Abstract
OBJECTIVE
To examine the long-term effects of type 1 diabetes treatment, metabolic control, and complications on health-related quality of life (HRQOL).
RESEARCH DESIGN AND METHODS
A total of 1,441 participants, initially 13–39 years of age, were followed for an average of 23.5 years as part of the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study. The Diabetes Quality-of-Life questionnaire (DQOL) was administered annually during DCCT and every other year during EDIC. Biomedical data, including HbA1c levels, exposure to severe hypoglycemia, intercurrent psychiatric events, and development of diabetes complications were collected at regular intervals throughout the follow-up.
RESULTS
Mean total DQOL scores were not significantly different between the former DCCT intensive and conventional treatment groups (DCCT baseline, 78 ± 8 vs. 78 ± 9; EDIC year 17, 75 ± 11 vs. 74 ± 11). Over the course of the study, a drop of ≥5 points in DQOL score from DCCT baseline maintained on two successive visits occurred in 755 individuals and was associated with increased HbA1c, albumin excretion rate, mean blood pressure, BMI, and occurrence of hypoglycemic events requiring assistance. Lower DQOL scores after 23.5 years of follow-up were associated with prior development of retinopathy (P = 0.0196), nephropathy (P = 0.0019), and neuropathy (P < 0.0001) as well as self-reported chest pain (P = 0.0004), decreased vision in both eyes (P = 0.0005), painful paresthesias (P < 0.0001), recurrent urinary incontinence (P = 0.0001), erectile dysfunction (P < 0.0001), and history of psychiatric events (P < 0.0001).
CONCLUSIONS
Among DCCT/EDIC participants, worsening metabolic control, serious diabetes complications and their associated symptoms, and development of psychiatric conditions led to decreased HRQOL.
The long-term impact of type 1 diabetes mellitus (T1DM), associated complications, and diabetes treatment modalities on health-related quality of life (HRQOL) are poorly understood. Although numerous studies of T1DM have evaluated HRQOL, many have been small and/or cross-sectional, and they typically involve only brief periods of follow-up (1–6). These previous studies have suggested that before the onset of chronic complications, patients with T1DM experience relatively small decrements in their HRQOL. Studies examining the effects of treatment, including comparisons of insulin type, frequency of injections, and pump use, have not shown consistent effects on HRQOL (1,2,5,7–10). Similarly, variations in level of glycemic control and/or frequency of exposure to severe hypoglycemia have not been consistently associated with HRQOL level (2,5,9). A small number of largely cross-sectional studies have indicated that diabetes complications are more strongly and consistently associated with lower quality of life (7, 11–14). However, there is little information about the long-term effects of treatment modalities or course of illness based on prospective follow-up of a well-characterized cohort of patients evaluated longitudinally over an extended time frame. Obtaining information from diabetes patients about their experiences over many years of illness can help guide clinicians and educators in developing interventions to address patient concerns about this personally demanding condition (2,5,15).
The Diabetes Control and Complications Trial (DCCT) and its long-term natural history follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC), provide an opportunity to address this gap in our understanding by examining the long-term impact of diabetes treatment, acute metabolic changes, and serious complications on HRQOL. Because of the possible deleterious effects of intensive diabetes management on the personal and emotional life of the participants, the DCCT developed the Diabetes Quality of Life (DQOL) scale (15), a measurement tool with greater sensitivity to the effects of diabetes treatment than was available in the generic measures of quality of life that were available at the time. The DQOL was tested for reliability and validated (15–17) and was subsequently used as the principal quality-of-life outcome assessment during the DCCT and EDIC (2,18,19). It has now become a commonly used measure of patient perceptions of their quality of life with translation into multiple languages and use with a wide variety of patients (20).
In this article, we report on observations that have been made over an average of >23 years on the impact on HRQOL. We address three primary research questions: 1) does prior DCCT assignment to conventional therapy in comparison with intensive therapy adversely impact HRQOL? 2) does worse glycemic control or episodes of severe hypoglycemia adversely impact HRQOL? and 3) does the development of long-term, advanced complications of diabetes adversely impact HRQOL? In addition to these primary research questions, we also examined the association of HRQOL with psychiatric events and symptomatic manifestations of diabetes complications.
RESEARCH DESIGN AND METHODS
Study sample
Between 1983 and 1989, 1,441 participants with T1DM, 13–39 years of age, were enrolled in the DCCT. The DCCT consisted of two cohorts: the primary prevention cohort had diabetes for 1–5 years, no retinopathy, and urinary albumin excretion <40 mg/24 h, and the secondary intervention cohort had diabetes for 1–15 years, very mild to moderate nonproliferative retinopathy, and urinary albumin excretion ≤200 mg/24 h at baseline (18). Approximately one-half of the subjects (N = 711) were randomly assigned to intensive therapy, and the remainder (N = 730) were assigned to conventional therapy. The treatment groups maintained a separation of median HbA1c levels of ∼2 percentage points (7.1 vs. 9.0%; 54.1 vs. 74.9 mmol/mol) during the 6.5-year average DCCT follow-up (18).
Since it had been shown to be highly effective in reducing diabetic microvascular complications, intensive therapy was recommended for all participants when the DCCT ended in 1993 (18,19). Participants were then returned to their own health care providers for diabetes care. In 1994, 1,375 (96%) of the 1,428 surviving members of DCCT volunteered to participate in EDIC for annual observational follow-up (19). This report incorporates data through EDIC year 17 (2010), representing an average of 23.5 years of follow-up from randomization into DCCT. By EDIC year 17, 1,287 subjects continued to participate in EDIC, and of these, 1,177 (91%) completed the DQOL survey. Ninety-five participants had died by the end of EDIC year 17. For purposes of these analyses, two subjects with acute, temporary renal failure unrelated to diabetes were excluded.
Nonparticipants, including those who died, did not differ from participants in most characteristics at DCCT baseline including sex, age, education, blood pressure, and cholesterol. Nonparticipants had significantly higher HbA1c levels and a higher frequency of current cigarette smokers (Supplementary Table 1). Furthermore, 48% of participants were in the conventional treatment group compared with 61% of nonparticipants.
Quality-of-life assessment
The DQOL is a self-administered multiple-choice 46-item assessment that has been described in detail (15–17). The DQOL has four primary subscales (satisfaction, impact, diabetes worry, and social/vocational worry) that assess different aspects of quality of life. The scoring system yields scale scores that range from 0 (lowest quality of life) to 100 (highest quality of life), identical to the procedure for scoring the Medical Outcome Survey 36-item short-form health survey (SF-36) quality-of-life measure (21–23). Total DQOL scores are presented in the Results section unless otherwise stated, and scores on the four subscales are presented in Supplementary Table 2.
Psychometric studies have indicated that the overall DQOL measure has excellent internal consistency (Cronbach α, 0.83–0.92) for both adults and adolescents (15–17). Test-retest reliability over an average period of 9 days was 0.92 for the overall measure (17). The DQOL has been shown to have convergent validity with conceptually relevant measures of well-being, psychiatric symptoms, and adjustment to illness (17). In addition, the DQOL discriminates between patients with different numbers of clinically evident complications (17) and is sensitive to different therapies for T2DM (17,24,25) and to a change in therapy for T1DM (i.e., pancreatic transplantation) (25). For this article, the primary outcome was the total DQOL score.
Past research has suggested that a difference of five points on the total DQOL score represents a clinically meaningful difference in HRQOL (15–17,20,24,25). Consequently, this parameter was used in our longitudinal analyses of the effects of time-dependent predictors on decrease in quality of life. Specifically, an event was defined as a drop from baseline ≥5 points on two consecutive evaluations. We used a sustained decrease in DQOL score to confirm that a change occurred over a substantial time frame and was not transitory. We have used this same approach for other outcomes such as nephropathy, where a measure like albumin excretion rate (AER) can change up and down over time (26). The DQOL was administered annually throughout the DCCT and biannually during EDIC.
Biomedical evaluations and assessment of diabetes complications
The methods and scheduling of physical examinations, outcomes assessments, and laboratory measurements have been previously described in detail and remained consistent throughout DCCT and EDIC (18,19,26–28). During the DCCT (quarterly) and EDIC (annually), glycated hemoglobin values were measured in a central laboratory by high-performance liquid chromatography (18,19). Retinopathy, assessed during EDIC years 11–14 by 7-field stereoscopic fundus photography according to the DCCT/EDIC protocol (26), was defined for these analyses as the presence of proliferative diabetic retinopathy (PDR) or worse, and/or a history of panretinal scatter-photocoagulation (laser) therapy. In addition, visual acuity (VA) was assessed to determine best corrected vision in the best and worst eye. Nephropathy was defined in this study as having any AER ≥300 mg/24 h through EDIC year 16 or end-stage renal disease (ESRD), defined as treatment with dialysis or transplantation for chronic renal failure (18,19,26). In EDIC years 13 to 14, board-certified neurologists and electromyographers conducted neurological evaluations and electrodiagnostic studies on all willing participants using the same protocol as was used in DCCT to determine the presence of clinical neuropathy (27,28).
The occurrence of severe hypoglycemia was documented quarterly during the DCCT and within 3 months of the annual visit during EDIC. It was defined in two ways: 1) any event requiring the assistance of another person, with either a blood glucose <50 mg/dL (2.78 mmol/L) and/or subsequent reversal of symptoms with oral carbohydrate, subcutaneous glucagon, or intravenous glucose; or 2) the same criteria plus unconsciousness, seizure, or coma (18,29). Twenty-seven percent of severe hypoglycemic episodes involved coma or seizure (30). In this article, the episodes of severe hypoglycemia requiring assistance and those also resulting in seizure or coma were analyzed separately.
Symptomatic data and changes since the last annual visit were self-reported each year during EDIC for the following symptoms: chest pain, decreased vision, paresthesias in hands/feet, recurrent urinary incontinence, and impotence.
Intercurrent psychosocial events and psychiatric symptoms
Information about intercurrent psychosocial events and psychiatric history was reported quarterly during the DCCT and annually during EDIC based on subject interviews. Information about education level, marital status, psychiatric treatment, psychiatric hospitalization, and suicide attempts was documented. A psychiatric event was defined as at least one occurrence in EDIC accompanied by inpatient or outpatient treatment for any psychiatric event during the same year.
Statistical analyses
Demographic and clinical characteristics were compared using the Wilcoxon rank-sum test to evaluate treatment group differences for ordinal and numeric variables (31). The contingency χ2 test was used for categorical variables; when the sample size was small, the Fisher exact test was used (31).
Cox proportional hazard models were used to determine the effects of demographic and biomedical assessments on the risk of a sustained five-point drop in total DQOL over two consecutive assessments during DCCT and EDIC. Each characteristic was modeled separately as a time-dependent covariate adjusting for its baseline value as well as sex, baseline age, and baseline mean years of education. Since QOL was only measured during odd years in EDIC, each model was stratified for calendar year of randomization, allowing subjects who entered into the study during the same calendar year to have the same visit sequence. Updated mean values are time-weighted, running means up to each study visit in the DCCT and EDIC.
Separate ANCOVA models were used to assess the relationship between total DQOL and both complication status and functional symptoms by year 17 of EDIC. Adjustments were made for sex, baseline age, baseline mean years of education, and baseline DQOL score. Least square means and SEs were compared for participants with and without the characteristic or complication of interest. Statistical analyses were performed using the SAS V 9.2 statistical analysis software (SAS Institute, Cary, NC).
RESULTS
Clinical characteristics of the participants
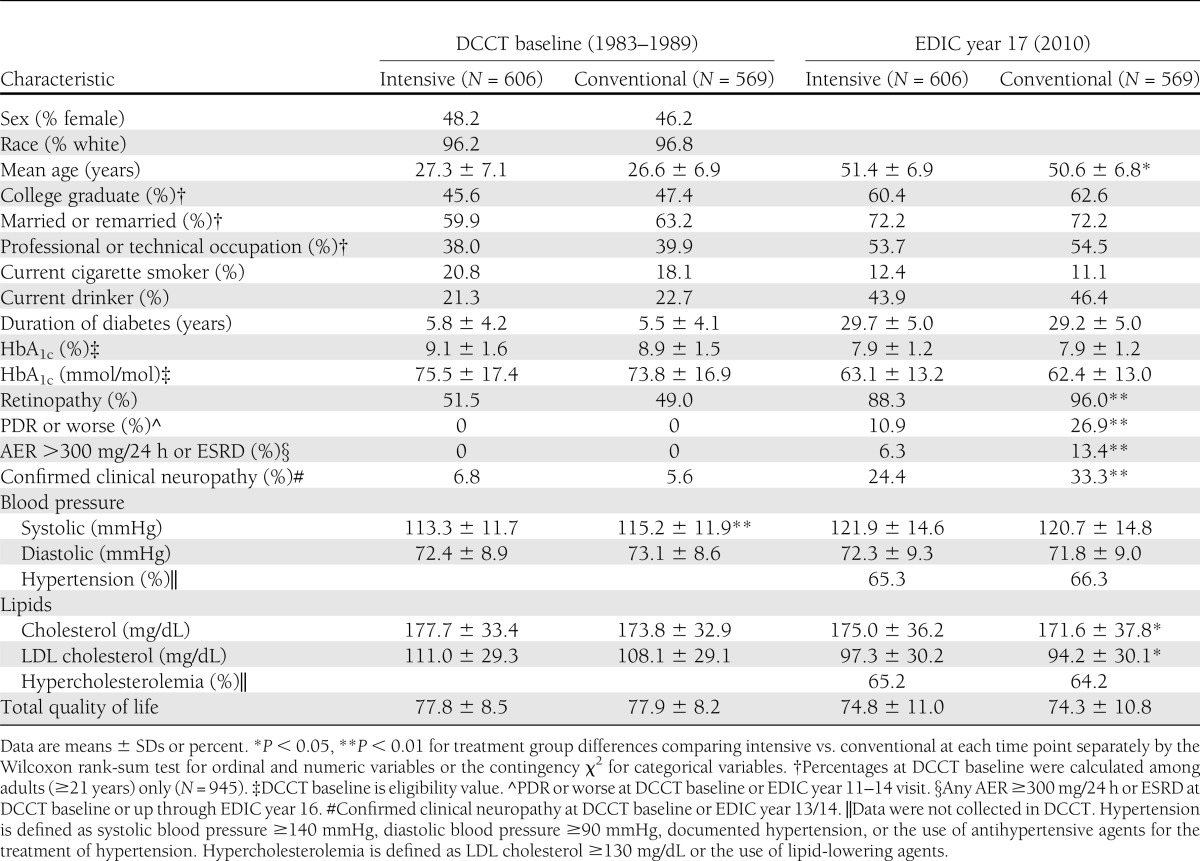
There were no significant differences between treatment groups in demographic or clinical characteristics at baseline, other than a clinically insignificant difference in systolic blood pressure (Table 1). At the 17-year EDIC follow-up, after an average of 23.5 years, 66% of all DCCT/EDIC participants had developed hypertension, 65% hypercholesterolemia, and 19% proliferative diabetic retinopathy or worse. The lower prevalence of retinopathy and nephropathy in the former intensive treatment group at EDIC year 17 reflects the previously published salutary effects of intensive therapy (19).
Table 1.
Characteristics of participants who completed the DQOL in EDIC year 17
Impact of prior treatment group assignment on HRQOL
No differences in total DQOL or DQOL subscale scores were found between the intensive and conventional groups at any time points. Mean total DQOL scores were not significantly different between the former DCCT intensive and conventional treatment groups at baseline (78 ± 8 vs. 78 ± 9), DCCT closeout (78.1 ± 8.8 vs. 78.2 ± 9.4), or EDIC year 17 (75 ± 11 vs. 74 ± 11). The group as a whole did not show a significant trend for decreasing DQOL scores over time (Table 1 and Supplementary Table 2).
There were 755 (52%) DCCT/EDIC participants who had a DQOL event (defined as a decrease in DQOL from baseline of ≥5 points on two consecutive evaluations) by EDIC year 17, with 293 (39%) occurring during DCCT and 462 (61%) during EDIC. There were no significant treatment group differences (P = 0.9339). Compared with females, males had a 13.8% lower risk of reaching a DQOL end point (hazard ratio 0.862 [95% CI 0.744–0.999]; P = 0.0484).
Impact of glycemic control and hypoglycemic episodes on HRQOL
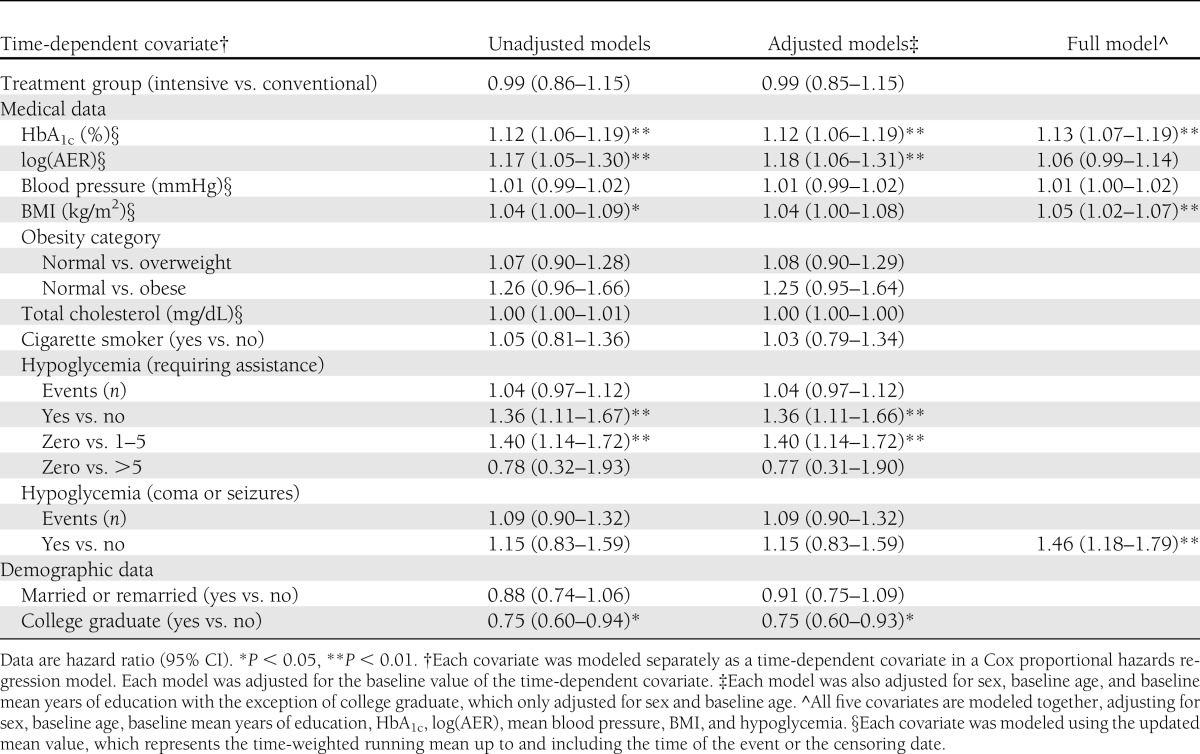
Higher values of HbA1c, log(AER), mean blood pressure, and BMI were all associated with a sustained drop of ≥5 points in DQOL score, before and after adjustment for sex, age, and mean years of education at DCCT baseline (Table 2). Subjects who experienced severe hypoglycemic events had a 36% higher risk of having a ≥5-point decrease in DQOL than those without such events. Analyses using the more stringent definition of severe events that included those that led to unconsciousness, coma, or seizure did not reveal an impact on DQOL scores. The effects of hypoglycemia, HbA1c, and BMI were significant even after adjustments of other covariates including log(AER) and mean blood pressure (Table 2).
Table 2.
Association of time-dependent covariates with risk of a sustained drop of ≥5 points in total DQOL since DCCT baseline
Impact of advanced complications on HRQOL
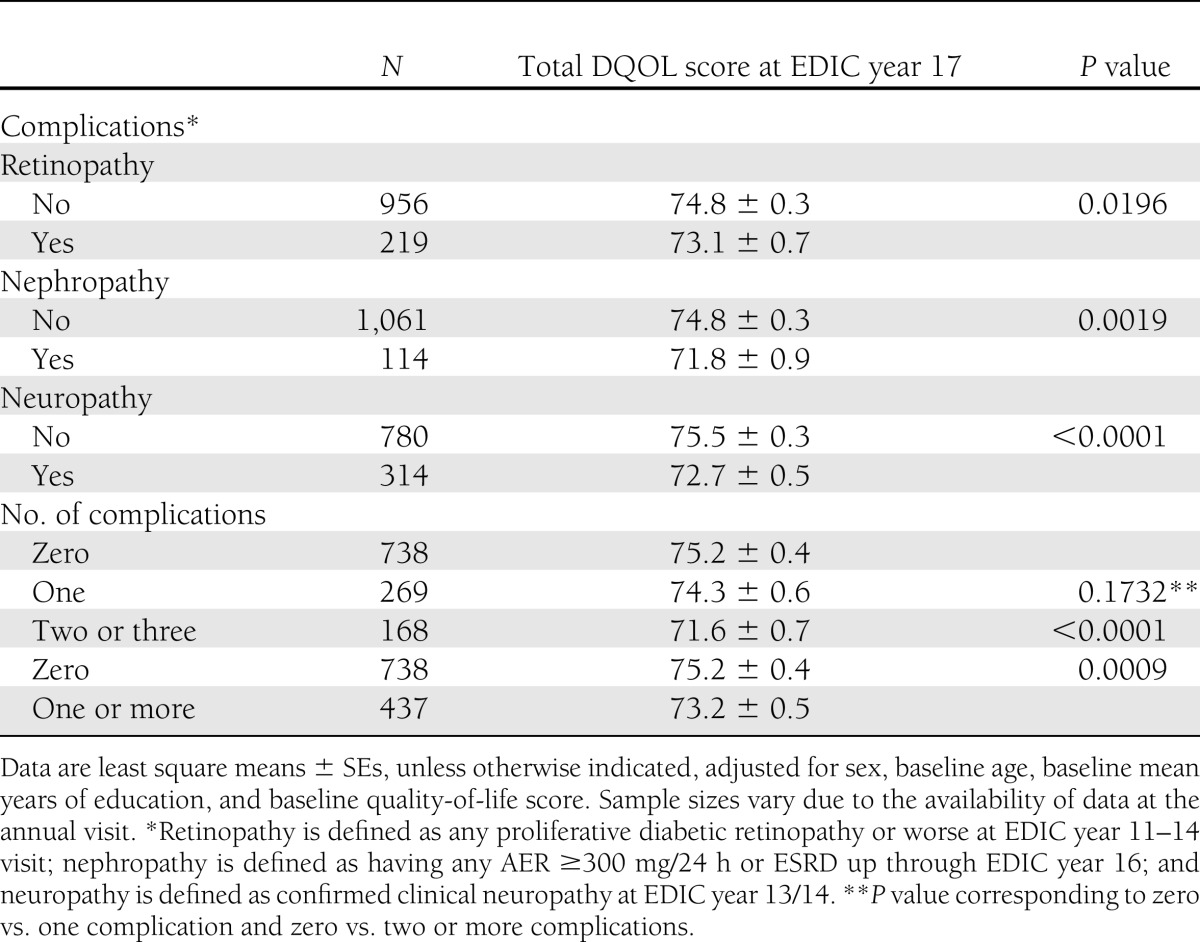
Development of advanced stages of retinopathy (P = 0.0196), nephropathy (P = 0.0019), and confirmed clinical neuropathy (P < 0.0001) were associated with lower total DQOL scores at year 17 of EDIC (an average of 23.5 years of follow-up), after adjusting for sex, age, and mean years of education at baseline (Table 3). There was a significant effect of the development of any complications (none vs. one or more) (P = 0.0009), with a linear trend showing that those subjects with all three complications had the lowest DQOL score of 71.6 ± 0.7 compared with no complications (P < 0.0001) (Table 3). By EDIC year 17, 13 subjects had chronic renal failure due to diabetes and were treated with either dialysis or transplantation. Their mean adjusted quality-of-life score was 74.6 ± 2.7.
Table 3.
Medical complications by total DQOL score at EDIC year 17 after an average of 23.5 years of follow-up
Relationship of self-reported symptoms and HRQOL
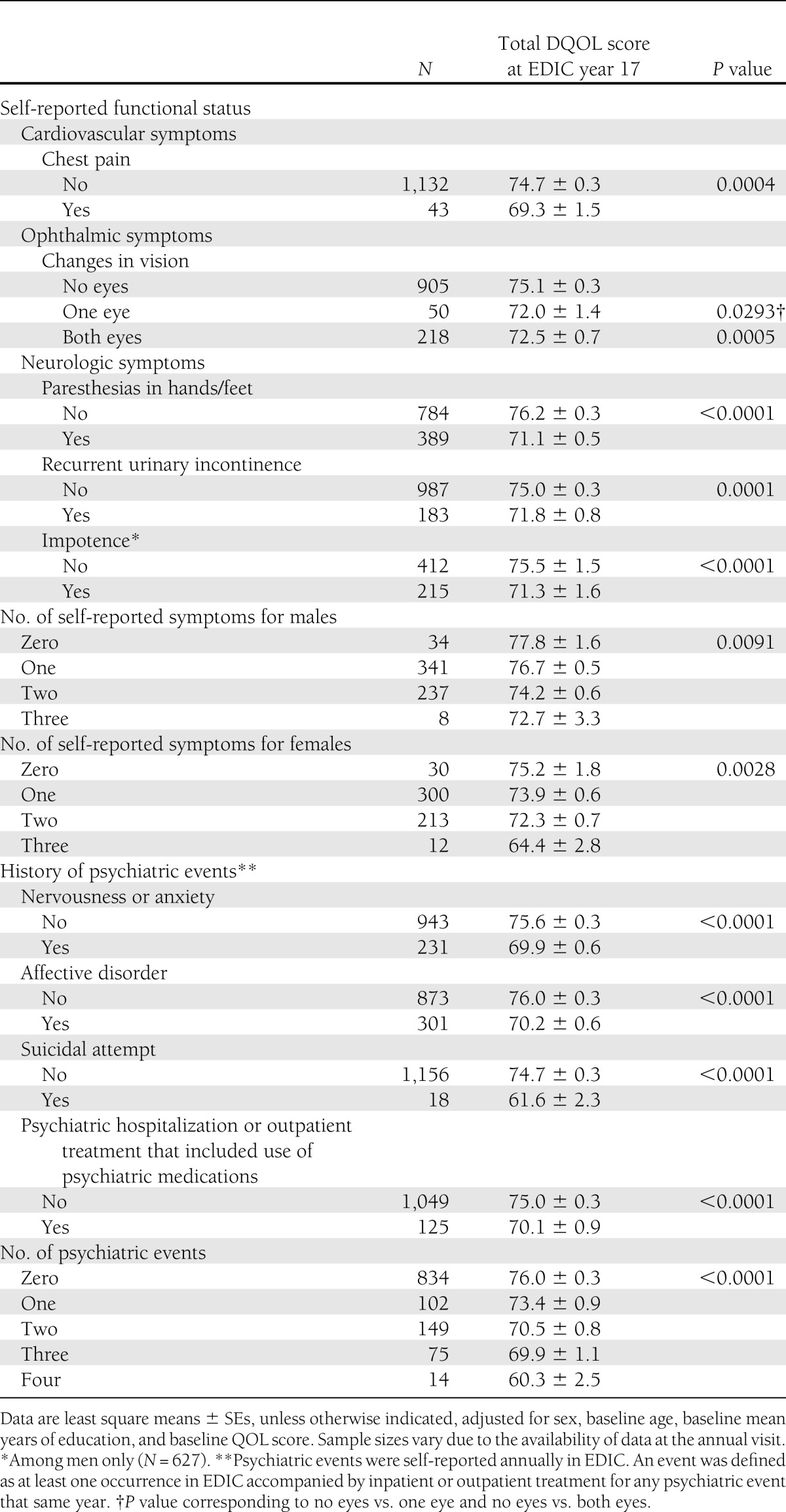
Self-reported symptoms associated with these complications at year 17 were also linked to decreased DQOL scores (Table 4). Specifically, chest pain (P = 0.0004), decreased vision in both eyes (P = 0.0005), paresthesias in hands or feet (P < 0.0001), urinary incontinence (P = 0.0001), and male erectile dysfunction (P < 0.0001) were associated with lower EDIC year 17 DQOL scores, after adjusting for sex, baseline age, baseline mean years of education, and baseline DQOL score. Among both men and women, increasing number of symptoms was associated with a lower DQOL score (men, P = 0.0091; women, P = 0.0028). Because the principal DCCT end point was progressive retinal disease, we also compared DQOL scores for participants at different levels of VA. The 14 participants with VA worse than 20/100 in the worse eye had a mean total DQOL score of 68.5 ± 7.9 compared with 74.9 ± 10.5, 74.6 ± 10.7, and 76.0 ± 12.5 for VA better or equal to 20/20, 20/20 to 20/40, and 20/40 to 20/100, respectively (P = 0.0661).
Table 4.
Self-reported functional status and history of psychiatric events occurring in EDIC by total DQOL score at EDIC year 17 after an average of 23.5 years of follow-up
Relationship of psychiatric events and HRQOL
Self-reported history of treated anxiety (P < 0.0001) and treated depression (P < 0.0001) were also associated with lower DQOL scores (Table 4). Two hundred subjects reported both anxiety and depression, and their mean adjusted quality-of-life score was 69.2 ± 0.7. Only a small number of participants were ever hospitalized for a psychiatric illness or reported suicidal attempts (27 attempts in 18 individuals), and both were associated with decreased DQOL scores (P < 0.0001). An increasing number of psychiatric events was reflected in a decreased DQOL score (P < 0.0001).
CONCLUSIONS
We present the longest prospective follow-up study of HRQOL in individuals with T1DM and describe their course of quality-of-life experiences over ∼25 years. The therapy applied to the intensive treatment group during DCCT and encouraged for the entire cohort during the 17 years of EDIC follow-up has resulted in relatively low rates of severe diabetes complications. Our findings support a salutary effect of the prevention and delay of complications on HRQOL (18,26–28,30,32). Decreases in HRQOL are particularly likely among patients with a higher prevalence of severe complications.
With regard to our first research question and consistent with our previous report at the end of the DCCT, there are no apparent adverse effects of intensive therapy on HRQOL (2). With regard to our second research question and in contrast to our initial HRQOL report based on an average of 6.5 years of treatment during DCCT (2), after an additional 17 years of follow-up, we now find an association between elevated HbA1c levels and severe hypoglycemia requiring assistance and a sustained decrease in DQOL scores.
With regard to our third research question, the development of advanced complications and the symptoms resulting from these complications, such as decreased VA, erectile dysfunction, and incontinence, were associated with decreases in HRQOL. Each of the three complications assessed (retinopathy, neuropathy, and nephropathy) were individually associated with lower DQOL scores in comparison with those without the complication; moreover, using a composite index, increasing numbers of advanced complications led to lower quality of life. Consistent with the assessment of complications, functional symptoms were also associated with lower quality of life. It is important to note that while almost all patients developed some degree of retinopathy (88% prior intensive group; 96% prior conventional group) and 19% required laser treatment or vitreoretinal surgery in at least one eye, only 4.8% (N = 52) had best corrected vision worse than 20/40 in one eye, and only 13 had best corrected vision worse than 20/100 in one eye. Patients with best corrected vision less than 20/100 in their worse eye had lower HRQOL than those with better VA.
Finally, the development of common psychiatric syndromes like depression is linked to HRQOL (5). This relationship was seen in the association of psychiatric conditions and their treatment on lower DQOL scores after an average of 23.5 years of follow-up.
The current study has unique strengths compared with previous studies of T1DM, including its long-term, consistent follow-up of a large cohort; detailed demographic and clinical information collected prospectively using standardized methods from relatively early in the course of illness; repeated measurement of HRQOL; the diversity of clinical outcomes; and the wide range of ages represented during the study period (13–65 years of age).
The study also has limitations that can affect the generalizability of its findings. These include the selection and self-selection of participants to enroll in a randomized clinical trial that required careful screening and adherence to a complex regimen over an extended period of time. Such patients have relatively high levels of motivation compared with the general population. Moreover, potential participants with psychosocial problems and limited social support would have been screened out. The DCCT/EDIC cohort had relatively high average socioeconomic status and education level of the group and was predominantly Caucasian. During DCCT, there was a high degree of assistance provided by the study nurse-coordinators and physicians. This was especially evident for the intensively treated group during DCCT. Such factors would tend to lessen the impact on HRQOL, so these findings may understate the effects in typical clinical populations, who also may have higher rates of severe complications.
Although our cohort, and in particular the intensive treatment group, received an unusual level of attention and support during the 6.5 years of the DCCT, during 17 of the average of 23.5 years represented by this study, the participants have been followed in typical practice settings with less support from the study. The return to usual care settings may increase the representativeness of this group of T1DM patients.
Our ability to examine the effects of treatment assignment, exposure to varying levels of glycemia, including severe hypoglycemia, and the development of medical complications provide a benchmark for comparison with other research studies and with patients followed in typical clinical settings. We have constructed a quantitative picture of the impact of different interventions, levels of metabolic control, and complications on patient experiences of illness. Smaller studies that have explored in greater depth the experiences of illness using qualitative methods augment these findings while evaluating specific aspects of illness (33). Other studies, typically cross-sectional or of shorter duration, but with broader representation of patient types, have provided information that is reasonably consistent with our findings. Specifically, complications exert a stronger effect on HRQOL than diabetes management approaches. Moreover, as in our study, intensification of treatment using multiple daily injections and insulin pumps does not lead to decreased quality of life (1,7,11,12,13). Indeed, intensive treatment and the subsequent reduction of symptomatic complications helps maintain the long-term quality of life of patients with diabetes.
The information derived from studies like this one can be useful for clinicians as the initiate newly diagnosed patients into treatment and work with patients collaboratively over time. Indeed, such personal experiences are increasingly viewed as critical for patients in making their own therapeutic decisions (34,35) and engaging their clinical care team in dealing with the rigors of long-term diabetes. Moreover, symptoms related to sexual and urologic functions may be embarrassing for patients to discuss, but have distinct impact on patient experience of illness and represent an important area for inquiry by treating health care professionals.
Acknowledgments
Abbott, Animas, Aventis, Becton Dickinson, Bayer, Can-Am, Eli Lilly, LifeScan, Medtronic MiniMed, Omron, and Roche contributed free or discounted supplies and/or equipment. The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translational Science Awards Program, National Center for Research Resources, and Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. This work is supported in part by funding from the Herbert Graetz Psychosocial Research Fund (Joslin Diabetes Center) and the Winthrop University Hospital Research Institute. No other potential conflicts of interest relevant to this article were reported.
A.M.J. wrote the manuscript. B.H.B. researched the data, performed analyses, and contributed to writing the manuscript. P.A.C. researched the data and contributed to writing the manuscript. R.A.G.-K. and M.E.L. contributed to writing the manuscript. A.M.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2109/-/DC1.
Clinical trial reg. no. NCT00360893, clinicaltrials.gov.
A complete list of participants in the DCCT/EDIC Research Group can be found at http://dx.doi.org/10.1056/NEJMoa1111732 (N Engl J Med 2011;365:2366–2376).
References
- 1.Jacobson AM. Impact of improved glycemic control on quality of life in patients with diabetes. Endocr Pract 2004;10:502–508 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group Influence of intensive diabetes treatment on quality-of-life outcomes in the diabetes control and complications trial. Diabetes Care 1996;19:195–203 [DOI] [PubMed] [Google Scholar]
- 3.El Achhab Y, Nejjari C, Chikri M, Lyoussi B. Disease-specific health-related quality of life instruments among adults diabetic: A systematic review. Diabetes Res Clin Pract 2008;80:171–184 [DOI] [PubMed] [Google Scholar]
- 4.Naughton MJ, Ruggiero AM, Lawrence JM, et al. SEARCH for Diabetes in Youth Study Group Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus: SEARCH for Diabetes in Youth Study. Arch Pediatr Adolesc Med 2008;162:649–657 [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JM, Yi-Frazier JP, Black MH, et al. SEARCH for Diabetes in Youth Study Group Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. J Pediatr 2012;161:201–207e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debaty I, Halimi S, Quesada JL, Baudrant M, Allenet B, Benhamou PY. A prospective study of quality of life in 77 type 1 diabetic patients 12 months after a hospital therapeutic educational programme. Diabetes Metab 2008;34:507–513 [DOI] [PubMed] [Google Scholar]
- 7.Huang ES, Brown SES, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care 2007;30:2478–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tharavanij T, Betancourt A, Messinger S, et al. Improved long-term health-related quality of life after islet transplantation. Transplantation 2008;86:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin RR, Peyrot M, STAR 3 Study Group Health-related quality of life and treatment satisfaction in the Sensor-Augmented Pump Therapy for A1C Reduction 3 (STAR 3) trial. Diabetes Technol Ther 2012;14:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna A, Bush AL, Swint JM, Peskin MF, Street RL, Jr, Naik AD. Hemoglobin A1c improvements and better diabetes-specific quality of life among participants completing diabetes self-management programs: a nested cohort study. Health Qual Life Outcomes 2012;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lloyd A, Nafees B, Gavriel S, Rousculp MD, Boye KS, Ahmad A. Health utility values associated with diabetic retinopathy. Diabet Med 2008;25:618–624 [DOI] [PubMed] [Google Scholar]
- 12.Hariprasad SM, Mieler WF, Grassi M, Green JL, Jager RD, Miller L. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol 2008;92:89–92 [DOI] [PubMed] [Google Scholar]
- 13.Davidov E, Breitscheidel L, Clouth J, Reips M, Happich M. Diabetic retinopathy and health-related quality of life. Graefes Arch Clin Exp Ophthalmol 2009;247:267–272 [DOI] [PubMed] [Google Scholar]
- 14.Fenwick EK, Xie J, Ratcliffe J, et al. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Invest Ophthalmol Vis Sci 2012;53:677–684 [DOI] [PubMed] [Google Scholar]
- 15.The DCCT Research Group Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care 1988;11:725–732 [DOI] [PubMed] [Google Scholar]
- 16.Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994;17:267–274 [DOI] [PubMed] [Google Scholar]
- 17.Jacobson AM. The diabetes quality-of-life measure. In Handbook of Psychology and Diabetes. Bradley C, Ed. Reading, U.K., Hardwood, 1994, p. 65–87 [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 19.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med 2009;26:315–327 [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 22.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66 [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–263 [DOI] [PubMed] [Google Scholar]
- 24.Huang IC, Liu JH, Wu AW, Wu MY, Leite W, Hwang CC. Evaluating the reliability, validity and minimally important difference of the Taiwanese version of the diabetes quality of life (DQOL) measurement. Health Qual Life Outcomes 2008;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan DM, Fogel H, Norman D, et al. Long-term metabolic and quality of life results with pancreatic/renal transplantation in insulin-dependent diabetes mellitus. Transplantation 1991;52:85–91 [DOI] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med 1995;122:561–568 [DOI] [PubMed] [Google Scholar]
- 28.The Diabetes Control and Complications Trial (DCCT) Research Group Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol 1995;38:869–880 [DOI] [PubMed] [Google Scholar]
- 29.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 30.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Jacobson AM, Musen G, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snedecor G, Cochran WG. Statistical Methods. 8th ed. Ames, IA, Iowa State University Press, 1989 [Google Scholar]
- 32.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritholz MD, Jacobson AM. Living with hypoglycemia. J Gen Intern Med 1998;13:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson AM. Diabetes: finding “a clean well-lighted place”. Lancet 2009;373:1746–1747 [DOI] [PubMed] [Google Scholar]
- 35.Hilliard M, Mann K, Peugh J, Hood K. How poorer quality of life in adolescence predicts subsequent type 1 diabetes management and control. Patient Educ Couns 2013;91:120–125 [DOI] [PMC free article] [PubMed] [Google Scholar]


