Abstract
OBJECTIVE
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) have additive insulinotropic effects when coadministered in health. We aimed to determine whether GIP confers additional glucose lowering to that of GLP-1 in the critically ill.
RESEARCH DESIGN AND METHODS
Twenty mechanically ventilated critically ill patients without known diabetes were studied in a prospective, randomized, double-blind, crossover fashion on 2 consecutive days. Between T0 and T420 minutes, GLP-1 (1.2 pmol/kg · min−1) was infused intravenously with either GIP (2 pmol/kg · min−1) or 0.9% saline. Between T60 and T420 minutes, nutrient liquid was infused into the small intestine at 1.5 kcal/min.
RESULTS
Adding GIP did not alter blood glucose or insulin responses to small intestinal nutrient. GIP increased glucagon concentrations slightly before nutrient delivery (P = 0.03), but not thereafter.
CONCLUSIONS
The addition of GIP to GLP-1 does not result in additional glucose-lowering or insulinotropic effects in critically ill patients with acute-onset hyperglycemia.
Hyperglycemia occurs frequently in the critically ill, even in the absence of pre-existing diabetes (1). Although insulin is an effective treatment, its use confers an increased risk of hypoglycemia, which is associated with increased mortality (1,2). In health, but not in patients with type 2 diabetes, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) have additive insulinotropic effects when coadministered, without increasing the risk of hypoglycemia (3,4). The objective of this study was to determine whether the addition of GIP to GLP-1 would result in additional glucose-lowering in enterally fed, critically ill patients without pre-existing diabetes.
RESEARCH DESIGN AND METHODS
Subjects
Mechanically ventilated critically ill patients were studied. Patients with known diabetes, HbA1c >6.5% (48 mmol/mol), previous small intestinal surgery, or evidence of acute or chronic pancreatitis were excluded.
Protocol
In this prospective, double-blind, crossover study performed on consecutive days, patients were randomly assigned to receive either the intervention (GIP at 2 pmol/kg · min−1) or control (intravenous [IV] 0.9% saline) in addition to IV GLP-1 at 1.2 pmol/kg · min−1. Patients were fasted for 6 h, and exogenous insulin was ceased 3 h prior to each study. Synthetic GIP and GLP-1 (Bachem, Weil am Rhein, Germany) were reconstituted in 0.9% saline by the Royal Adelaide Hospital Pharmacy, which undertook computer generated randomization. The investigators remained blinded to treatment allocation. Study solutions were administered via low absorbance tubing. Between T60 and T420, a mixed liquid nutrient (Ensure Plus; Abbott Nutrition) was infused into the small intestine at 1.5 kcal/min (5). Arterial blood samples were obtained during each study.
This study protocol was approved by the Research Ethics Committee of the Royal Adelaide Hospital with informed consent obtained from patients’ next of kin. The protocol was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12611000735954).
Data analysis
Blood glucose concentrations were measured immediately using a blood gas analyzer (ABL800 FLEX; Radiometer). Serum insulin was measured by ELISA, while plasma glucagon, GIP, and GLP-1 were measured using radioimmunoassays.
Statistical analysis
Based on previous data, 20 completed subjects were required (5). Data are presented as mean ± SD or median (range) as appropriate. As data for inferential analysis were normally distributed, comparisons were made using the Student paired t test. Because the islet cell effects of GIP are glucose-dependent (6,7), the effect of GIP in the subgroup of patients in whom peak blood glucose concentrations were >10 mmol/L was also assessed.
RESULTS
Twenty patients (age 52 ± 16 years; sex: 12 male; HbA1c: 5.7% [range 4.8–6.5%], 39 [29–48)] mmol/mol; Acute Physiology and Chronic Health Evaluation II score: 17 [9–30]; days in ICU when studied: 6 [2–15] days; admission diagnostic group: sepsis, 9; trauma, 7; asthma, 2; neurological, 2) were studied.
Blood glucose
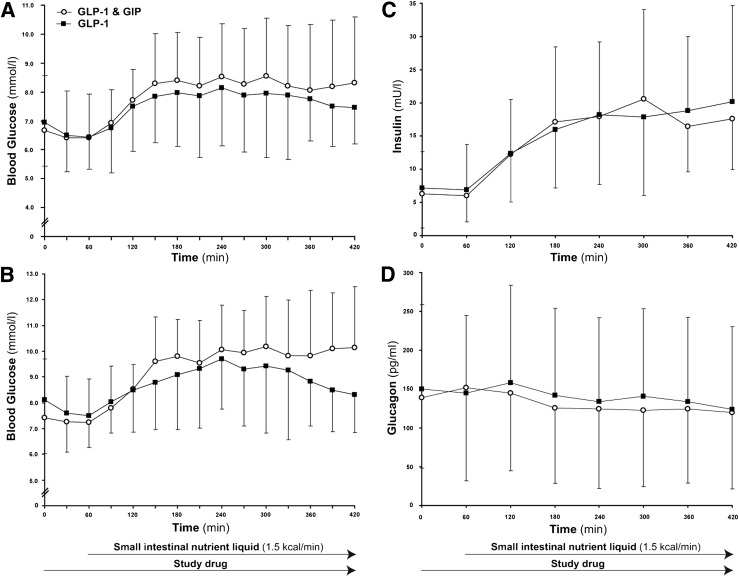
Baseline blood glucose concentrations were similar on both days (P = 0.38). In nine patients, the peak blood glucose was >10 mmol/L at least on one study day. GIP had no effect on fasting, peak, or overall glycemic response in either the entire cohort (Fig. 1A) or the group with glycemic excursions >10 mmol/L (Fig. 1B).
Figure 1.
Effects of the coinfusion of GIP and GLP-1 (open circles) when compared with GLP-1 alone (filled squares). A: Peak (P = 0.43) and overall glycemic response to nutrient infusion (P = 0.34) were similar. B: Overall glycemia in the subgroup (P = 0.55) of patients with excursions >10 mmol/L was comparable (n = 9). C: Serum insulin concentrations were similar (P = 0.86). D: Overall plasma glucagon concentrations were comparable (P = 0.39). Data are mean ± SD. Area under the curve calculated using the trapezoidal rule. Comparisons using paired Student t tests; n = 20 for all except the subgroup described.
Insulin
Baseline insulin concentrations were similar on the 2 days (P = 0.81). GIP had no effect on fasting or overall insulin concentrations in either the entire cohort (Fig. 1C) or the subgroup of patients with glycemic excursions >10 mmol/L (P = 0.96).
Glucagon
Baseline glucagon concentrations were similar on the 2 days (P = 0.48). The addition of GIP led to a minor increase in glucagon before nutrient was given (T60: 152 ± 93 vs. 145 ± 113 pg/mL; P = 0.03), but there was no difference at the end of the infusion (P = 0.55). Overall, glucagon responses to the infusion were also comparable regardless of GIP administration (Fig. 1D). There was no difference in the glucagon response between the 2 days in patients with glycemic excursions >10 mmol/L (P = 0.38).
GIP and GLP-1
Baseline GIP and GLP-1 concentrations were similar on both days (P = 0.79 and 0.35, respectively). While there was a sustained twofold rise above fasting plasma GIP concentrations in response to intraduodenal nutrient during the control infusion (P < 0.001), GIP concentrations were fourfold greater during GIP infusion (P < 0.001). On both days, GLP-1 concentrations increased ∼40% in response to GLP-1 infusions, with the addition of GIP having no effect on plasma GLP-1 concentrations (P = 0.88).
CONCLUSIONS
This study indicates that IV administration of GIP to GLP-1 does not lower blood glucose concentrations in the critically ill more than exogenous GLP-1 alone. In keeping with this, the addition of GIP does not affect insulin or suppress glucagon response to enteral nutrient. While these observations are at variance with the outcome of studies performed in healthy volunteers (3,4), they are consistent with the reported effects of concurrent administration of GLP-1 and GIP in patients with type 2 diabetes (4,8).
The doses of GIP and GLP-1 used were based on previous studies. We have reported that GLP-1 at 1.2 pmol/kg · min−1 attenuates, but does not abolish, the glycemic response to enteral nutrition in critically ill patients with and without type 2 diabetes (5,9,10). While this is the first report on the effects of GIP in the critically ill, in healthy volunteers, the coinfusion of GIP at doses >1 pmol/kg · min−1 is additive to the insulinotropic effect of GLP-1 (3,4). We confirmed that GIP concentrations reached pharmacological levels, so it appears unlikely that the negative outcome of our study reflects an insufficient dose of GIP.
The reason GIP failed to lower glucose concentrations may relate to antecedent glycemic control, as the insulinotropic effect of GIP is markedly attenuated in various conditions associated with chronic hyperglycemia, such as latent autoimmune diabetes, chronic pancreatitis, and monogenic diabetes (11,12). Moreover, in vivo, hyperglycemia acutely reduces the expression of GIP receptors on β-cells, providing a plausible explanation as to why even pharmacological concentrations of GIP are not insulinotropic during chronic hyperglycemia (13). Højberg et al. (14) recently reported that the insulinotropic property of GIP increased several-fold following 4 weeks of near-normal glycemia in patients with type 2 diabetes. These data and ours indicate that the response of the β-cell to pharmacological doses of GIP is acutely susceptible to the effects of antecedent glycemia. In both healthy humans and patients with pre-existing hyperglycemia and hyperglucagonemia, exogenous GIP is reportedly glucagonotropic, which would counteract any insulinotropic effect (6,8,11,15). However, we observed a modest glucagontropic effect during fasting (T60), which is unlikely to be of clinical significance.
There are limitations to our study. We cannot exclude the possibility that GIP alone lowers glycemia in the critically ill compared with placebo. Our cohort was relatively small and heterogeneous, and the exposure to exogenous GIP was relatively short (7 h). It therefore remains possible, albeit intuitively unlikely, that an insulinotropic effect would be apparent during more prolonged GIP exposure.
In conclusion, this study indicates that the addition of GIP to exogenous GLP-1 does not yield any additional glucose-lowering effect, nor potentiate insulin secretion, in critically ill patients. The implication is that the insulinotropic capacity of GIP, unlike GLP-1, is diminished in critically ill patients with hyperglycemia, as is the case in patients with type 2 diabetes.
Acknowledgments
This study was supported by a research grant from the National Health and Medical Research Council of Australia (to A.M.D., Project 1025648).
J.J.M. has received consulting or lecture fees from the following companies: AstraZeneca, Berlin-Chemie, BMS, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, Novartis, Roche, and Sanofi. M.H. has participated in advisory boards and/or symposia for Novo Nordisk, Sanofi, Novartis, Eli Lilly, MSD, Boehringer Ingelheim, Satiogen, and AstraZeneca and received honoraria for this activity. No other potential conflicts of interest relevant to this article were reported.
M.Y.L. was responsible for acquisition of data, statistical analysis, and drafting the manuscript. J.D.F. and K.S. contributed to subject enrollment. M.J.C., C.K.R., J.J.M., and M.H. contributed to the study design and critical revision of the manuscript for important intellectual content. M.M.U., M.J.S., and A.V.Z. contributed to the acquisition of data. A.M.D. was responsible for the study conception and design, obtaining funding, acquisition of data, interpretation, and manuscript review. A.M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013. This study was also presented at the ANZICS/ACCCN Intensive Care Annual Scientific Meeting, Adelaide, Australia, 25–27 October 2012.
Footnotes
Clinical trial reg. no. ACTRN12611000735954, www.anzctr.org.au.
References
- 1.Deane AM, Horowitz M. Dysglycaemia in the critically ill—significance and management. Diabetes Obes Metab 2013;15:792–801 [DOI] [PubMed] [Google Scholar]
- 2.Finfer S, Liu B, Chittock DR, et al. NICE-SUGAR Study Investigators Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–1118 [DOI] [PubMed] [Google Scholar]
- 3.Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab 1993;76:912–917 [DOI] [PubMed] [Google Scholar]
- 4.Elahi D, McAloon-Dyke M, Fukagawa NK, et al. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept 1994;51:63–74 [DOI] [PubMed] [Google Scholar]
- 5.Deane AM, Chapman MJ, Fraser RJ, Burgstad CM, Besanko LK, Horowitz M. The effect of exogenous glucagon-like peptide-1 on the glycaemic response to small intestinal nutrient in the critically ill: a randomised double-blind placebo-controlled cross over study. Crit Care 2009;13:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier JJ, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 2003;46:798–801 [DOI] [PubMed] [Google Scholar]
- 7.Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011;60:3103–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mentis N, Vardarli I, Köthe LD, et al. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes 2011;60:1270–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deane AM, Summers MJ, Zaknic AV, et al. Exogenous glucagon-like peptide-1 attenuates the glycaemic response to postpyloric nutrient infusion in critically ill patients with type-2 diabetes. Crit Care 2011;15:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deane AM, Chapman MJ, Fraser RJ, et al. Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med 2010;38:1261–1269 [DOI] [PubMed] [Google Scholar]
- 11.Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab 2011;300:E1038–E1046 [DOI] [PubMed] [Google Scholar]
- 12.Vilsbøll T, Knop FK, Krarup T, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. J Clin Endocrinol Metab 2003;88:4897–4903 [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Livak MF, Bernier M, et al. Ubiquitination is involved in glucose-mediated downregulation of GIP receptors in islets. Am J Physiol Endocrinol Metab 2007;293:E538–E547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009;52:199–207 [DOI] [PubMed] [Google Scholar]
- 15.Chia CW, Carlson OD, Kim W, et al. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 2009;58:1342–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]