Abstract
OBJECTIVE
Fetuin-A levels are associated with higher risk of type 2 diabetes, but it is unknown if the association is causal. We investigated common (>5%) genetic variants in the fetuin-A gene (AHSG) fetuin-A levels, fasting glucose, and risk of type 2 diabetes.
RESEARCH DESIGN AND METHODS
Genetic variation, fetuin-A levels, and fasting glucose were assessed in 2,893 Caucasian and 542 African American community-living individuals 65 years of age or older in 1992–1993.
RESULTS
Common AHSG variants (rs4917 and rs2248690) were strongly associated with fetuin-A concentrations (P < 0.0001). In analyses of 259 incident cases of type 2 diabetes, the single nucleotide polymorphisms (SNPs) were not associated with diabetes risk during follow-up and similar null associations were observed when 579 prevalent cases were included. As expected, higher fetuin-A levels were associated with higher fasting glucose concentrations (1.9 mg/dL [95% CI, 1.2–2.7] higher per SD in Caucasians), but Mendelian randomization analyses using both SNPs as unbiased proxies for measured fetuin-A did not support an association between genetically predicted fetuin-A levels and fasting glucose (−0.3 mg/dL [95% CI, −1.9 to 1.3] lower per SD in Caucasians). The difference between the associations of fasting glucose with actual and genetically predicted fetuin-A level was statistically significant (P = 0.001). Results among the smaller sample of African Americans trended in similar directions but were statistically insignificant.
CONCLUSIONS
Common variants in the AHSG gene are strongly associated with plasma fetuin-A concentrations, but not with risk of type 2 diabetes or glucose concentrations, raising the possibility that the association between fetuin-A and type 2 diabetes may not be causal.
Fetuin-A (also known as α-Heremans-Schmid glycoprotein) is secreted from the liver and reversibly binds the insulin receptor tyrosine kinase in peripheral tissues, thereby inhibiting the insulin-induced intracellular signal cascade and producing peripheral insulin resistance (1–3). Elevated fetuin-A levels have been associated with the risk of incident type 2 diabetes in several human populations (4,5). Recently, we also observed an association between plasma fetuin-A concentrations and risk of type 2 diabetes in a large sample of community-living older adults who participated in the Cardiovascular Health Study (CHS) and were followed for a median of 11 years (6).
Thus far, little is known about the potential causal relation between fetuin-A and type 2 diabetes. In the absence of experimental evidence that selective lowering of fetuin-A improves insulin sensitivity and reduces risk of type 2 diabetes, investigations that use genetic variants with strong influence on circulating fetuin-A levels as unconfounded proxies (i.e., Mendelian randomization) may shed light on the potential causal role of fetuin-A in type 2 diabetes.
The locus (3q27) where the gene encoding fetuin-A (AHSG) is situated has been identified in human linkage studies of phenotypes such as the metabolic syndrome and type 2 diabetes (7,8). These findings were followed by direct sequencing of gene-encoding regions and the identification of nine highly correlated single nucleotide polymorphisms (SNPs) with a minor allele frequency (MAF) >5% (9). Case-control studies have suggested that a few of these SNPs (rs1071592, rs4917/rs4918, and rs2248690) are associated with type 2 diabetes and insulin resistance (9,10), but none of these studies had plasma measurements of fetuin-A available to evaluate the potential causal nature of the direct association between fetuin-A and type 2 diabetes. In general, carriers of the minor alleles of promoter variant rs2248690 (T; frequency in Caucasians, ∼26%) and the tightly linked nonsynonymous amino acid substitution variants rs4917 (T; frequency in Caucasians, ∼33%) and rs4918 (G; frequency in Caucasians, ∼34%), have lower levels of circulating fetuin-A compared with carriers of the normal wild-type alleles (11–14).
By incorporating information for polymorphisms at the AHSG locus with our previous analysis of plasma fetuin-A in relation to risk of incident type 2 diabetes in the CHS (6), we aimed to address this potential causative association.
RESEARCH DESIGN AND METHODS
Study population
The CHS is a community-based study of older adults designed to evaluate risk factors for development and progression of cardiovascular disease. The study design and protocols have been described previously (15,16). In brief, eligibility required 65 years of age or older, expectation to remain in the area for 3 years after recruitment, no active cancer treatment, and the ability to provide consent. Between 1989 and 1990, 5,201 participants were recruited from 4 communities using Medicare eligibility lists in each area (Sacramento, California; Forsyth County, North Carolina; Washington County, Maryland; and Allegheny County, Pennsylvania). An additional 687 African American participants were recruited in 1992–1993. We considered the 1992–1993 visit as the baseline study visit for this analysis. Among 5,265 individuals who participated at this visit, we excluded individuals who had not given consent for genetic analyses, those whose samples failed genotypic quality control (n = 1,026), and those with insufficient blood specimen for fetuin-A measurement (n = 390). For analyses of fasting glucose, we additionally excluded those with missing glucose levels at baseline and participants who reported using insulin or oral hypoglycemic agents. The sample size for these analyses was 3,438 (2,893 Caucasians and 542 African Americans). In prospective analysis of incident type 2 diabetes, we excluded participants with prevalent or missing information for type 2 diabetes at baseline (n = 236) and those with no follow-up for incident type 2 diabetes (n = 109), resulting in a study sample of 3,093 participants (2,627 Caucasians and 466 African Americans).
The study was approved by the Investigational Review Boards of the four clinical sites and the Data Coordinating Center at the University of Washington.
Measurements
Fetuin-A.
Plasma was collected at the 1992–1993 study visit after participants had fasted overnight and was stored at −70°C until 2010, when it was thawed and measured for fetuin-A using an ELISA kit (Epitope Diagnostics, San Diego, CA). The assay uses a two-site “sandwich” technique with polyclonal antibodies that bind different epitopes of human fetuin-A. Plasma samples were measured twice in each participant, and results were averaged. We observed coefficients of variation between 3.3 and 9.1%.
Genotypes.
CHS is part of the National Heart, Lung, and Blood Institute–funded Candidate Gene Association Resource (CARe) study (17). DNA was collected at baseline, and genotyping was performed using a gene-centric 50K SNP array (18). This genotyping array was designed to capture genetic variation at ∼2,000 genetic loci of relevance to a range of cardiovascular, metabolic, and inflammatory syndromes. At the time of chip development, a multistage approach for SNP selection was taken for the selection of SNPs within the candidate loci for the IBC array. For a given locus, known or putative functional SNPs were included first and then additional tagging SNPs were added to capture the known variation at the locus (with MAF >0.02 and r2 ≥ 0.8). Priority was given to nonsynonymous and functional variants if possible. This chip included 13 variants at the AHSG locus (rs12486044, rs16860926, rs2248690, rs2518136, rs34819441, rs35457250, rs35890379, rs4831, rs4917, rs4918, rs6444151, rs6795506, and rs7633550). We excluded SNPs with MAF <5%, leaving 7 SNPs in Caucasians and 9 SNPs in African Americans. Tables showing pairwise linkage disequilibrium (LD) as measured by D′ and r2 are provided (Supplementary Tables 1 and 2). From the CARe project, principal components also were generated based on ancestry-related SNPs.
Glucose and incident type 2 diabetes.
Glucose was measured in fasting blood samples obtained during the annual clinic examinations in 1992–1993, 1996–1997, 1998–1999, and 2005–2006 and in nonfasting blood samples in 1994–1995 (19). Medication use was assessed at baseline and annually thereafter by medication inventory through 2007 (20). We classified participants as having type 2 diabetes if fasting glucose was ≥126 mg/dL, casual glucose was ≥200 mg/dL, or individuals used insulin or oral hypoglycemic agents.
Statistical analysis
We used means and proportions of the demographics and type 2 diabetes risk factors to describe the study population according to ethnicity. We assessed the genotype and MAF for the variants and conducted tests for Hardy-Weinberg equilibrium. All analyses were performed separately for Caucasian and African American participants. We estimated mean fetuin-A concentration across genotypes and per minor allele copy in linear regression models adjusted for age, sex, and field center site. Multivariable Cox regression models were fit to evaluate the risk of incident type 2 diabetes with adjustment for age, sex, and field center site. We used Schoenfeld residuals to evaluate proportional hazards assumptions and found no appreciable evidence of violations of assumptions of proportionality. To see if results were stronger when prevalent cases of type 2 diabetes (n = 579) were included in addition to incident diabetes as the outcome of interest, we also estimated odds ratios from logistic regression analyses including both prevalent and incident type 2 diabetes cases. To account for multiple testing, we used Bonferroni-corrected thresholds for statistical significance (P < 0.007 in Caucasians and P < 0.006 in African Americans).
To perform a Mendelian randomization analysis of fetuin-A and fasting glucose levels, we chose two generally unrelated SNPs and used them as a single instrument; rs4917, a nonsynonymous amino acid coding variant, and the promoter variant rs2248690 were strongly associated with fetuin-A levels in our data, were not in strong LD, and previous literature suggests strong associations or even regulatory potential of these SNPs in relation to fetuin-A levels (11–14,21). We first explored if the two SNPs were associated with any of the potential confounders listed in Table 1, and we found no statistically significant associations. We used the two-stage least-squares (ivregress function in Stata) approach to estimate the difference in fasting glucose per 1 SD difference in genetically predicted fetuin-A concentrations (22). We assumed an additive genetic model (i.e., fetuin-A concentration increasing linearly with each additional minor allele of the genotypes). We compared the differences in fasting glucose per 1 SD difference from analyses using measured fetuin-A concentrations and instrumentally predicted fetuin-A (i.e., endogeneity) using the Wooldridge test. Analyses were repeated with adjustment for population stratification by including the top 10 principal components as covariates. All analyses were conducted using Stata version 11.1 (StataCorp, College Station, TX).
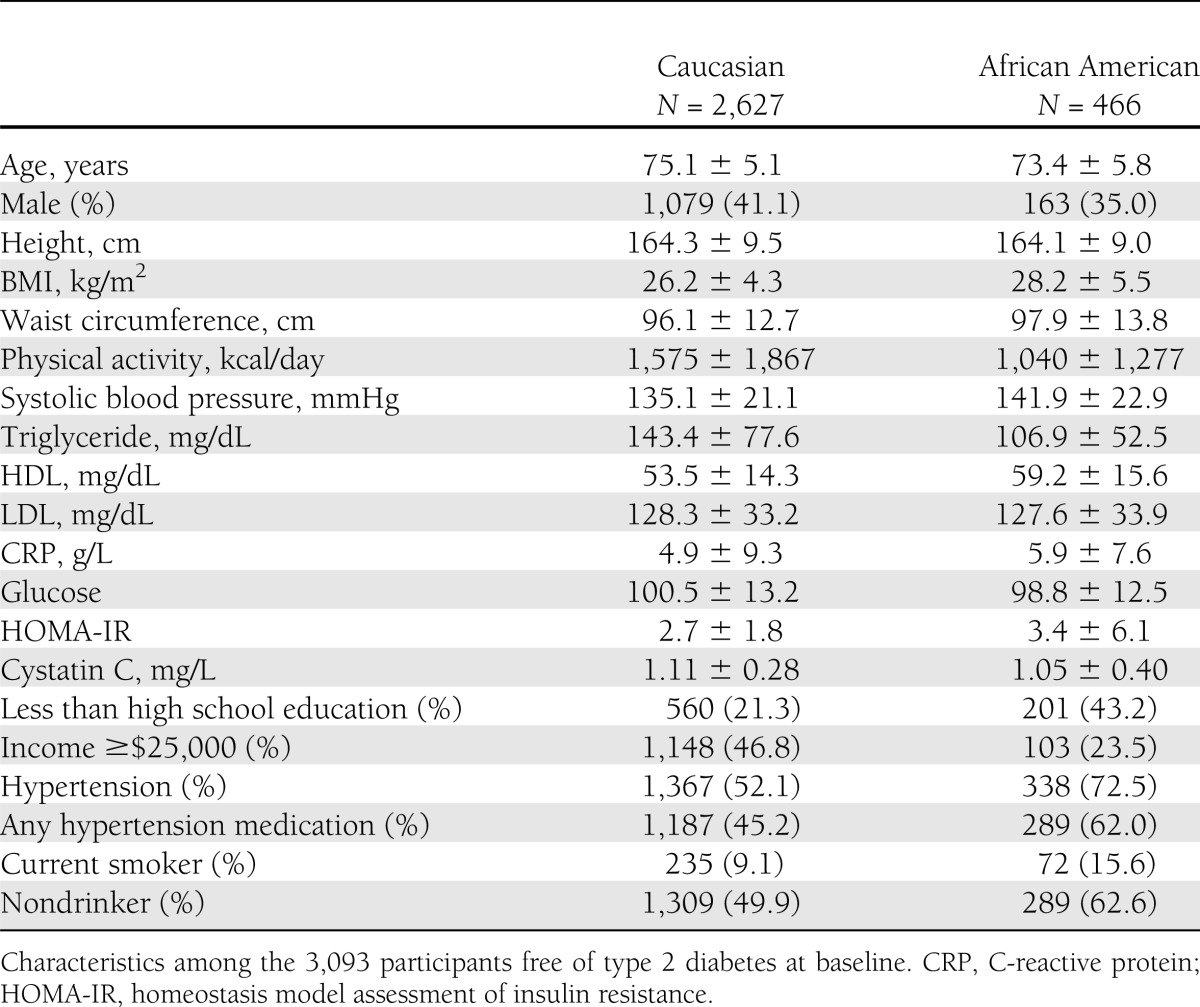
Table 1.
Baseline characteristics of older adults at baseline (1992): the CHS
RESULTS
The majority of the participants were Caucasian (85%) and female (60%) (Table 1). The mean was 75 years of age for participants of Caucasian descent and 73 years of age for participants of African American descent. The mean fetuin-A concentration was 0.48 ± 0.10 g/L in Caucasians and 0.43 ± 0.09 g/L in African Americans, and the distributions were approximately normal within the study samples.
Association of ASHG SNPs with plasma fetuin-A concentrations
Table 2 lists the frequencies of the genotypes and minor alleles for the genetic variants that had MAF >5%. The MAFs differed slightly between the Caucasian and African American subset, and two polymorphisms were observed with MAF >5% in the African American sample but not in the Caucasian sample (rs16860928 and rs6795506). The three SNPs (rs4917, rs4918, and rs2248690) that have been consistently associated with fetuin-A concentrations in previous studies were frequent in both subsamples (MAFs >25%). Borderline significant deviation from Hardy-Weinberg equilibrium was observed for rs4918 in both subsamples (P = 0.04 and 0.05 for Caucasians and African Americans, respectively) and for rs4917 in Caucasians (P = 0.04). Among Caucasians, all the measured SNPs were in high LD when based on D′ (all D′ >0.9), whereas r2 measures were not as high for SNPs that varied in frequency. The only SNPs in complete LD were rs4917 with rs4918 and rs2248690 with rs12486044, respectively. As expected, the LD measures were not as high in the African Americans (Supplementary Tables 1 and 2).
Table 2.
AHSG genotypes, MAFs (polymorphisms >5% MAF), and plasma fetuin-A concentrations according to AHSG genotype and per variant allele
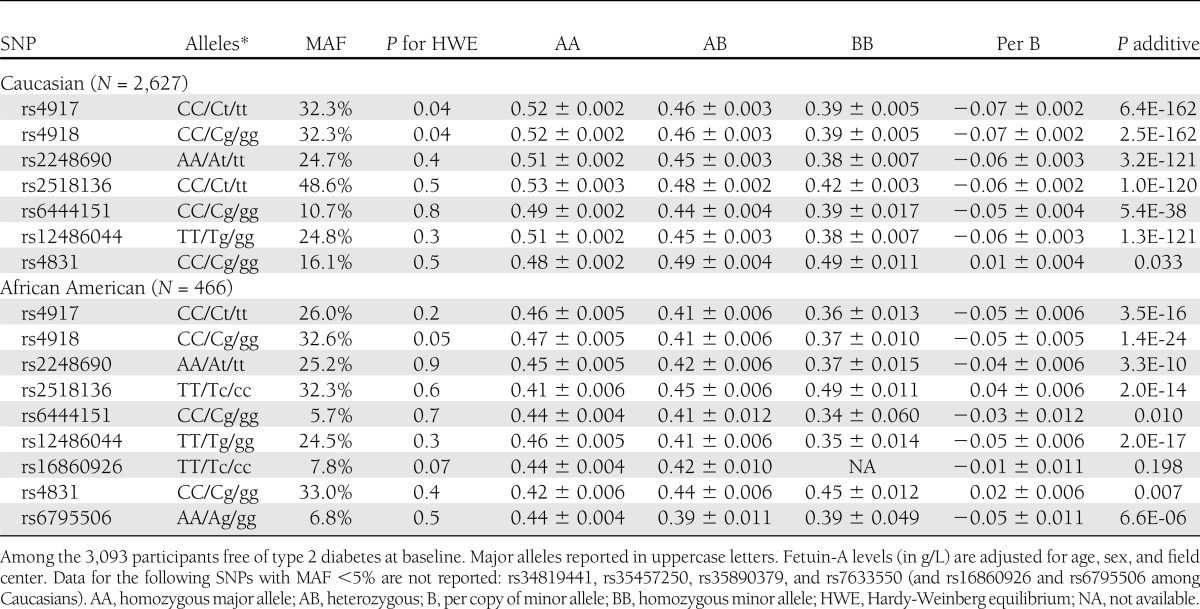
Mean plasma fetuin-A concentrations according to genotypes are also shown in Table 2. In Caucasians, the minor allele of all SNPs were strongly associated with lower fetuin-A concentrations, except for the rs4831 variant, which was weakly associated with slightly higher fetuin-A concentrations in both Caucasians and African Americans. After taking multiple testing into consideration, the rs4831 was not statistically significantly associated with fetuin-A levels. The direction of associations for the minor alleles were the same for all SNPs in African Americans, except for the rs2518136 variant allele, which was associated with higher fetuin-A, and the rs16860926 variant, which was only observed in African Americans and was not statistically significantly associated with fetuin-A concentrations. Carriers of the minor alleles for the tightly linked rs4917 (T) and rs4918 (G) variants had lower fetuin-A concentrations (−0.07 g/L [∼13%] per minor allele in Caucasians and −0.05 g/L [∼11%] in African Americans). Carriers of the rs2248690 T allele also had lower fetuin-A concentrations (−0.06 g/L in Caucasians and −0.04 g/L in African Americans).
Association of ASHG SNPs with incident type 2 diabetes
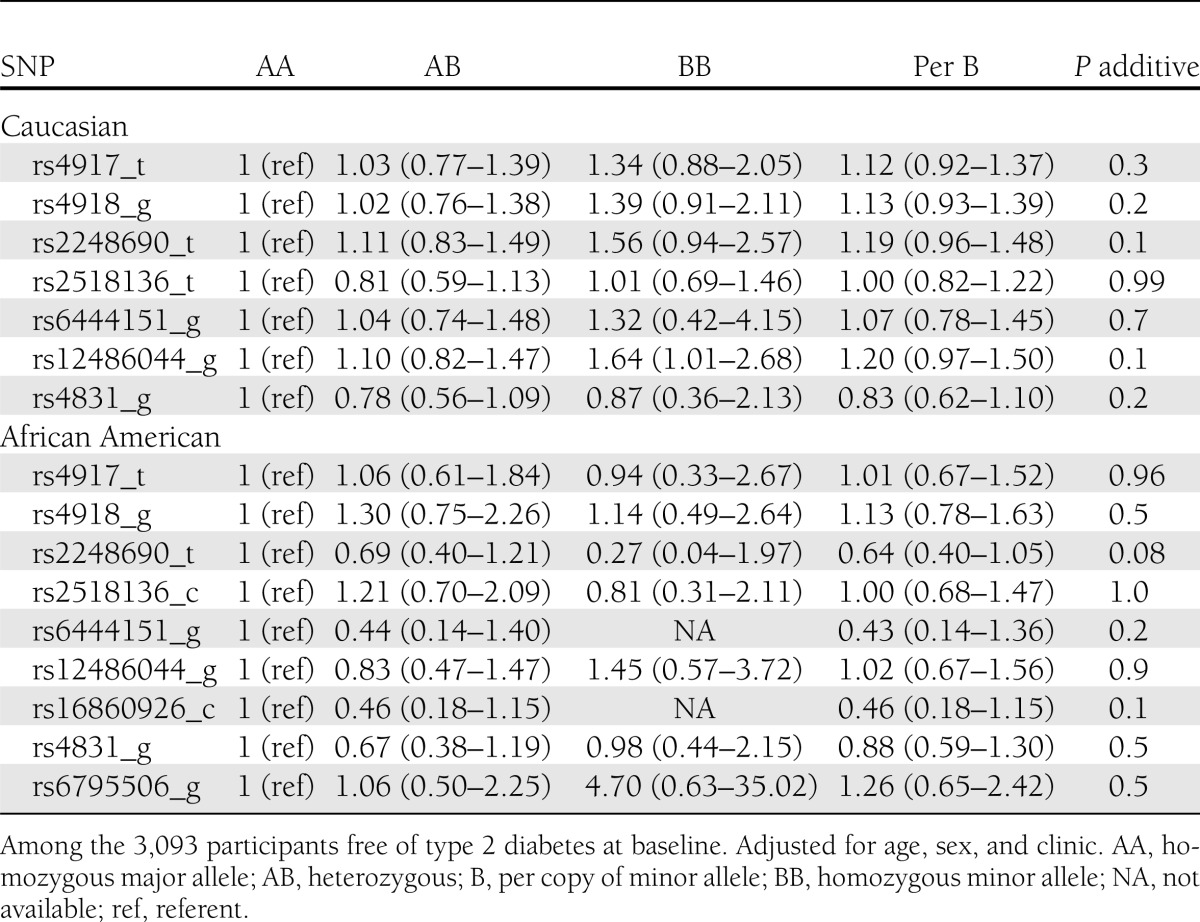
We did not detect any significant associations between the common genetic variants in AHSG and risk of type 2 diabetes in prospective analyses of either race (Table 3) or in analyses that included the 579 prevalent type 2 diabetes cases (data not shown). Rather than being associated with a lower risk of type 2 diabetes, as would be expected based on the direction of association observed between the genetic variants with lower plasma fetuin-A concentrations, the variant alleles tended to be associated with greater risk of type 2 diabetes by evaluation of the point estimates, albeit none reached statistical significance. The risk of type 2 diabetes per additional copy of the rs4917 minor allele was 1.12 (95% CI, 0.92–1.37) in Caucasians and null in African Americans (hazard ratio [HR] 1.01; 95% CI, 0.67–1.52). The rs2248690 variant allele was associated with a HR of 1.19 (95% CI, 0.96–1.48) per minor allele in Caucasians, whereas the opposite was observed for African Americans (HR 0.64; 95% CI, 0.40–1.05). Additional adjustment for population stratification did not alter these estimates appreciably (data not shown).
Table 3.
Association of AHSG genotypes and minor alleles with risk of type 2 diabetes in the CHS
AHSG SNPs and fasting glucose
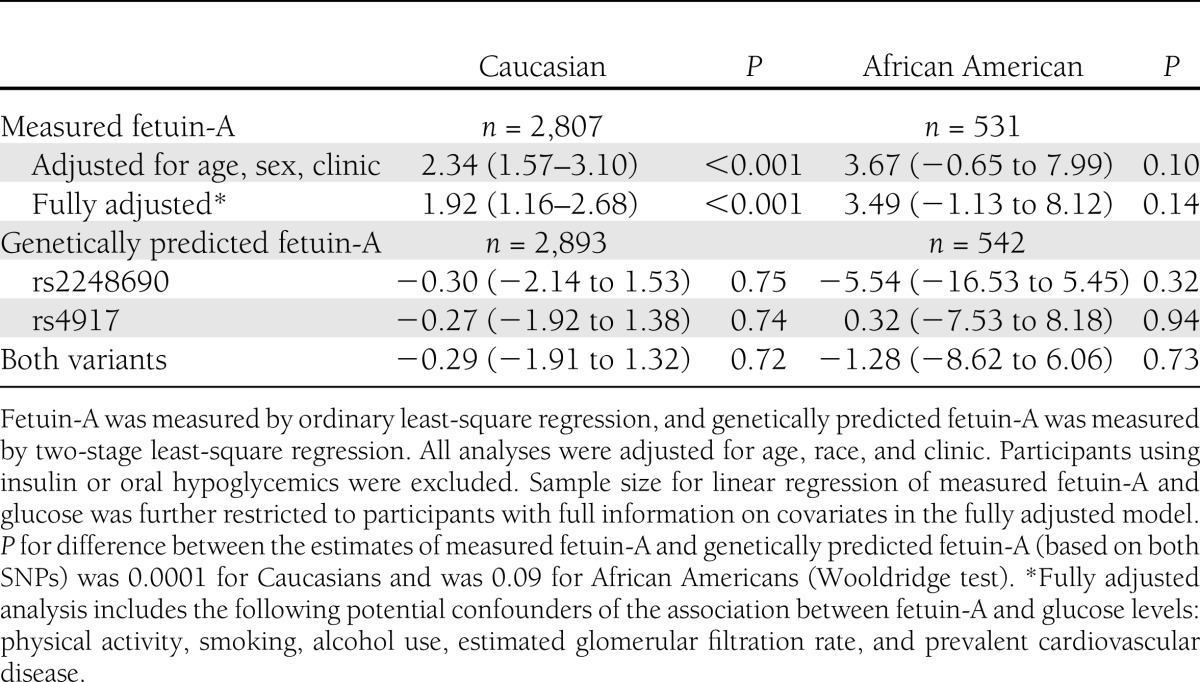
We next performed Mendelian randomization analyses using rs4917 and rs2248690 as instruments and using fasting glucose as the outcome for maximal power. Each 1-SD greater measured plasma fetuin-A concentration was associated with 1.9 mg/dL (95% CI, 1.2–2.7) higher glucose concentration in Caucasians and 3.5 mg/dL (−1.1 to 8.1) higher glucose concentration in African Americans (Table 4). In contrast, using the two AHSG SNPs as instruments, a 1-SD increment in genetically predicted fetuin-A concentration was associated with slightly lower glucose levels (−0.3 mg/dL [95% CI, −1.9 to 1.3] lower glucose per SD in Caucasians and −1.3 mg/dL [95% CI, −8.6 to 6.1] lower in African Americans), although neither association reached statistical significance. Although the Mendelian randomization analysis had broad CIs, the coefficients for measured and genetically predicted fetuin-A concentrations were significantly different from one another in Caucasians (P = 0.0001), with a similar but not statistically significant trend in the smaller African American sample (P = 0.09). Inclusion of the top 10 principal components as covariates capturing potential confounding by population stratification did not change the results (data not shown).
Table 4.
Differences in fasting glucose per 1 SD difference in fetuin-A and per 1 SD difference in genetically predicted fetuin-A
In our previous investigation from CHS, we reported that the association between plasma fetuin-A and risk of type 2 diabetes was stronger in individuals younger than 75 years of age (6). We did not observe any statistically significant interactions between the AHSG SNPs and age on the risk of incident diabetes (all P for interaction >0.4) or on fasting glucose levels (P > 0.2). Whereas the association of plasma fetuin-A and fasting glucose was strongest in the participants younger than 75 years of age, the genetically predicted fetuin-A levels were not associated with fasting glucose irrespective of age in this study (data not shown).
CONCLUSIONS
In a large biracial cohort of community-living older persons, we confirmed previously reported associations between genetic variants in the AHSG gene with plasma fetuin-A concentrations and between plasma fetuin-A concentrations and both fasting glucose and incident diabetes. However, using the genetic variants as instruments to assess the potentially causal association of fetuin-A with fasting glucose showed that the associations with measured and genetically predicted fetuin-A levels differed. Thus, although we cannot exclude a potentially small association between genetically predicted fetuin-A and glucose, the estimate was statistically significantly different from the positive association observed between measured fetuin-A and fasting glucose.
This study confirms reported findings that minor alleles for the tightly linked rs4917/rs4918 variants and rs2286490 are strongly associated with lower plasma fetuin-A concentrations (12–14). The rs4917 is a nonsynonymous amino acid coding variant that likely affects the transcription of the AHSG protein. It is in perfect LD with rs4918, and together these two variants capture a double nonsynonymous amino acid substitution that previously has been associated with lower serum levels of fetuin-A in vivo (11). The promoter variant rs2248690 A allele has previously been found to upregulate the promotor activity, resulting in higher fetuin-A levels compared with the T allele (21). The minor T variant may change a transcription binding site for a co-repressor, leading to lower expression of the AHSG protein and thus significantly lower fetuin-A levels.
We also found similar associations for the other commonly occurring variants across the AHSG gene, indicating a genetic region with a high extent of LD, especially in Caucasians.
We (6) and others (4,5) previously have reported that higher plasma fetuin-A concentrations are associated with greater risk of type 2 diabetes. Here, we extend these findings by reporting that SNPs associated with lower fetuin-A concentrations were not associated with a lower risk of type 2 diabetes or fasting glucose concentrations, suggesting that genetically elevated fetuin-A may not be causally related to type 2 diabetes.
Other studies also have explored candidate SNPs in AHSG in relation to type 2 diabetes and markers of insulin sensitivity with conflicting results. In a case-control study, the rs1071592 minor allele was statistically significantly less prevalent among individuals with type 2 diabetes compared with normoglycemic controls (9). Minor alleles at rs4918 and rs2248690 showed the same tendencies, although associations did not reach statistical significance. Surprisingly, no association was observed for rs4917 despite its being in near complete LD with rs4918 (9). None of the investigated AHSG SNPs were associated with homeostasis model assessment of insulin resistance or glucose levels in the ∼700 normoglycemic controls (9). In a Danish study of >7,000 healthy participants, the finding of an association with risk of type 2 diabetes was not replicated for the rs1071592 variant; rs4917/rs4918 exhibited lower insulin resistance and fasting insulin but were not associated with risk of type 2 diabetes (10).
There are a number of possible reasons for the disparity in the associations of plasma fetuin-A concentrations and fetuin-A–associated SNPs with fasting glucose concentrations and risk of type 2 diabetes. One possibility is that the observed association between elevated plasma fetuin-A concentrations and risk of type 2 diabetes is confounded by other determinants of fetuin-A concentrations, whereas the fetuin-A genotype is not subject to confounding by similar factors. For example, fetuin-A, which is derived from hepatocytes, appears to increase with hepatic steatosis and obesity, which themselves have been independently associated with risk of type 2 diabetes (23–25). Importantly, the AHSG SNPs do not appear to be associated with other glucose-related traits available in CHS, and the locus has not been implicated in nonalcoholic fatty liver disease. This suggests that these AHSG SNPs meet the necessary criteria for Mendelian randomization analyses of not being associated with potential confounders and not influencing the outcome through another potential intermediate. Although using genetic markers as instrumental variants for fetuin-A level is limited by the degree to which genetics contribute to the interindividual variation in circulating fetuin-A levels (26), our CIs (which reflect the other sources of variability) excluded large effects of genetically determined fetuin-A on glucose.
It is also possible that the differences between the relationships of measured plasma fetuin-A concentrations and the fetuin-A genotype with fasting glucose concentrations and risk of type 2 diabetes relate to our study of older community-living individuals. Participants in this study had an average of 75 years of age at blood draw; therefore, we may not have captured the appropriate study population if the studied genetic variants impact risk of type 2 diabetes primarily earlier in life. Two variants (the rs4917/rs4918 polymorphisms) were slightly out of Hardy-Weinberg equilibrium, which might indicate an unlikely scenario of survival bias. We found no similar issues for the remainder of the variants.
Another explanation may be related to the fact that individuals who carry the major AHSG allelic variants, and thus have lived with elevated fetuin-A levels into older age, may have developed compensatory mechanisms that buffer against the diabetes risk associated with higher plasma fetuin-A concentrations. It is possible that it would take years to develop feedback mechanisms that dampen the effect of continuously elevated fetuin-A concentrations on the insulin receptor, blunting an association between the studied genetic variants and type 2 diabetes in an elderly population used in the CHS. We did not find any evidence of effect modification when we stratified by younger than 75 years of age compared with older than 75 years of age to explore the associations of the genetic variants with glucose and diabetes. However, to test this hypothesis formally, a study population with a larger distribution in age would be necessary.
In conclusion, we observed that genetic variants in the gene encoding fetuin-A were strongly associated with lower plasma fetuin-A concentrations but were not associated with fasting plasma glucose concentrations, prevalent diabetes, or incident diabetes in community-living older persons. Future studies are needed to identify nongenetic determinants of plasma fetuin-A concentrations and to evaluate if such factors may confound the association of plasma fetuin-A concentrations and diabetes risk.
Acknowledgments
This study was supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI) (R01 HL094555 to L.D., J.R.K., S.J.Z., J.H.I., and K.J.M.), and genotyping was funded by the NHLBI CARe project (Broad Institute of Massachusetts Institute of Technology and Harvard, N01HC65226). The CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and by grant HL080295 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by AG023629 from the National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
M.K.J. assisted in study design, assisted in interpretation of the data, and wrote all versions of the manuscript. T.M.B. conducted statistical analysis, assisted in interpretation of the data, and critically edited the manuscript. L.D., J.R.K., and S.J.Z. assisted in obtaining funding for the project, assisted in interpretation of the data, and critically edited the manuscript. E.B.R. assisted in interpretation of the data and critically edited the manuscript. D.S.S. directed the study's implementation, including quality-control procedures; assisted in interpretation of the data; and critically edited the manuscript. B.M.P. assisted in the study's implementation, assisted in interpretation of the data, and critically edited the manuscript. J.H.I. conceived the idea for this study, designed the study, assisted in obtaining funding for the study, assisted in directing the data analysis, interpreted the results, and critically edited all versions of the manuscript. K.J.M. assisted in obtaining funding for the project, assisted in interpretation of the data, critically edited the manuscript, conceived the idea for this study, designed the study, assisted in obtaining funding for the study, assisted in directing the data analysis, interpreted the results, and critically edited all versions of the manuscript. T.M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2323/-/DC1.
*A complete list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Srinivas PR, Wagner AS, Reddy LV, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 1993;7:1445–1455 [DOI] [PubMed] [Google Scholar]
- 2.Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 1989;58:631–640 [DOI] [PubMed] [Google Scholar]
- 3.Mathews ST, Srinivas PR, Leon MA, Grunberger G. Bovine fetuin is an inhibitor of insulin receptor tyrosine kinase. Life Sci 1997;61:1583–1592 [DOI] [PubMed] [Google Scholar]
- 4.Stefan N, Fritsche A, Weikert C, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 2008;57:2762–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ix JH, Wassel CL, Kanaya AM, et al. Health ABC Study Fetuin-A and incident diabetes mellitus in older persons. JAMA 2008;300:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ix JH, Biggs ML, Mukamal KJ, et al. Association of fetuin-a with incident diabetes mellitus in community-living older adults: the Cardiovascular Health Study. Circulation 2012;125:2316–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vionnet N, Hani EH, Dupont S, et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet 2000;67:1470–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA 2000;97:14478–14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiq A, Lepretre F, Hercberg S, Froguel P, Gibson F. A synonymous coding polymorphism in the alpha2-Heremans-schmid glycoprotein gene is associated with type 2 diabetes in French Caucasians. Diabetes 2005;54:2477–2481 [DOI] [PubMed] [Google Scholar]
- 10.Andersen G, Burgdorf KS, Sparsø T, et al. AHSG tag single nucleotide polymorphisms associate with type 2 diabetes and dyslipidemia: studies of metabolic traits in 7,683 white Danish subjects. Diabetes 2008;57:1427–1432 [DOI] [PubMed] [Google Scholar]
- 11.Osawa M, Tian W, Horiuchi H, Kaneko M, Umetsu K. Association of alpha2-HS glycoprotein (AHSG, fetuin-A) polymorphism with AHSG and phosphate serum levels. Hum Genet 2005;116:146–151 [DOI] [PubMed] [Google Scholar]
- 12.Fisher E, Stefan N, Saar K, et al. Association of AHSG gene polymorphisms with fetuin-A plasma levels and cardiovascular diseases in the EPIC-Potsdam study. Circ Cardiovasc Genet 2009;2:607–613 [DOI] [PubMed] [Google Scholar]
- 13.Verduijn M, Prein RA, Stenvinkel P, et al. Is fetuin-A a mortality risk factor in dialysis patients or a mere risk marker? A Mendelian randomization approach. Nephrol Dial Transplant 2011;26:239–245 [DOI] [PubMed]
- 14.Stenvinkel P, Wang K, Qureshi AR, et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney Int 2005;67:2383–2392 [DOI] [PubMed] [Google Scholar]
- 15.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–366 [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 17.Musunuru K, Lettre G, Young T, et al. NHLBI Candidate Gene Association Resource Candidate gene association resource (CARe): design, methods, and proof of concept. Circ Cardiovasc Genet 2010;3:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE 2008;3:e3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–270 [PubMed] [Google Scholar]
- 20.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M, The Cardiovascular Health Study Collaborative Research Group Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol 1992;45:683–692 [DOI] [PubMed] [Google Scholar]
- 21.Inoue M, Takata H, Ikeda Y, et al. A promoter polymorphism of the alpha2-HS glycoprotein gene is associated with its transcriptional activity. Diabetes Res Clin Pract 2008;79:164–170 [DOI] [PubMed] [Google Scholar]
- 22.Stock JH, Wright JH, Yogo M. A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat 2002;20:518–529 [Google Scholar]
- 23.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012;35:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz Y, Yonal O, Kurt R, et al. Serum fetuin A/α2HS-glycoprotein levels in patients with non-alcoholic fatty liver disease: relation with liver fibrosis. Ann Clin Biochem 2010;47:549–553 [DOI] [PubMed] [Google Scholar]
- 25.Haukeland JW, Dahl TB, Yndestad A, et al. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. Eur J Endocrinol 2012;166:503–510 [DOI] [PubMed] [Google Scholar]
- 26.Kaess BM, Enserro DM, McManus DD, et al. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham Heart Study. J Clin Endocrinol Metab 2012;97:E1943–E1947 [DOI] [PMC free article] [PubMed] [Google Scholar]


