Abstract
OBJECTIVE
Elevated plasma free fatty acids (FFAs) are one important link between excess visceral adiposity, insulin resistance, and the development of type 2 diabetes. Effects of lifestyle interventions on FFA metabolism are poorly known. This open-label study was conducted to test the effects of a 1-year healthy eating/physical activity intervention program on plasma FFA homeostasis in 117 viscerally obese men with dyslipidemia associated with insulin resistance (waist circumference ≥90 cm, triglycerides ≥1.69 mmol/L, and/or HDL-cholesterol <1.03 mmol/L).
RESEARCH DESIGN AND METHODS
Body weight, body composition, and fat distribution were assessed by dual-energy X-ray absorptiometry/computed tomography. Oral loads of lipid (60 g fat/m2 body surface area) and glucose (75 g) were measured before and after the intervention.
RESULTS
After 1 year of lifestyle intervention, visceral adiposity was reduced by −26% (95% CI −29 to −23), whereas cardiorespiratory fitness improved by +20% (95% CI +16 to +24). After 1 year, the suppression of FFAs after the glucose load improved, whereas insulin concentrations were drastically reduced. After the oral lipid load, the late increase in FFA was reduced together with reduced circulating insulin. We calculated an insulin sensitivity index to reflect the concentration of insulin needed to manage plasma FFAs after the oral lipid load, which increased after the intervention and was associated with improved glucose tolerance, independent of changes in visceral or total adiposity.
CONCLUSIONS
A 1-year healthy eating/physical activity intervention improved the suppression of FFAs after oral glucose and lipid load tests in viscerally obese men, possibly due to improved responsiveness to insulin. This insulin-mediated regulation of postprandial plasma FFA levels could be a link between visceral obesity and impaired glucose homeostasis.
Excess visceral adiposity has been associated with deteriorated cardiometabolic risk profile (1,2) and increased risk of developing type 2 diabetes and cardiovascular diseases (3,4). In both fasting and postprandial conditions (5), obese patients have increased concentrations of plasma free fatty acids (FFAs) compared with lean patients (6,7). Although visceral adiposity lipolysis accounts for less than 20% of systemic circulating FFA in obese patients, these FFAs are released directly into the portal vein, thereby exposing the liver to more FFA than would be predicted from systemic FFA (6). Moreover, in vitro studies have shown that visceral adipocytes are characterized by increased lipolysis compared with subcutaneous adipocytes (8) because visceral fat is much less sensitive to lipolysis inhibition by insulin than subcutaneous fat (9). Thus, increased visceral fat increases delivery of deleterious levels of FFA to the liver via the portal vein, leading to elevated hepatic triglyceride concentration and hepatic insulin resistance. Therefore, abdominal adiposity is an important determinant of postprandial plasma FFA flux (10). Increased plasma FFA, especially in the postprandial state, seems to be one important link between excess visceral adiposity, ectopic fat deposition, insulin resistance, and the development of type 2 diabetes (see Carpentier [11] and Giacca et al. [12] for review).
Few studies have addressed the effect of lifestyle intervention on FFA homeostasis, particularly in relation to changes in visceral adiposity. An ancillary study from the LookAHEAD trial was conducted in obese patients with type 2 diabetes who participated to the intensive lifestyle intervention arm. In this study, Albu et al. (13) assessed insulin sensitivity and FFAs during a hyperinsulinemic euglycemic clamp in 26 men and 32 women. One year of intensive lifestyle intervention elicited an increase in exogenous insulin-mediated glucose uptake as well as a decrease in FFA concentrations during the clamp, demonstrating improved insulin sensitivity of both glucose metabolism and lipolysis inhibition. Furthermore, Johnson et al. (14) performed a study of 19 obese patients with impaired glucose tolerance (12 in the intervention and 7 in the control arm) to evaluate the effects of 4 weeks of aerobic exercise training on fat distribution and metabolism. They found that fasting FFA concentrations decreased in the intervention group in association with a reduction in hepatic triglyceride concentration. While these studies show that fasting FFA concentrations decrease after a diet or exercise intervention program, or both, it is still not known whether postprandial FFA metabolism may be improved with a lifestyle intervention.
The current study was conducted to test the effects of a 1-year healthy eating/physical activity intervention program on plasma FFA homeostasis in viscerally obese men. Two complementary lipid and glucose oral loads were performed at baseline and after 1 year of intervention to test plasma FFA response to dynamic, oral, thus physiologic tests, in parallel with observed changes in fat accumulation and distribution.
RESEARCH DESIGN AND METHODS
Study design
Caucasian men (n = 144) between the ages of 30 and 65, with abdominal obesity (waist circumference ≥90 cm), elevated triglyceride concentrations (≥1.69 mmol/L), low HDL-cholesterol (<1.03 mmol/L), or all three, were recruited by media solicitation to participate in an open-label, uncontrolled, 1-year intensive lifestyle modification program with 3 years of follow-up. This study focuses on the results after 1 year in the 117 men that completed the first year of intervention. Subjects dropped out mainly because of an inability of some participants to attend the regular follow-up visits planned with the study dietitians and kinesiologist.
Subjects with type 2 diabetes, with BMI values <25 or >40 kg/m2, or those taking medication targeting glucose, lipid metabolism, or blood pressure (BP) management were excluded. Four patients developed type 2 diabetes after 1 year (15). Diabetes was diagnosed by the 1-year oral glucose tolerance test (OGTT). Thus, subjects did not take any antidiabetic medications during the follow-up period of the current study. Informed written consent was obtained from all participants before their inclusion in the study, which had been approved by the medical ethics committees of Université Laval and Institut universitaire de cardiologie et de pneumologie de Québec (Québec, QC, Canada).
Subjects were counseled individually once every 2 weeks during the first 4 months of the study, with subsequent monthly visits to improve their nutritional and physical activity/exercise habits. Each visit included an interactive session with a registered dietitian followed by a meeting with a kinesiologist. The nutritional counseling was personally adapted to elicit a 500-kcal daily energy deficit, including less than 30% of kilocalories from lipids, preferably unsaturated. The daily caloric intake was estimated at baseline and at year 1 by a 3-day dietary record including one nonworking day. The aim of the physical activity program was 160 min/week of moderate-intensity endurance exercise. Men received a personalized training program that was adapted according to subjects’ history, preferences, maximal treadmill test results, and the ratings of their self-perceived exhaustion (modified Borg scale [16]). Participants were asked to wear a pedometer daily and to target 10,000 steps/day.
Anthropometric measurements and body composition
Height, weight, hip circumference (17), and waist circumference above the iliac crest (18) were measured according to standardized procedures. Fat mass and fat-free mass were assessed using dual-energy X-ray absorptiometry (Lunar Prodigy; GE, Madison, WI). BP was measured 3 min apart on the nondominant arm with an appropriate cuff size after the patient had been resting in a seated position for 5 min.
Computed tomography
Cross-sectional areas of visceral adipose tissue and abdominal subcutaneous adipose tissue were assessed by computed tomography, using previously described procedures (19,20). The partial volumes of visceral and subcutaneous adipose tissues between L2/L3 and L4/L5 were calculated using the product of the mean of L2/L3 and L4/L5 cross-sectional areas multiplied by the distance separating the two slices, as previously described (21).
Cardiorespiratory fitness
Cardiorespiratory fitness was assessed using a submaximal standardized exercise test on a TMX 425 treadmill (Trackmaster, Newton, KS) linked to a QuarkB2 monitor (Cosmed, Rome, Italy). In this study, two variables were retained as fitness end points to evaluate cardiorespiratory fitness: 1) the subject’s heart rate (mean of the last 3 min) at a standardized treadmill stage (3.5 mph, 2% slope) and 2) the estimated metabolic equivalent of task reached by the subject at a heart rate of 150 bpm and calculated using American College of Sports Medicine formulas (16).
Oral glucose tolerance test
After a 12-h overnight fast, participants were given a 75-g OGTT with plasma samples taken at 0, 15, 30, 45, 60, 90, and 120 min for assessment of insulin, glucose, and FFA concentrations. Patients were asked to avoid alcohol consumption and strenuous exercise during the 48 h before the test. Plasma FFA was measured enzymatically (Wako HR series NEFA-HR [2]; Wako Chemicals USA, Inc., Richmond, VA) (22). The detection threshold of FFAs was 0.12 mmol/L in our assay. A 0.12 mmol/L value was assigned to measurements below this detection threshold. Plasma insulin was determined by radioimmunoassay. The area under the curve of FFAs during the OGTT was determined by the trapezoid method between 0 and 120 min. Two FFA homeostasis indices were calculated via conceptually extrapolation from widely used plasma glucose/insulin homeostasis indices: FFA-resistance index (RI) was extrapolated from homeostasis model assessment–insulin resistance (HOMA-IR) (23) using the formula FFA-RI = log (fasting plasma FFA × fasting plasma insulin), with insulin as picomoles per liter and FFAs as millimoles per liter (24). Insulin sensitivity index (ISI) of FFA (ISI FFA-OGTT) was extrapolated from the ISI of Matsuda (ISI-Matsuda) (25) using the formula ISI FFA-OGTT = 100/ √ [(fasting plasma insulin × fasting FFA) × (mean plasma insulin during OGTT × mean FFA during OGTT)], with insulin as International units per liter and FFAs as millimoles per liter.
Oral lipid tolerance test
After a 12-h overnight fast, participants were asked to consume a standardized meal (breakfast) containing 60 g fat/m2 body surface area. The meal comprised 64% energy content from fat, 18% from carbohydrates, and 18% from proteins (26,27). Blood samples were collected before breakfast and at every 2-h period (for a total of 8 h) to measure plasma FFA and insulin concentrations. Patients were asked to avoid alcohol consumption and strenuous exercise in the 48 h before the test. As for the OGTT, for the oral lipid tolerance test (OLTT) we built an index derived from the ISI-Matsuda index to estimate the insulin sensitivity of plasma FFA homeostasis. We used the following formula: ISI FFA-OLTT = 100/ √ [(fasting plasma insulin UI/L × fasting FFA mmol/L) × (mean plasma insulin UI/L during OLTT × mean FFA mmol/L during OLTT)].
Plasma lipoprotein–lipid profile
Fasting plasma triglycerides, total cholesterol, VLDL-cholesterol, LDL-cholesterol, and HDL-cholesterol concentrations were determined according to standardized procedures (28–30).
Statistical analyses
In the main text and tables, data are expressed as mean (SD) for normally distributed variables and median (interquartile range) for nonnormally distributed variables. The changes in variables over the 1-year lifestyle intervention are reported as mean (95% CI). In the figures, data are reported as mean ± SEM. Statistical significance of changes after 1 year was evaluated by paired t tests. A two-factor repeated measures ANOVA was conducted to analyze time effect (changes occurring in successive samples of the test), visit effect (after vs. before the lifestyle intervention), and time × visit interaction in OGTT and OLTT tests. Pearson correlation coefficients were computed to examine relationships between changes in cardiometabolic risk markers and changes in fasting plasma insulin, fasting plasma FFA, FFA-RI, ISI FFA-OGTT, and ISI FFA-OLTT. A multivariable linear regression model was built to look for the independent and respective contributions of visceral adipose tissue, cardiorespiratory fitness (exercise output at 150 bpm expressed in metabolic equivalents of tasks), and fat mass changes to the changes in FFA-RI, ISI FFA-OGTT, and ISI FFA-OLTT (dependent variables). Then, a multivariable linear regression model was built to look for the independent and respective contributions of either FFA-RI, ISI FFA-OGTT, or ISI FFA-OLTT and visceral adipose tissue/fat mass changes to the changes in cardiometabolic risk markers (dependent variables). Results were reported by partial and total R2. The normal distribution of residuals was verified using the Shapiro-Wilk test and their stem and leaf distributions. A logarithmic transformation was performed in case of skewed distribution (fasting plasma insulin, triglycerides, VLDL-cholesterol, ISI-Matsuda, ISI FFA-OGTT, ISI FFA-OLTT, and HOMA-IR). The significance level was set as P < 0.05. All analyses were performed using SAS statistical package version 9.2 (SAS Institute, Cary, NC).
RESULTS
Of the 144 subjects who initially started the program, 117 completed the first year of intervention (81% completion rate). Men who completed the 1-year intervention were not different at baseline from the men who did not, except for a higher self-reported daily caloric intake among the men who completed the 1-year intervention compared with those who did not (3,028 ± 656 vs. 2,677 ± 617 kcal for men who completed versus those who did not complete the 1-year intervention, respectively; P = 0.02).
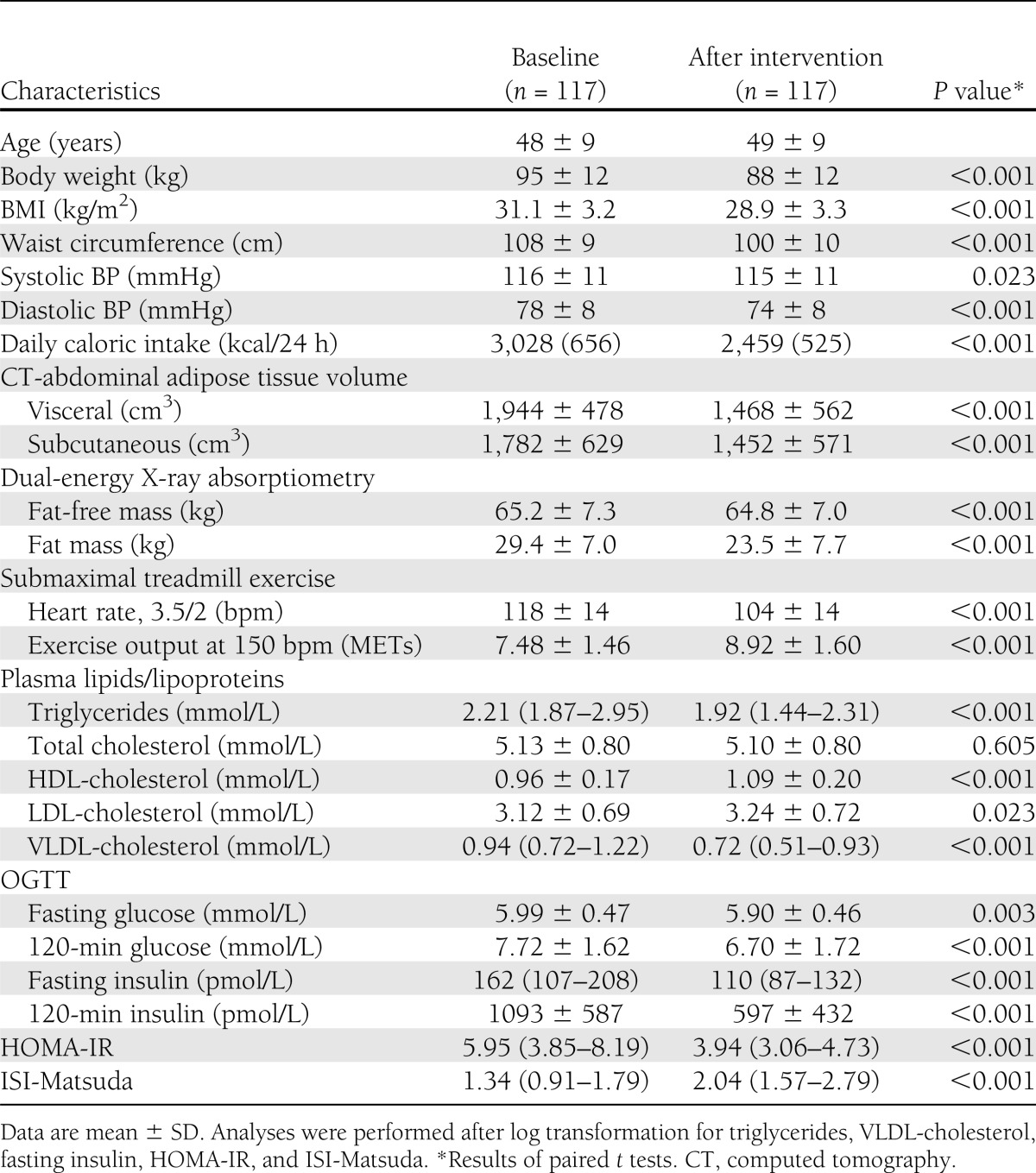
Characteristics of subjects and their 1-year evolution are reported in Table 1. As we have previously reported (1,15), visceral adiposity was reduced by −26% (95% CI −29 to −23), whereas cardiorespiratory fitness improved by +20% (+16 to +24) (both P < 0.001) after 1 year. Many other anthropometric and cardiometabolic risk markers were significantly improved after the 1-year lifestyle intervention program.
Table 1.
Subjects’ anthropometric, adiposity, body composition, and cardiorespiratory fitness characteristics before (baseline) and after the 1-year lifestyle intervention
Descriptive results of plasma FFAs after 1 year of lifestyle intervention
Variables related to plasma FFA homeostasis are reported in Table 2. Plasma insulin concentrations following the oral lipid load were significantly lower after 1 year of intervention (Fig. 1A). In parallel, the shape of the curve of FFA concentration changed significantly after the 6th hour of the test (Fig. 1B), and the 8-h FFA concentration was significantly reduced. ISI FFA-OLTT significantly improved after the 1 year intervention (Table 2).
Table 2.
FFA concentrations before (baseline) and after the 1-year intervention during oral lipid tolerance and OGTTs in the sample of viscerally obese men
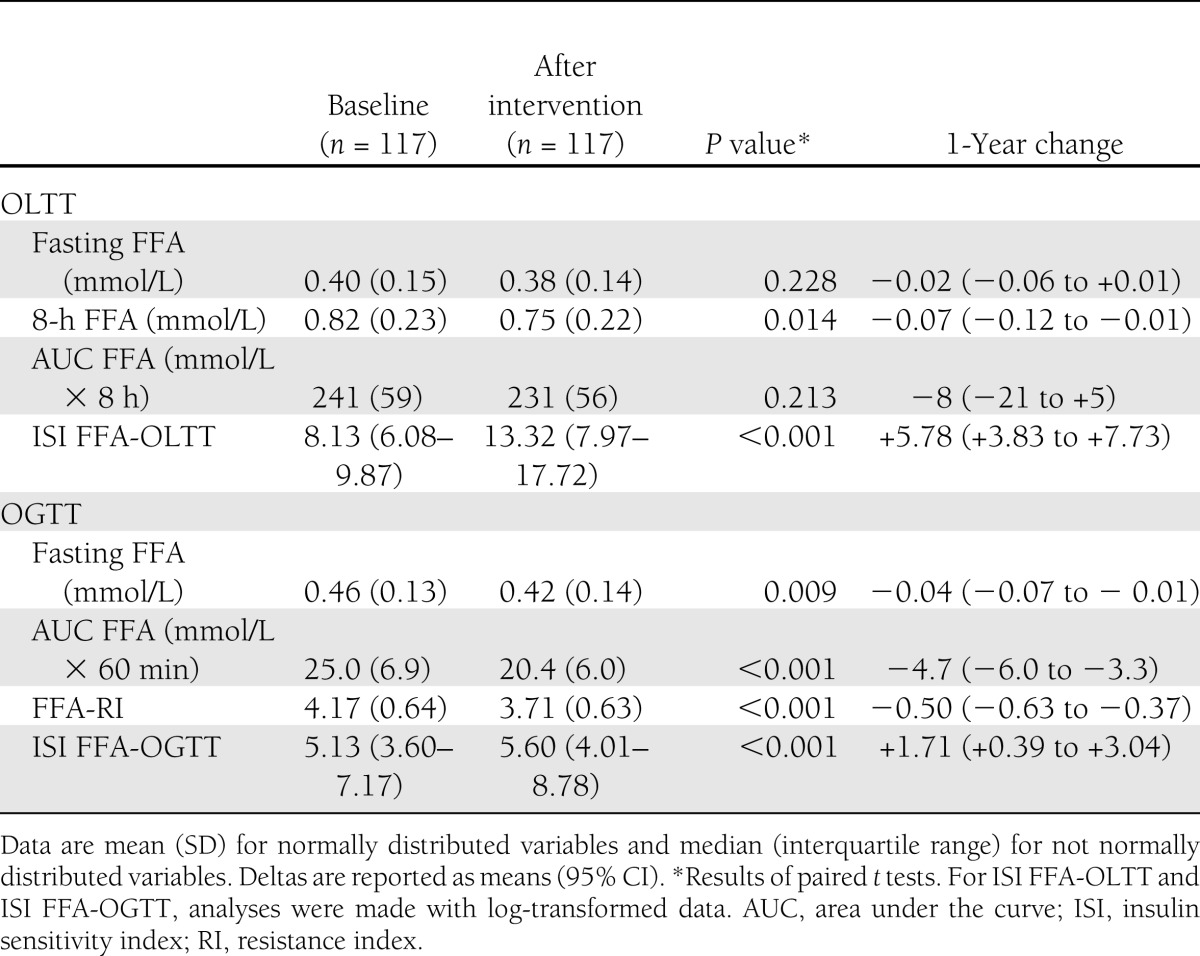
Figure 1.
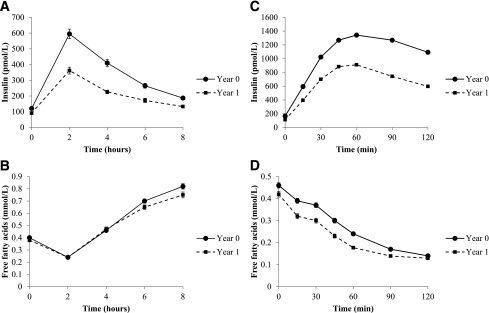
Changes in plasma insulin and plasma FFAs during OLTT and OGTT. Data are means ± SEM. Analyses were performed by repeated-measure ANOVA. A: Plasma insulin in the OLTT. The concentration of insulin changed during the test (time effect, P < 0.001) and between baseline and follow-up visits (visit effect, P < 0.001). The curve’s shape also changes after the lifestyle intervention (time × visit interaction, P < 0.001). B: Plasma FFAs in the OLTT. The effect of time during the test was significant (P < 0.001). The visit effect was not significant (P = 0.119). The time × visit interaction was significant (P = 0.006) for plasma FFAs, meaning that the shape of the curve changed after 1 year of intervention: the two lines were no longer superimposed from 6 h after the lipid load. C: Plasma insulin in the OGTT. The concentration of insulin changed during the test (time effect, P < 0.001) and between baseline and follow-up visits (visit effect, P < 0.001). The curve’s shape also changed after the lifestyle intervention (time × visit interaction, P < 0.001). D: Plasma FFAs in the OGTT. The concentration of FFAs changed during the test (time effect, P < 0.001) and between baseline and follow-up visits (visit effect, P < 0.001). The shape of the curve also changed after the lifestyle intervention (time × visit interaction, P < 0.001).
During the OGTT, the increase in insulin concentration led to a decrease in plasma FFA to a nadir often below the detection threshold of our assay (0.12 mmol/L) (Fig. 1C and D). During the OGTT, both plasma FFA and plasma insulin concentrations decreased after 1 year of intervention. This was corroborated by a significant reduction in FFA-RI and improvement of ISI FFA-OGTT after the 1-year lifestyle intervention (Table 2).
Regression analyses
Changes in fasting FFA as well as changes in fasting insulin, FFA-RI, ISI FFA-OGTT, and ISI FFA-OLTT were significantly correlated to the changes of most anthropometric and cardiometabolic variables in univariate correlations (Supplementary Table 1).
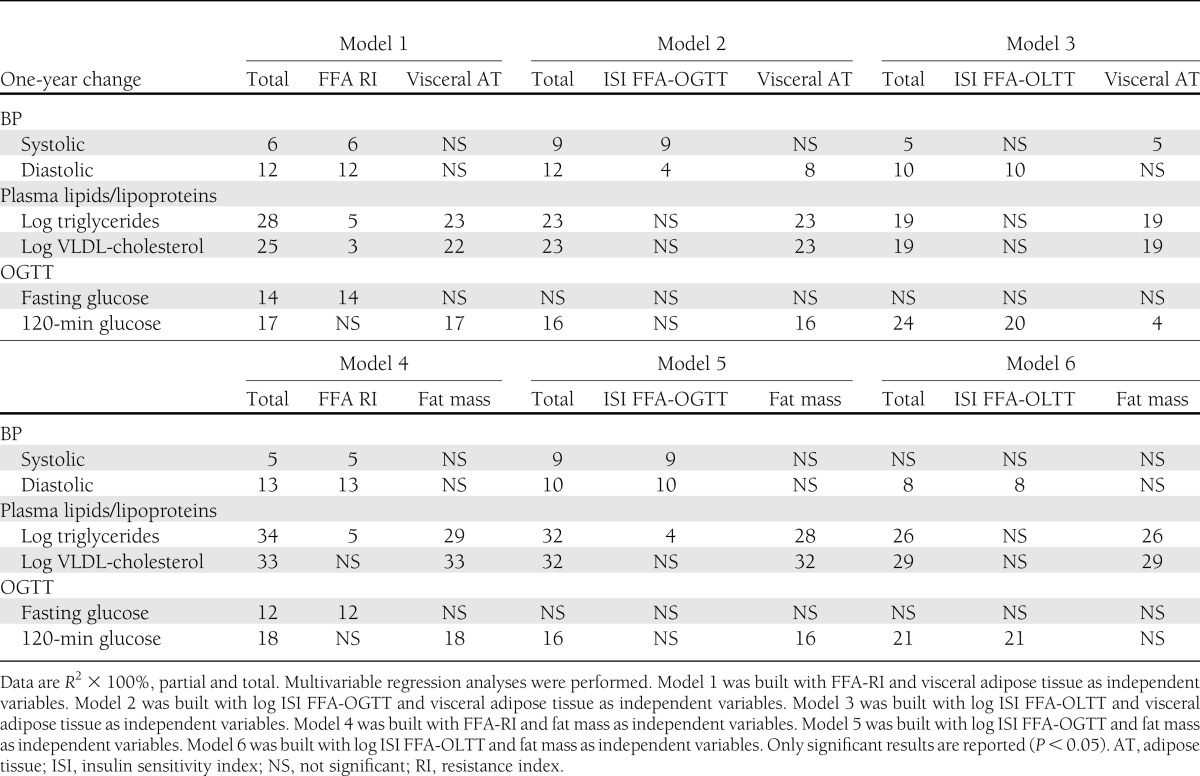
Multivariable linear models were built to address the relative contribution of visceral adipose tissue, fat mass, and cardiorespiratory fitness changes (independent variables) to changes in FFA-RI, ISI FFA-OGTT, and ISI FFA-OLTT (dependent variables). Changes in fat mass explained 25% of the variance of FFA-RI changes and 26% of the variance of ISI FFA-OGTT changes, whereas neither visceral adipose tissue nor cardiorespiratory fitness changes were independently associated with FFA-RI changes or ISI FFA-OGTT changes. On the other hand, visceral adipose tissue changes explained 10% of the variance of ISI FFA-OLTT changes, whereas neither fat mass nor cardiorespiratory fitness changes were independently associated with ISI FFA-OLTT changes.
We used multivariable linear regression models to assess the relative contribution of visceral adipose tissue/fat mass changes and FFA-RI/ISI FFA-OGTT/ISI FFA-OLTT changes to the 1-year changes in cardiometabolic variables, as reported in Table 3. In these analyses, we considered only the variables that were associated with FFA-RI, ISI FFA-OGTT, and ISI FFA-OLTT in univariate correlations. Changes in triglyceride concentrations and systolic and diastolic BP were associated with changes in FFA-RI, ISI FFA-OGTT, or ISI FFA-OLTT independent of visceral adipose tissue/fat mass changes. Fasting glucose changes were specifically associated with changes in FFA-RI and not with changes in visceral adiposity or fat mass. A greater percentage of change in 120-min OGTT glucose was explained by a change in ISI FFA-OLTT than by a change in fat mass/visceral adipose tissue, whereas it was not associated with changes in FFA-RI or ISI FFA-OGTT.
Table 3.
Respective percentage contribution of 1-year changes in FFA homeostasis indices and in fat mass/visceral adiposity to changes in cardiometabolic risk markers in response to the lifestyle intervention
CONCLUSIONS
This 1-year physical activity/healthy eating intervention program had a significant effect on plasma FFA homeostasis, which was measured using two complementary tests: oral lipid and glucose loads. During the OGTT, fasting FFA and concentrations after the load were lower after 1 year of intervention in spite of decreased plasma insulin concentrations. Thus, lower concentrations of plasma insulin were better able to modulate plasma FFA concentrations, suggesting an improvement in insulin sensitivity of lipolysis. After 1 year of intervention, the postprandial rise of FFA during the OLTT was superimposable until the 6th postprandial hour and only moderately reduced at the 8th hour. Nevertheless, plasma insulin concentrations were markedly lower during the test after 1 year of intervention. These results suggest that intracellular adipose tissue lipolysis and/or FFA spillover from chylomicron-triglyceride lipolysis following a lipid-rich meal were more efficiently suppressed by plasma insulin after the lifestyle intervention. From these observations, we built three plasma FFA homeostasis indices reflecting fasting insulin resistance of FFA homeostasis (FFA-RI), global insulin sensitivity of FFA homeostasis during OGTT (ISI FFA-OGTT), and FFA homeostasis during OLTT (ISI FFA-OLTT). One-year changes in these indices were associated with changes in numerous anthropometric variables and cardiometabolic risk markers. These indices, reflecting FFA plasma concentrations in response to endogenous insulin in three different physiologic conditions, remained independently associated with improvement in BP, triglyceride concentrations, and glucose tolerance, independent of changes in visceral and total body fat mass.
Effects of lifestyle intervention on plasma FFA
Several previous studies have demonstrated an improvement in FFA concentrations after weight loss. Kelley et al. (31) measured the effects of a 6-month behavioral intervention on glucose and FFA homeostasis assessed by euglycemic hyperinsulinemic clamps in 39 overweight and obese patients with type 2 diabetes treated with either placebo or orlistat. They found that the suppression of FFA during the clamp was improved after the behavioral intervention in both groups, with an additional benefit in the group treated with orlistat. Fasting plasma FFA concentrations were a strong correlate of insulin sensitivity both before and after weight loss. Similarly, Albu et al. (13) presented an ancillary investigation of the LookAHEAD trial in which they measured FFA during an euglycemic hyperinsulinemic clamp in 26 men and 32 women with obesity and type 2 diabetes participating in the intensive lifestyle intervention arm of LookAHEAD. Both fasting and clamp FFA were reduced after the lifestyle intervention. Changes in FFA during the clamp were associated with changes in liver fat, but not with changes in body weight, fat mass, or subcutaneous/visceral adiposity. In accordance with the previous findings of Kelley et al., the magnitude of FFA suppression by insulin during the euglycemic hyperinsulinemic clamp and the decrease in hepatic fat were independent determinants of improved peripheral insulin sensitivity. Two studies have tested the effect of an exercise intervention alone on change in intrahepatic lipid content independent of body weight loss. Johnson et al. (14) conducted a 4-week aerobic cycling exercise intervention in 12 obese subjects compared with 4 weeks of stretching sessions in 7 obese control subjects. Visceral adipose tissue mass and intrahepatic lipid content decreased by 12% and 21%, respectively, in the aerobic cycling group only, with no difference in body weight between the groups. Fasting FFA decreased by 14% in the aerobic cycling exercise group, which was significantly associated with the decrease in intrahepatic lipid content. The correlation between fasting FFA and visceral adiposity change was not reported. Another study by Hallsworth et al. (32) tested the effect of 8 weeks of resistance exercise (n = 11) compared with no intervention (n = 10) in subjects with moderate nonalcoholic fatty liver disease. The exercise intervention was effective in lowering intrahepatic lipid content; however, fasting FFAs as well as OGTT FFAs did not change with the exercise intervention.
The current study expands upon the previous findings of Kelley et al. (31) and Albu et al. (13) to show that viscerally obese men without type 2 diabetes improve their FFA homeostasis, as shown by a reduction in fasting FFAs and a greater suppression of FFAs during the OGTT after a combined healthy eating and physical activity intervention. Considering the role of elevated FFA in ectopic fat deposition and the development of insulin resistance and type 2 diabetes (11), this improvement in FFA homeostasis is particularly important in patients with a high risk of developing type 2 diabetes, such as the men with excess visceral adiposity who participated in this study.
We found that improvement in fasting FFA and in FFA insulin sensitivity indices after the 1-year intervention were associated with a reduction in total fat mass and visceral and subcutaneous abdominal adiposity as well as improvements in cardiometabolic risk markers, such as triglycerides, BP, and fasting and 120-min OGTT glucose, independent of changes in visceral or total adiposity.
We acknowledge that indices of FFA insulin sensitivity used in this study have never been validated in controlled studies using an insulin clamp experiment with FFA tracers to measure the sensibility of lipolysis suppression by insulin in euglycemic conditions as well as in conditions of high circulating levels of triglyceride-rich lipoproteins. Thus, findings of this study, while plausible, require cautious interpretation. Indeed, the specificity of these indices to reflect insulin sensitivity of lipolysis, lipoprotein lipase activity, or both need to be further explored. Of note, the fasting FFA-RI was previously used in one study by Abdul-Ghani et al. (24) that compared fasting FFAs in patients with normal glucose tolerance, impaired fasting glucose, and impaired glucose tolerance. The product of fasting insulin and log transformed fasting FFAs was higher in both impaired fasting glucose and impaired glucose tolerance conditions than in normal glucose tolerance, reflecting resistance to the antilipolytic effect of insulin.
FFA after OLTT and the effects of the 1-year lifestyle intervention
In the postprandial state, insulin regulates plasma FFA concentrations by suppressing intracellular adipose tissue lipolysis (33) and by reducing FFA spillover from chylomicron triglyceride lipolysis (34,35). The rise of FFAs after OLTT in spite of the rise of plasma insulin concentration is likely to reflect the spillover of FFAs from intravascular lipolysis of triglyceride-rich lipoproteins. It has been shown previously that this spillover is increased in the offspring of patients with type 2 diabetes compared with subjects without any family history of type 2 diabetes (35). In this study we observed that a 1-year lifestyle intervention did not decrease the overall area under the curve of FFA after OLTT, whereas a previous study demonstrated that sitagliptin (a DPP4 inhibitor) treatment was able to decrease FFAs after an oral lipid load (36). However, after 1 year of intervention, late postprandial plasma FFA concentrations were lower compared with baseline results before the lifestyle intervention. Importantly, insulin concentrations decreased markedly, demonstrating much improved postprandial plasma FFA sensitivity to insulin, as estimated by the decrease in the ISI FFA-OLTT. It is, however, not possible to determine which mechanism (e.g., reduced intracellular lipolysis or reduced FFA spillover) drove this response.
Relationships of changes in plasma FFA/insulin homeostasis with glucose tolerance
It has been demonstrated previously that waist circumference, a surrogate marker of visceral fat, was the main determinant of systemic plasma FFA turnover as measured by FFA tracer (10). FFA concentrations also correlate with total adipose tissue stores, as shown in previous studies (7) as well as in the present work. However, fasting glucose changes after 1 year were associated only with changes in fasting FFA-RI and not with changes in visceral or total adiposity. Changes in 120-min OGTT-glucose were strongly associated with the changes in ISI FFA-OLTT compared with changes in visceral adiposity or fat mass. An elevation of the 120-min OGTT-glucose concentration is associated with peripheral, and particularly with muscular, insulin resistance as well as with impaired insulin secretion (15,37). Here we show that improvement in insulin-mediated suppression of plasma FFA after an oral lipid load is associated with lower 120-min OGTT-glucose after a 1-year lifestyle intervention. Such associations cannot prove causation; however, previous works have shown that insulin resistance of adipose tissue resulting in postprandial elevation of plasma FFAs may be an important pathophysiological link between obesity and altered glucose metabolism (11). Using FFA tracer during euglycemic normal and hyperinsulinemic clamps, Brassard et al. (35) demonstrated that subjects at high risk of developing type 2 diabetes had increased plasma FFA turnover at high—but not at low—insulin concentrations during intravenous fat infusion compared with control subjects with the same BMI and waist circumference. Using tracer methodologies, Normand-Lauzière et al. (10) also found a progressive increase in postprandial plasma FFA turnover from normal glucose tolerance to prediabetes and to type 2 diabetes. The experimental evidence maintains that increased plasma FFA has a role in the development of insulin resistance and β-cell dysfunction in humans (12). Our results suggest that one potential mechanism of lifestyle intervention-mediated improvement in insulin sensibility could be the improvement in FFA metabolism.
Strengths and weaknesses
Of all subjects, 81% completed the first year of intervention. This retention rate is comparable to those of pharmacological weight loss studies; for example, the ADAGIO-Lipids study, which evaluated the effects of rimonabant, a CB1 receptor antagonist, in viscerally obese dyslipidemic men, also reported a retention rate of 80% after 1 year of treatment (20). Compared with previous publications addressing the effects of lifestyle interventions on FFA metabolism, the current study included a larger number of participants who were exempt from type 2 diabetes medications. We presented the results of two complementary tests assessing plasma FFAs and plasma insulin concentrations after oral lipid and glucose loads, allowing us to address the insulin-mediated inhibition of postprandial FFA concentrations. The precise mechanistic significance of FFA ISIs after oral glucose and lipid loads, however, needs to be validated in future studies.
One year of a combined healthy eating and physical activity intervention resulted in an improvement in plasma FFA/insulin homeostasis after oral glucose and lipid loads. Improvement in FFA metabolism was associated with improved glucose tolerance, independent of changes in visceral or total adiposity. Postprandial plasma FFA may be a mechanistic link between obesity and impaired glucose homeostasis.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR). A.-L.B. is a postdoctoral fellow supported by a fellowship from Agiràdom (Meylan, France) and the Rhône-Alpes region (France). J.-A.N. is a postdoctoral fellow supported by a fellowship from the CIHR, the Fondation Bullukian, and the Institut Appert. P.P. is a senior clinical researcher of the Fonds de recherche en santé du Québec (FRSQ). A.C.C. is the CIHR-GSK Research Chair in Diabetes.
No potential conflicts of interest relevant to this article were reported.
A.-L.B. and G.B. analyzed and interpreted the results and wrote the paper. J.-A.N., J.S., and A.C.C. interpreted the results and revised the manuscript. N.A. designed and conducted the study. A.T., J.B., and P.P. designed the study, interpreted the results, and revised the manuscript. J.-P.D. designed and conducted the study, interpreted the results, and revised the manuscript. J.-P.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2353/-/DC1.
References
- 1.Borel AL, Nazare JA, Smith J, et al. Visceral and not subcutaneous abdominal adiposity reduction drives the benefits of a 1-year lifestyle modification program. Obesity (Silver Spring) 2012;20:1223–1233 [DOI] [PubMed] [Google Scholar]
- 2.Smith JD, Borel AL, Nazare JA, et al. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab 2012;97:1517–1525 [DOI] [PubMed] [Google Scholar]
- 3.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 [DOI] [PubMed] [Google Scholar]
- 4.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care 2005;28:2322–2325 [DOI] [PubMed] [Google Scholar]
- 5.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 1993;42:1567–1573 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am 2008;37:635–646, viii–ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauriege P, Galitzky J, Berlan M, Lafontan M. Heterogeneous distribution of beta and alpha-2 adrenoceptor binding sites in human fat cells from various fat deposits: functional consequences. Eur J Clin Invest 1987;17:156–165 [DOI] [PubMed] [Google Scholar]
- 9.Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes 2002;51:755–761 [DOI] [PubMed] [Google Scholar]
- 10.Normand-Lauzière F, Frisch F, Labbé SM, et al. Increased postprandial nonesterified fatty acid appearance and oxidation in type 2 diabetes is not fully established in offspring of diabetic subjects. PLoS One 2010;5:e10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentier AC. Postprandial fatty acid metabolism in the development of lipotoxicity and type 2 diabetes. Diabetes Metab 2008;34:97–107 [DOI] [PubMed] [Google Scholar]
- 12.Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 2011;300:E255–E262 [DOI] [PubMed] [Google Scholar]
- 13.Albu JB, Heilbronn LK, Kelley DE, et al. Look AHEAD Adipose Research Group Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes 2010;59:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–1112 [DOI] [PubMed] [Google Scholar]
- 15.Borel AL, Nazare JA, Smith J, et al. Improvement in insulin sensitivity following a 1-year lifestyle intervention program in viscerally obese men: contribution of abdominal adiposity. Metabolism 2012;61:262–272 [DOI] [PubMed] [Google Scholar]
- 16.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription 5th edition. Baltimore, Williams & Wilkins, 1995
- 17.Lohman TG, Roche A, Martorell R. Anthropometric Standardization Reference Manual, Champaign, Human Kinetics, 1988 [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 19.Ferland M, Després JP, Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr 1989;61:139–148 [DOI] [PubMed] [Google Scholar]
- 20.Després JP, Ross R, Boka G, Alméras N, Lemieux I, ADAGIO-Lipids Investigators Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol 2009;29:416–423 [DOI] [PubMed] [Google Scholar]
- 21.Paré A, Dumont M, Lemieux I, et al. Is the relationship between adipose tissue and waist girth altered by weight loss in obese men? Obes Res 2001;9:526–534 [DOI] [PubMed] [Google Scholar]
- 22.Dole VP, Meinertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem 1960;235:2595–2599 [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol 2008;45:147–150 [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 26.Couillard C, Bergeron N, Prud’homme D, et al. Postprandial triglyceride response in visceral obesity in men. Diabetes 1998;47:953–960 [DOI] [PubMed] [Google Scholar]
- 27.Couillard C, Bergeron N, Prud’homme D, et al. Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol 1999;19:2448–2455 [DOI] [PubMed] [Google Scholar]
- 28.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 1955;34:1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 30.Riepponen P, Marniemi J, Rautaoja T. Immunoturbidimetric determination of apolipoproteins A-1 and B in serum. Scand J Clin Lab Invest 1987;47:739–744 [PubMed] [Google Scholar]
- 31.Kelley DE, Kuller LH, McKolanis TM, Harper P, Mancino J, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care 2004;27:33–40 [DOI] [PubMed] [Google Scholar]
- 32.Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles JM, Wooldridge D, Grellner WJ, et al. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 2003;52:675–681 [DOI] [PubMed] [Google Scholar]
- 34.Carpentier AC, Frisch F, Cyr D, et al. On the suppression of plasma nonesterified fatty acids by insulin during enhanced intravascular lipolysis in humans. Am J Physiol Endocrinol Metab 2005;289:E849–E856 [DOI] [PubMed] [Google Scholar]
- 35.Brassard P, Frisch F, Lavoie F, et al. Impaired plasma nonesterified fatty acid tolerance is an early defect in the natural history of type 2 diabetes. J Clin Endocrinol Metab 2008;93:837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab 2011;13:366–373 [DOI] [PubMed] [Google Scholar]
- 37.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]


