatment groups (Table 2). Hazard ratios (HRs) for both the lower two doses of corticosteroids using competing risk or Cox proportional hazards regression analyses were similar. The HR for the higher-dose grouping of corticosteroid ≥0.83 using Cox proportional hazards regression analysis demonstrated an increased risk (HR 2.46 [95% CI 1.45–4.18], P = 0.001) by comparison with competing risk (SHR 1.94 [1.14–3.28], P = 0.014), highlighting the increasing influence of the competing risk of death, for this dose.
Figure 2.
Cumulative incidence of time to hospitalization for a diabetes complication stratified by total cumulative dose (DDD) of corticosteroids in the 12 months from study entry.
Table 2.
Competing risk and Cox proportional hazards regression analyses for time to hospitalization for diabetes complication, stratified by total dose (DDD) of corticosteroid/day in the 12 months from study entrya
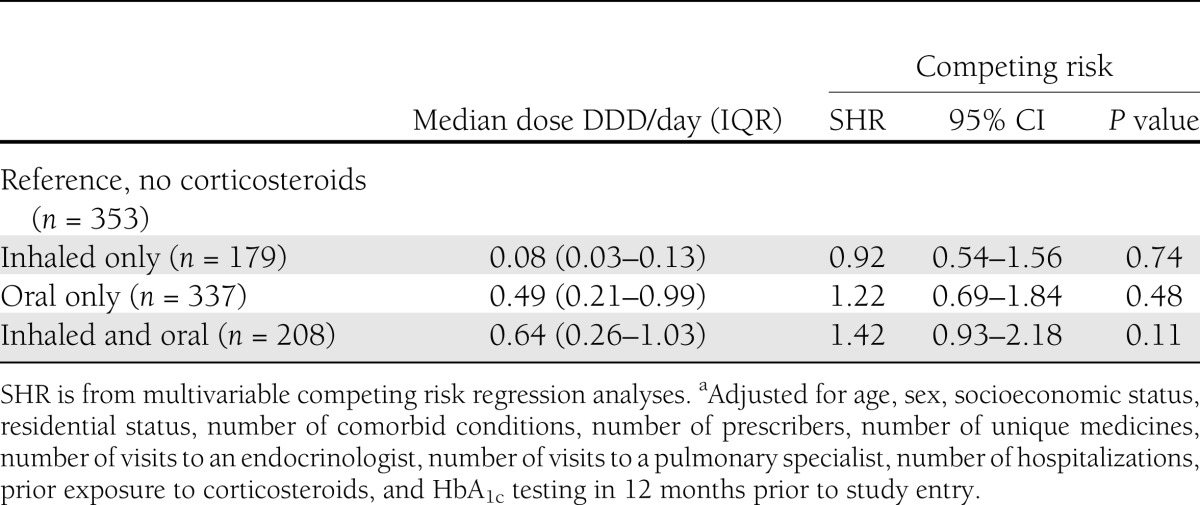
We examined the risk of diabetes hospitalizations in those who received inhaled only, oral only, and both. Inhaled corticosteroid only was received by 24.8%, with a median total dose of 0.08 DDD/day (IQR 0.03–0.13); 46.5% received an oral corticosteroid only, with a median total dose of 0.49 DDD /day (IQR 0.21–0.99); and 28.7% received both an inhaled and oral corticosteroid with a median total dose of 0.64 DDD/day (IQR 0.07–3.04) (Table 3). Competing risk analysis stratified by corticosteroid use (inhaled, oral, and both) showed no significant association between corticosteroid use and risk of a diabetes-related hospitalization (Table 3).
Table 3.
Competing risk regression analyses for time to hospitalization for diabetes complication, stratified by inhaled corticosteroids, oral corticosteroids, or botha
CONCLUSIONS
In this large population-based study of older people with COPD and diabetes, a dose of corticosteroids of ≥0.83 DDD/day in the 12 months after starting diabetes medication was associated with a 94% increased risk of having a hospitalization for a diabetes complication. This increased risk was not evident for doses <0.83 DDD/day. To our knowledge, this is the first study to have examined the effect of corticosteroid dose on longer-term diabetes complications for patients with diabetes and comorbid COPD. Older patients and those with a comorbidity are generally excluded from randomized clinical trials used to generate the evidence for clinical guidelines, decreasing the applicability of trial results to real-world populations (23). The results of this study suggest that, when they are necessary to use, corticosteroids should be used in their minimally effective dose for older patients with diabetes and COPD.
The use of inhaled corticosteroids for patients with COPD has been the subject of much debate (5). Inhaled corticosteroids have been shown to reduce the frequency of exacerbations and decline in quality of life in people with COPD but have no effect on the rate of decline in lung function or mortality (5). Although they are recommended for use in those with moderate to severe COPD, inhaled corticosteroids are used widely, with reports of use in >70% of patients with COPD (24).
Whereas systemic corticosteroids are known to increase diabetes risk (25), the effects of inhaled corticosteroids are less well known. In a recent large population–based study of 388,584 patients with respiratory disease, treatment with inhaled corticosteroids was associated with a 34% (RR 1.34 [95% CI 1.29–1.39]) increased risk of diabetes onset (defined by commencement of an oral hypoglycemic agent), with the highest dose associated with the greatest risk (11). Furthermore, in a subset of patients with respiratory disease and newly diagnosed diabetes treated with oral hypoglycemic agents, inhaled corticosteroid use was associated with a 34% (RR 1.34 [1.17–1.53]) increased likelihood of progression to insulin use, with higher doses associated with a 54% increased risk (11). This demonstrates the short-term systemic effects of inhaled corticosteroid use, resulting in hyperglycemia and the subsequent need for the use of oral antidiabetic agents or intensification of treatment. We stratified our analyses by type of corticosteroid dispensed (inhaled, oral, or those who received both) and found no significant association with risk of diabetes-related hospitalization. However, the median doses of each of these groups were lower than ≥0.83 DDD/day, consistent with our results, where an association of increased risk of diabetes-related hospitalization was only observed at these higher doses.
Previous studies have shown that corticosteroids are associated with insulin resistance, hyperglycemia, and loss of diabetic control in a dose-dependent manner in patients with diabetes (10,25). Corticosteroids have been shown to inhibit a number of steps in the insulin signaling network, leading to insulin resistance. These include increased proteolysis, lipolysis, and free fatty acid production, which can contribute to insulin resistance. Corticosteroids can also directly increase hepatic gluconeogenesis, leading to hyperglycemia (26). The results of the current study provide an insight into potential safety concerns of corticosteroid use in a real-world setting for the treatment of COPD in patients with comorbid diabetes, where both oral and systemic corticosteroids are used. This highlights the potential for longer-term adverse effects of higher doses of both oral and systemic corticosteroids on diabetes complications in older patients with diabetes and COPD.
Diabetes and COPD are common comorbid disorders, and both the prevalence and incidence of type 2 diabetes are higher in patients with COPD, by comparison with those without COPD (27,28). The presence of comorbidity adds considerable complexity to the therapeutic management, with >60% of those with diabetes having a comorbid condition that may cause a treatment conflict, affecting both the benefits and risks associated with treatment choices (2). The presence of both diabetes and COPD has been reported to be associated with greater care complexity (6). Greater numbers of comorbid conditions or the presence of comorbid cardiovascular disease, hypertension, anxiety, or depression are associated with increased respiratory impairment and risk of hospitalization and mortality (5,28). Our results highlight the importance of the need for shared decision making in this population, where treatment prioritization of the more symptomatic conditions, such as COPD, needs to be balanced with both the short-term and long-term harms associated with use of corticosteroids for patients with comorbid diabetes. Although we have shown that longer-term adverse effects on diabetes complications were only evident at higher doses of corticosteroids, use of corticosteroids (both inhaled and oral) in this patient population should be associated with close monitoring of blood glucose levels and a review of efficacy within 4–8 weeks of commencing inhaled therapy. Regular use of high-dose corticosteroids should be avoided where possible (≥0.83 DDD/day) for those individuals with comorbid diabetes.
Our study has a number of limitations. The identification of patients with COPD in our study was based on dispensings of either- the short or long-acting anticholinergic agent ipratropium or tiotropium, respectively. We have not included patients who are only taking β-2 agonists as these drugs may also be used for asthma or in those with mild or newly diagnosed COPD, and as such, we may have excluded patients with less severe COPD in our study. A recent Australian study of patients with COPD showed that the majority (89%) used tiotropium or ipratropium as their regular COPD therapy (29). The use of an intention-to-treat analysis may have resulted in misclassification of corticosteroid exposure during follow-up; however, the median dose of corticosteroids within the second year after study entry was 0.27 DDD/day (IQR 0.09–0.66), similar to that of the first 12 months, suggesting that this was minimized.
Although we included a number of covariates in our analyses to adjust for several potential confounders, residual confounding from unmeasured covariates may still exist. The issue of confounding by indication also deserves consideration; those with more severe COPD are more likely to receive a corticosteroid, which may be associated with the outcome of a diabetes-related hospitalization. Despite a lack of evidence to support that severity of COPD is on the causal pathway to an increased likelihood of a diabetes-related hospitalization, there is, however, the potential that a nonbiological causal bias exists; that is, competing priorities, whereby those who have more severe COPD may have less capacity to focus on management of their comorbid diabetes and therefore have poorer diabetic control. A number of covariates were included in our analyses as a proxy for COPD disease severity, including number of unique medicines (which included β-agonists and antibiotics), number of visits to a pulmonary specialist, and number of hospitalizations (including those that are COPD related). We also adjusted for potential differences in diabetes severity at study entry due to prior corticosteroid exposure in our analyses.
Our study end point mainly included microvascular diabetes complications and did not include macro/cardiovascular complications, as almost all of the study population (>92%) had pre-existing cardiovascular comorbidity at study entry. The results of the current study are likely to be applicable to the older Australian population; age-specific comparisons of DVA gold card holders with no service-related disability with the wider Australian population have shown similar rates of general practitioner visits, use of prescriptions, and hospitalizations (30).
In conclusion, use of a higher dose of corticosteroids is associated with an increased risk of diabetes complications in diabetes patients with comorbid COPD. Use of corticosteroids in patients with diabetes and COPD should be accompanied by regular review to ensure that minimally effective doses are used, efficacy of treatment, and close monitoring of blood glucose control. Treatment with higher doses of corticosteroids in patients with diabetes and COPD should be limited to optimize the balance between risk of benefits and longer-term adverse effects.
Acknowledgments
This study was funded by a National Health and Medical Research Council/Australian Research Council Ageing Well Ageing Productively Program grant (APP ID 401832).
No potential conflicts of interest relevant to this article were reported.
G.E.C. contributed to the study design and analysis plan, researched data, and wrote, reviewed, and edited the manuscript. A.K.P. contributed to the study design and analysis plan, performed data and statistical analysis, and reviewed and edited the manuscript. A.I.V. contributed to the study design and analysis plan and reviewed and edited the manuscript. A.L.G. contributed to the study design and reviewed and edited the manuscript. E.E.R. contributed to the study design and analysis plan, researched data, and reviewed and edited the manuscript. G.E.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the Australian Government DVA for providing the data used in this study.
References
- 1.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med 2007;22:1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caughey GE, Roughead EE, Vitry AI, McDermott RA, Shakib S, Gilbert AL. Comorbidity in the elderly with diabetes: identification of areas of potential treatment conflicts. Diabetes Res Clin Pract 2010;87:385–393 [DOI] [PubMed] [Google Scholar]
- 3.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006;29:725–731 [DOI] [PubMed] [Google Scholar]
- 4.Fortin M, Soubhi H, Hudon C, Bayliss EA, van den Akker M. Multimorbidity’s many challenges. BMJ 2007;334:1016–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabe KF, Wedzicha JA. Controversies in treatment of chronic obstructive pulmonary disease. Lancet 2011;378:1038–1047 [DOI] [PubMed] [Google Scholar]
- 6.Grant RW, Wexler DJ, Ashburner JM, Hong CS, Atlas SJ. Characteristics of “complex” patients with type 2 diabetes mellitus according to their primary care physicians. Arch Intern Med 2012;172:821–823 [DOI] [PubMed] [Google Scholar]
- 7.McKenzie DK, Abramson M, Crockett AJ, et al.; on behalf of The Australian Lung Foundation. The COPD-X Plan: Australian and New Zealand Guidelines for the Management of Chronic Obstructive Pulmonary Disease v2.30 Brisbane, Australian Lung Foundation, 2011
- 8.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 [DOI] [PubMed] [Google Scholar]
- 9.The NHMRC and the Diabetes Australia Guideline Development Consortium. National evidence based guidelines for the management of type 2 diabetes mellitus [Internet]. Available from http://www.nhmrc.gov.au/publications/synopses/di7todi13syn.htm Accessed 12 December 2011
- 10.Slatore CG, Bryson CL, Au DH. The association of inhaled corticosteroid use with serum glucose concentration in a large cohort. Am J Med 2009;122:472–478 [DOI] [PubMed] [Google Scholar]
- 11.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010;123:1001–1006 [DOI] [PubMed]
- 12.Australian Government Department of Veterans' Affairs. Treatment population statistics, quarterly report June 2010 [Internet]. Available from http://www.dva.gov.au/aboutDVA/Statistics/Documents/TpopJun10.pdf Accessed June 6th 2011
- 13.World Health Organization Collaborating Centre for Drug Statistics Methodology. Anatomical therapeutic chemical code classification index with defined daily doses [Internet]. Available from http://www.Whocc.No/atcddd Accessed 25 June 2010
- 14.Australian Government Department of Health and Ageing. Schedule of pharmaceutical benefits. PBS for health professionals. 2010. Available from http://www.pbs.gov.au/html/healthpro/home Accessed 1 July 2011
- 15.World Health Organization International Classification of Diseases. International statistical classification of diseases and related health problems 10th revision. Geneva, World Health Org., 2007
- 16.Diabetes Australia, Royal Australian College for General Practitioners. Diabetes Management in General Practice. Guidelines for Type 2 Diabetes 16th ed. Canberra, Diabetes Australia, 2010 [Google Scholar]
- 17.World Health Organization Collaborating Centre for Drug Statistics Methodology. Defined daily dose: definition and general considerations [Internet]. Available from http://www.Whocc.No/ddd/definition_and_general_considera/ Accessed 25 June 2011
- 18.Mash BRJ, Bheekie A, Jones PW. Inhaled vs oral steroids for adults with chronic asthma. Cochrane Database Syst Rev 2001;(1):CD002160. [DOI] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risks in survival analysis. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 20.Australian Bureau of Statistics (ABS) Information Paper: Census of Population and Housing - Socioeconomic Indexes for Areas, Australia, 2001. Canberra, Australia, ABS, 2003 [Google Scholar]
- 21.Vitry AI, Wong SA, Roughead EE, Ramsay E, Barratt J. Validity of medication-based co-morbidity indices in the Australian elderly population. Aust N Z J Public Health 2009;33:126–130 [DOI] [PubMed] [Google Scholar]
- 22.Szychowski JM, Roth DL, Clay OJ, Mittelman MS. Patient death as a censoring event or competing risk event in models of nursing home placement. Stat Med 2010;29:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005;294:716–724 [DOI] [PubMed] [Google Scholar]
- 24.Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J 2009;34:13–16 [DOI] [PubMed] [Google Scholar]
- 25.Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med 2002;17:717–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferris HA, Kahn CR. New mechanisms of glucocorticoid-induced insulin resistance: make no bones about it. J Clin Invest 2012;122:3854–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 2004;27:2478–2484 [DOI] [PubMed] [Google Scholar]
- 28.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008;32:962–969 [DOI] [PubMed] [Google Scholar]
- 29.Ta M, George J. Management of chronic obstructive pulmonary disease in Australia after the publication of national guidelines. Intern Med J 2011;41:263–270 [DOI] [PubMed] [Google Scholar]
- 30.Australian Institute of Health and Welfare (AIHW). Health Care Usage and Costs. A Comparison of Veterans and War Widows and Widowers with the Rest of the Community Canberra, AIHW, 2002 (Cat. no. Phe42)