Abstract
OBJECTIVE
To examine the effect of a combined exercise program on C1q/tumor necrosis factor-related protein (CTRP) 3 and CTRP-5 levels and novel adiponectin paralogs suggested to be links between metabolism and inflammation and to evaluate sex differences and association with cardiometabolic risk factors in humans with use of a newly developed ELISA.
RESEARCH DESIGN AND METHODS
This cross-sectional study explored the implications of CTRP-3 and CTRP-5 on cardiometabolic parameters in 453 nondiabetic Korean adults. In addition, we evaluated the impact of a 3-month combined exercise program on CTRP-3 and CTRP-5 levels in 76 obese women. The exercise program consisted of 45 min of aerobic exercise at an intensity of 60–75% of the age-predicted maximum heart rate (300 kcal/session) and 20 min of resistance training (100 kcal/session) five times per week.
RESULTS
Both CTRP-3 and CTRP-5 concentrations were significantly higher in women (P < 0.001) than in men (P = 0.030). In a multiple stepwise regression analysis, CTRP-3 levels were independently associated with age, sex, and triglyceride, LDL cholesterol, adiponectin, and retinol-binding protein 4 (RBP4) levels (R2 = 0.182). After 3 months of a combined exercise program, cardiometabolic risk factors, including components of metabolic syndrome, insulin resistance, and RBP4 levels, decreased significantly. In particular, CTRP-3 levels decreased significantly (median [interquartile range] 444.3 [373.8–535.0] to 374.4 [297.2–435.9], P < 0.001), whereas CTRP-5 levels were slightly increased (34.1 [28.6–44.3] to 38.4 [29.8–55.1], P = 0.048).
CONCLUSIONS
A 3-month combined exercise program significantly decreased CTRP-3 levels and modestly increased CTRP-5 levels in obese Korean women.
Adipose tissue was previously regarded as a simple energy storage site, but now it is established as an active endocrine organ that controls systemic energy homeostasis by secretion of adipokines (1). Adiponectin is a well-known adipokine that has anti-inflammatory and antidiabetic effects. Recently, members of the C1q/tumor necrosis factor-related protein (CTRP) family have been reported to share structural homology with adiponectin. To date, 15 CTRP family members have been found that might play major roles in metabolism and inflammation (2).
CTRP-3 is expressed and secreted in monocytes and adipocytes and circulates in human plasma (2). CTRP-3 is a potent anti-inflammatory adipokine that inhibits proinflammatory pathways induced by fatty acids, lipopolysaccharides (LPS), and toll-like receptor (TLR) ligands in adipocytes and monocytes (3). Moreover, administration of CTRP-3 lowers glucose concentrations in both insulin-sensitive wild-type mice and insulin-resistant ob/ob mice without affecting insulin or adiponectin levels (4). Although CTRP-3 inhibits LPS-induced release of interleukin-6 and tumor necrosis factor-α in primary human monocytes, this anti-inflammatory effect is lost in patients with type 2 diabetes (5). Recently, with the use of a newly developed ELISA, we found that circulating CTRP-3 concentrations were significantly elevated in patients with type 2 diabetes or prediabetes compared with a normal glucose tolerance group (6). These results suggested that CTRP-3 may have dual effects on glucose metabolism and inflammation in both rodents and humans (2). Previous studies suggested that the beneficial effects of exercise on insulin sensitivity and other cardiometabolic risk factors might be partially mediated by changes in adipokines (7). However, to the best of our knowledge, no previous studies have evaluated the influence of exercise on CTRP-3 along with changes in cardiometabolic risk factors, including insulin resistance.
CTRP-5 is expressed in adipocytes, retinal pigment and ciliary epithelium, and myocytes (2). CTRP-5 concentrations are higher in obese and diabetic rodents, such as ob/ob and db/db mice and Otsuka Long-Evans Tokushima Fatty (OLETF) rats (8). Similar to adiponectin, CTRP-5 induces phosphorylation of AMP-activated protein kinase and enhances glucose uptake by GLUT4 (8). Furthermore, CTRP-5 stimulates fatty acid oxidation in rat myocytes by the phosphorylation of acetyl-CoA carboxylase (8). In a microarray gene-profiling study, CTRP5 was identified as a candidate gene for obesity in humans (9). However, because of the unavailability of valid laboratory methods to measure circulating CTRP-5 levels, the clinical implications of CTRP-5 in humans have not been elucidated.
In the current study, we examined sex differences in CTRP-3 and CTRP-5 levels, and explored their relationship with cardiometabolic risk variables in nondiabetic Asian subjects the with use of a newly developed ELISA. This study also evaluated the effects of a combined aerobic and resistance exercise program on CTRP-3 and CTRP-5 levels along with other cardiometabolic risk factors and surrogate markers of atherosclerosis in obese women.
RESEARCH DESIGN AND METHODS
Study subjects were participants of the Korean Sarcopenic Obesity Study, an ongoing prospective, observational, cohort study. The details of the study design and objectives have been published previously (10,11). Participants were healthy volunteers without diabetes who resided in Seoul, Korea. None of the participants had a history of cardiovascular disease (myocardial infarction, unstable angina, stroke, or cardiovascular revascularization), stage 2 hypertension (resting blood pressure ≥160/100 mmHg), malignancy, or severe renal or hepatic disease.
A prospective study was performed to examine the effect of a 3-month combined exercise program on circulating CTRP-3 and CTRP-5 concentrations in obese women. Women between 30 and 60 years of age with a BMI >25 kg/m2 were recruited for the exercise study. All were otherwise healthy and had a stable body weight (<2 kg change in weight in the past 6 months) and a sedentary lifestyle (<20 min of exercise twice a week). Women who smoked cigarettes or had diabetes, cardiovascular disease, or chronic kidney or liver disease; were pregnant or breastfeeding; had any other major illnesses; or were taking medication that could have affected laboratory test results were excluded. Informed consent was obtained from all participants before the start of the study. The ethics committee of the study institution, in accordance with the World Medical Association Declaration of Helsinki, approved the study.
Exercise protocol
All exercise sessions were supervised by a professional exercise physiologist. Participants were encouraged to train five times a week, with a training session consisting of a brief warm-up, ∼45 min of aerobic exercise at an intensity of 60–75% of the age-predicted maximum heart rate (∼300 kcal/day) and a 20-min muscle strength training program (∼100 kcal/day), and a brief cooldown. The training program started at 40% of the observed maximal heart rate and gradually increased to 60–75% of the maximal heart rate by week 12. Aerobic exercise included treadmill walking/running (M901T; Motus Co., Seoul, Korea) and cycling (CY8866R; Sunny Co., Seoul, Korea).
Anthropometric and biochemical parameters
BMI was calculated as kilograms body weight over height (in meters) squared, and waist circumference was measured at the midpoint between the lower border of the rib cage and iliac crest. All blood samples were obtained in the morning after an 8-h overnight fast and were immediately stored at −70°C for subsequent assays. Levels of high-sensitivity C-reactive protein (hsCRP) and serum insulin were measured with two different electrochemiluminescence immunoassays (Daiichi Pure Chemicals Co., Tokyo, Japan; Roche Diagnostics, Basel, Switzerland). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the following equation: fasting insulin (µU/L) × fasting glucose (nmol/L) / 22.5 (12). Sandwich human retinol-binding protein 4 (RBP4) (Cat. #AG-45A-0035), adiponectin (Cat. #AG-45A-0001), and competitive CTRP-3 (Cat. #AG-45A-0042) ELISAs were purchased from AdipoGen, Inc. (Incheon, Korea) with respect to having the following assay parameters: RBP4, intra-assay coefficient of variance (CV) (1.7–3.7%) and interassay CV (7.0–8.8%); adiponectin, intra-assay CV (2.9–3.8%) and interassay CV (2.8–5.5%); and competitive CTRP-3, intra-assay CV (6.1–8.3%) and the interassay CV (2.4–8.2%). RBP4 ELISA was established with the use of a human RBP4-specific IgA monoclonal antibody in a sandwich format, giving rise to comparable recovery of human serum or plasma RBP4 to the one determined by quantitative Western blot.
Development of human CTRP-5 ELISA
To avoid potential cross-reactivity with other CTRP family members, a cDNA sequence encoding the NH2-terminal half of human CTRP-5 was amplified and cloned into pET21a+ (Novagen, Madison, WI). A cDNA sequence encoding the mature peptide of human CTRP-5 was cloned as well. Both CTRP-5 proteins were purified through a Ni-sepharose column. A polyclonal antibody was produced by immunizing rabbits with the NH2-terminal half CTRP-5 protein antigen. The mature CTRP-5 protein was used for ELISA standard as well as for an immobilized antigen. A competitive ELISA format was subsequently designed. Fifty microliters of the human serum in 1:2 dilutions together with the polyclonal antibody was applied to wells followed by the addition of 50 μL of the standard or diluted sample and 50 μL of a detection antibody and incubated for 1 h at 37°C. After washing, 100 μL of 3,3′,5,5′-tetramethylbenzidine was added and color visualization developed. The assay sensitivity was 1 ng/mL. Although the degree of precision of the ELISA system in terms of intra-assay CV was between 4.0 and 10.0%, interassay CV was between 6.3 and 9.0%. Linearity was in the range of 79–108%. Specificity was determined by human adiponectin, CTRP-1, CTRP-2, CTRP-3, CTRP-6, CTRP-7, CTRP-9, CTRP-10, CTRP-11, and CTRP-13. No cross-reactivity was noted. The same holds true for >20 adipokines or metabolic hormones (data not shown). The CTRP-5 assay was provided by AdipoGen, Inc. (Cat. #AG-45A-0031).
Measurement of visceral and subcutaneous fat area
Visceral and subcutaneous fat area was quantified by CT scan (Brilliance 64; Philips Medical Systems, Cleveland, OH). Visceral fat area (VFA), total abdominal fat area, and subcutaneous fat area (SFA) were calculated from a 10-cm CT slice image between the fourth and fifth lumbar vertebrae that was obtained during suspended respiration.
Pulse wave velocity
Ankle-brachial index and brachial-ankle pulse wave velocity (baPWV) were measured with a Colin waveform analyzer (model BP-203RPE II; Nippon Colin, Komaki, Japan). The reproducibility of this method has been previously reported (13).
Statistical analysis
Each variable was assessed for a normal distribution. Data are mean ± SD or median (interquartile range [25–75%]). Differences between groups were tested with an independent two-sample t test or Mann-Whitney U test for continuous variables, and differences in the distribution of categorical variables were tested with a χ2 test. Spearman correlation test was performed to determine the relationships of CTRP-3 or CTRP-5 levels with other study variables. Multiple stepwise regression analyses with CTRP-3 or CTRP-5 as the dependent variable were performed to identify the independently associated factors in study participants. Differences between before and after the 3-month combined aerobic and resistance exercise program were tested with paired t test or Wilcoxon signed rank test. All statistical results were based on two-sided tests. Data were analyzed with SAS version 9.2 (SAS Institute, Cary, NC) statistical software. P < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
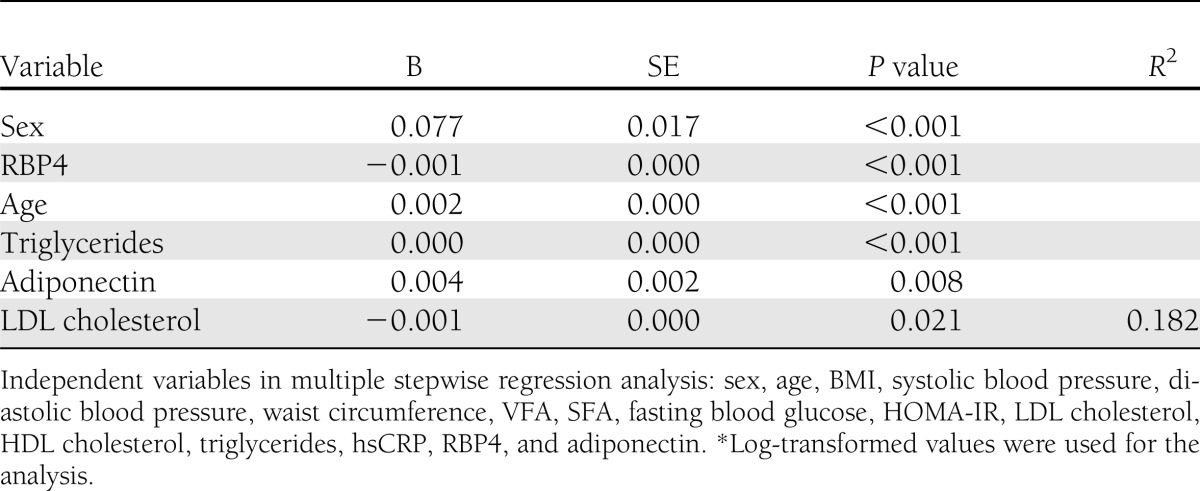
The clinical and biochemical characteristics of the study participants are presented in Table 1. The men were older and had higher body weight, BMI, waist circumference, blood pressure, and fasting plasma glucose, triglyceride, and liver transaminase enzyme levels than the women. Furthermore, hsCRP, ankle-brachial index, and baPWV values were significantly higher in men than in women.
Table 1.
Clinical and laboratory characteristics of the study participants
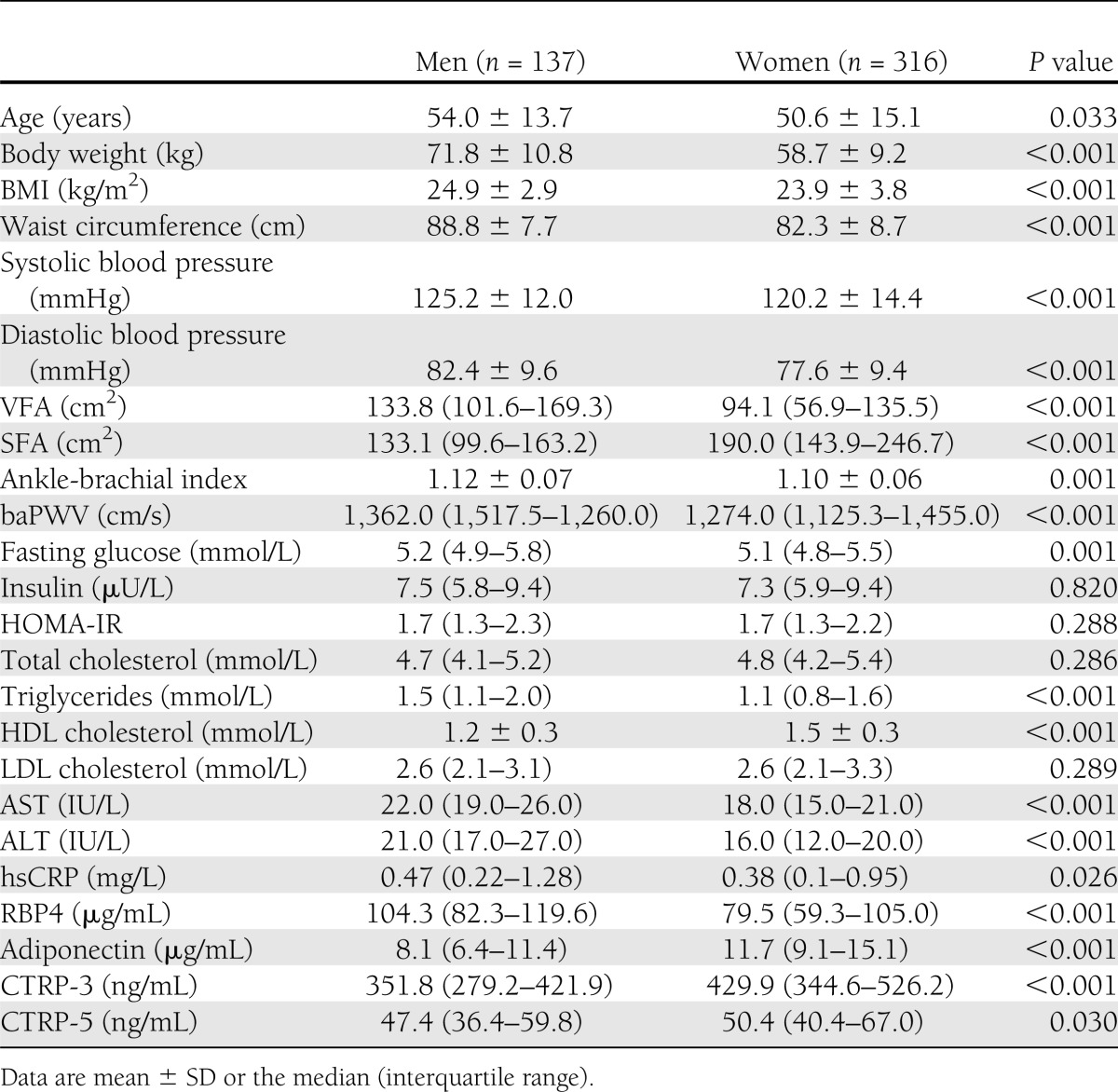
Distribution of CTRP-3 and CTRP-5 according to sex
Circulating concentrations of RBP4 were significantly higher (P < 0.001), whereas adiponectin levels were significantly lower (P < 0.001) in men than in women (Table 1). Figure 1 shows the distribution of CTRP-3 (Fig. 1A) and CTRP-5 (Fig. 1B) according to sex. Women had significantly higher CTRP-3 levels than men (median 429.9 [interquartile range 344.6–526.2] vs. 351.8 [279.2–421.9] ng/mL, P < 0.001). CTRP-5 levels were also slightly higher in women than in men (50.4 [40.4–67.0] vs. 47.4 [36.4–59.8] ng/mL, P = 0.030).
Figure 1.
Distributions of CTRP-3 (A) and CTRP-5 (B) in men and women.
Determining factors for CTRP-3 and CTRP-5
In a stepwise multiple regression analysis, log-transformed CTRP-3 levels were independently associated with age, sex, and triglyceride, LDL cholesterol, adiponectin, and RBP4 levels (R2 = 0.182) (Table 2). In contrast, log-transformed CTRP-5 values were not significantly associated with other metabolic parameters (data not shown). When obesity was defined as a BMI of ≥25 kg/m2 according to the criteria recommended by the Korean Society for the Study of Obesity (14), CTRP-3 and CTRP-5 levels were not significantly different in participants with and without obesity (P = 0.130 and 0.335, respectively [data not shown]).
Table 2.
Multiple linear stepwise regression analysis with CTRP-3* value as a dependent variable
Effects of an exercise program on physiological and biochemical parameters and adipokines levels
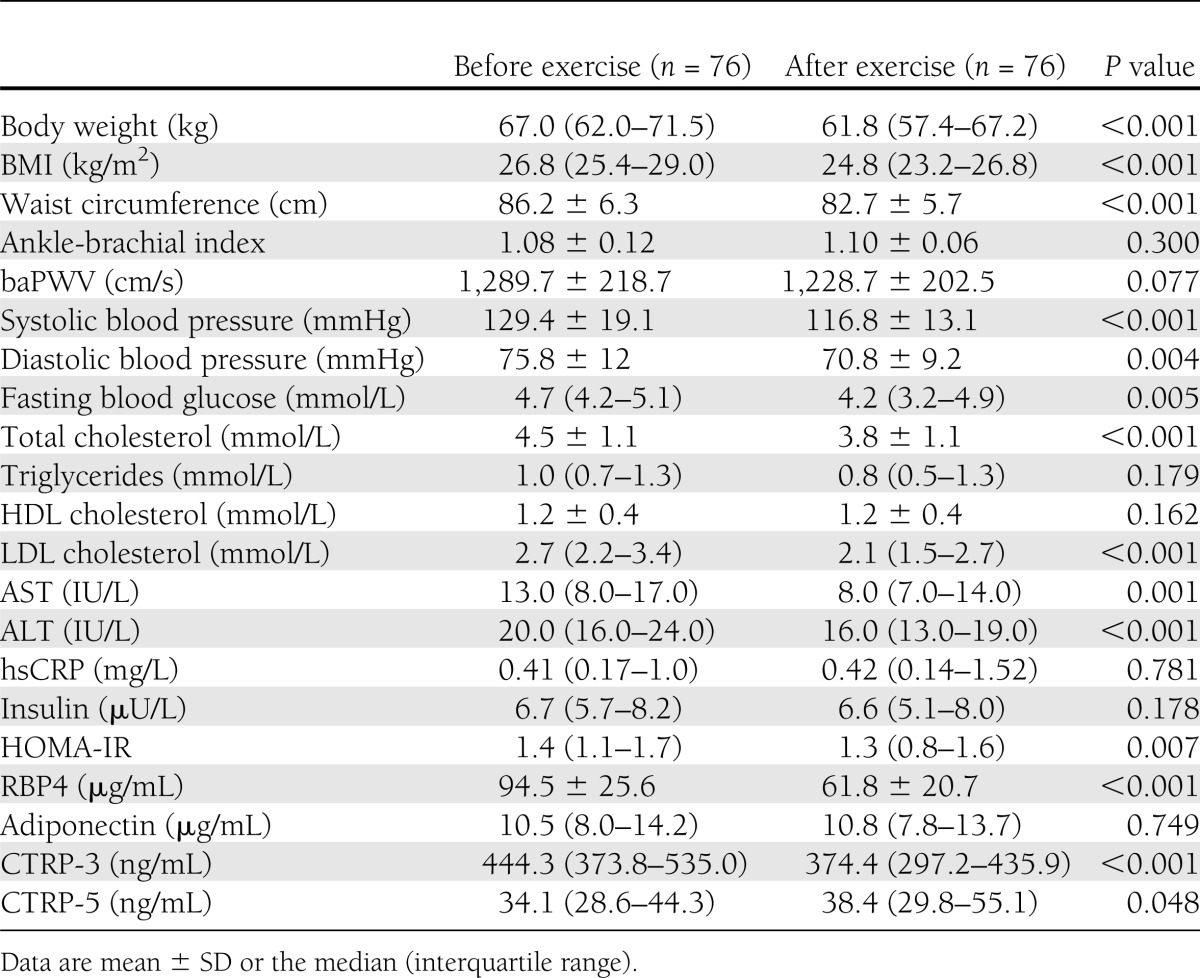
After 3 months of a combined aerobic and resistance exercise program, body weight, BMI, waist circumference, systolic and diastolic blood pressure, and fasting plasma glucose, total cholesterol, LDL cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and HOMA-IR levels decreased significantly (Table 3). In addition, RBP4 levels declined significantly (P < 0.001). In particular, CTRP-3 levels decreased significantly (median 444.3 [interquartile range 373.8–535.0] to 374.4 [297.2–435.9], P < 0.001), whereas CTRP-5 levels increased slightly (34.1 [28.6–44.3] to 38.4 [29.8–55.1], P = 0.048) after the combined exercise program (Supplementary Fig. 1). However, adiponectin levels did not change significantly.
Table 3.
Clinical and laboratory parameters of study participants before and after a 3-month combined aerobic and resistance exercise program
CONCLUSIONS
Despite the multiple beneficial effects of adiponectin, the phenotype in adiponectin-null mice is relatively mild, even under diet- or genetically induced metabolic challenges. On the other hand, adiponectin gain-of-function studies reported definite improvements in the metabolic phenotypes. Therefore, this discrepancy suggests that compensatory mechanisms may exist that partially counteract the deficiency of adiponectin (15). The CTRP family of proteins comprises adiponectin paralogs that may allow cross talk between metabolic pathways and the innate immune system (16). Recently, the CTRP family was proposed as a promising drug target in adipose tissue inflammation, insulin resistance, and type 2 diabetes (2).
CTRP-3 (also called CORS-26, cartducin, or cartonectin) is synthesized and secreted by mesenteric adipose tissue in humans (17). Schmid et al. (18) found that CTRP-3 is positively regulated by insulin, whereas chronic LPS exposure inhibits CTRP-3 expression. CTRP-3 mRNA expression is strongly induced during adipocyte differentiation, and knockdown of CTRP-3 in preadipocytes leads to dedifferentiation into a more proinflammatory and immature phenotype, which is characterized by upregulation of monocyte chemoattractant protein-1 (MCP-1) release and reduction of lipid droplet size (3). CTRP-3 reduces interleukin-6 and tumor necrosis factor-α secretion in LPS-treated monocytic cells, and suppression of nuclear factor–κB (NF-κB) signaling may explain the anti-inflammatory actions of CTRP-3 (19). Wölfing et al. (20) reported that CTRP-3 stimulates the secretion of adiponectin and resistin in adipocytes, which is not caused by an induction of peroxisome proliferator–activated receptor-γ (PPAR-γ) protein expression. In the current study, CTRP-3 concentrations were positively associated with adiponectin levels, whereas they were negatively associated with RBP4 levels in multiple regression analysis. Further research should explore the interaction of adipokines, especially within the CTRP family of proteins.
CTRP-5 is expressed in adipocytes, with the highest expression being observed in the stromal vascular fraction (21). Serum CTRP-5 concentrations are higher in obese and diabetic rodents (8). In addition, the chromosomal localization of CTRP-5 falls within the linkage locus for obesity in Pima Indians, which suggests that CTRP5 might represent a candidate gene for obesity in humans (9). However, another study reported that CTRP-5 levels are not increased in the adipose tissue of ob/ob mice or in rosiglitazone-treated animals (21). Furthermore, injection of mice with CTRP-5 has no effect on their glucose levels (21). In the current study, which included a relatively large sample size, circulating CTRP-5 levels measured with a newly developed ELISA were not increased in obese human subjects compared with those of normal body weight (P = 0.335). On the other hand, depletion of mitochondrial DNA results in insulin resistance as well as increases CTRP-5 expression and secretion in myocytes (8). Moreover, phosphorylation of AMP-activated protein kinase and acetyl-CoA carboxylase is enhanced by CTRP-5 in human myocytes (8). Together, these results suggest that CTRP-5 is a metabolic mediator in the context of obesity and related insulin resistance (2). However, the current study did not show a significant relationship between circulating CTRP-5 levels and insulin resistance index or other cardiometabolic risk factors. Moreover, circulating CTRP-5 concentrations were not associated with adiponectin or RBP4 levels in Korean adults without diabetes. Despite the structural similarities between adiponectin and the CTRP proteins, the metabolic effects of CTRP-5 were not mediated by AdipoR1 or AdipoR2 (8). In addition, although CTRP-5 has been identified as a marker of mitochondrial dysfunction in muscle, it is not yet clear whether muscle is a primary source of systemic CTRP-5 (2).
The current study showed sex-specific differences in CTRP-3 and CTRP-5 levels, which are similar to trends found in adiponectin and several other adipokine levels. For a given BMI, men have more visceral and hepatic adipose tissue, whereas women have more subcutaneous adipose tissue (22). This contrast may be attributable to different sex hormones and adipokines, which lead to more insulin-sensitive characteristics in women (22). Previous studies have shown that adiponectin is higher in women than in men. Even in children, adiponectin concentrations in girls are significantly greater than in boys (23). Kos et al. (24) reported that adipose tissue–derived RBP mRNA expression is sex specific and regulated by leptin. In the Framingham Heart Study, RBP4 concentrations were higher in men and associated with insulin resistance and hsCRP (25). The results of the current study are similar to previous reports in that adiponectin levels were higher and RBP4 levels were lower in women than in men. Wong et al. (21) reported that CTRP-5 protein levels were higher in the serum of female mice despite similar mRNA expression of CTRP-5 in the adipose tissue of female and male mice. In agreement with their findings, the current study showed higher circulating concentrations of CTRP-3 and CTRP-5 in women than in men. These results suggest that the CTRP family of proteins might be one of the underlying factors explaining the difference in metabolic phenotypes between men and women.
Because adipokines influence insulin sensitivity and inflammation, regulation of adipokines may play a pivotal role in the etiology of insulin resistance, which is associated with obesity and type 2 diabetes. Exercise and weight reduction are common nonpharmacological interventions for the treatment of insulin resistance (26). Therefore, exercise may improve insulin resistance by regulating the circulating levels and/or functions of adipokines (26). The current study results are consistent with those of Graham et al. (27), who reported that exercise reduces circulating RBP4 concentrations only in subjects who also experience improved insulin resistance. In previous studies, we observed a reduction in adipokine levels, such as visfatin and adipocyte fatty acid–binding proteins, which was associated with insulin resistance after a combined exercise program (28,29). In the current study, a 3-month combined exercise program significantly decreased CTRP-3 levels in obese Korean women. We previously identified a paradoxical increase of CTRP-3 levels in insulin-resistant patients with type 2 diabetes, which may be a compensatory mechanism reminiscent of insulin or leptin resistance (6). Recently, Chalupova et al. (30) reported that CTRP-1 levels were significantly higher in subjects with metabolic syndrome than in healthy subjects. On the other hand, CTRP-5 concentrations increased modestly after exercise. Lim et al. (31) recently reported that CTRP-5 levels, as measured with immunoblotting, decreased significantly after exercise in 28 women. There is no clear explanation for the discrepancy between the previous results and those of the current study. However, the method (immunoblotting vs. ELISA), number of subjects (28 vs. 76) and type of exercise program (aerobic vs. combined) were different between the two studies, and the CTRP-5 levels during exercise showed only modest changes in both studies. In the current study, circulating adiponectin levels did not change significantly despite improved insulin sensitivity after 3 months of a combined exercise program, suggesting that exercise-induced improvements in insulin resistance develop independently of changes in circulating adiponectin levels (26).
This study has several limitations. First, we used a cross-sectional design; thus, it was not possible to draw conclusions about causality. Second, the prospective exercise study only included obese Korean women. Therefore, the results of the exercise study may not apply to men or other ethnic populations.
In conclusion, similar to adiponectin, both circulating CTRP-3 and CTRP-5 concentrations showed sexual dimorphism, which is higher in women than in men. A 3-month combined aerobic and resistance exercise program significantly decreased CTRP-3 levels and modestly increased CTRP-5 levels, which was accompanied by improvements in cardiometabolic risk factors in obese Korean women.
Acknowledgments
This research was supported by the Brain Korea 21 Project of the Ministry of Education and Human Resources Development, Republic of Korea (A102065-1011-1070100) (to S.H.B. and K.M.C.); the 21C Frontier Functional Proteomics Project (FPR08A1-070) (to K.S.P.); and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012006363) (to K.M.C.).
J.W.P., N.L., and B.-S.Y. are employed by AdipoGen, Inc.; however, this did not alter the authors’ adherence to all Diabetes Care policies on sharing of data and materials. No other potential conflicts of interest relevant to this article were reported.
H.Y.C. and K.M.C. wrote the manuscript and performed the research. J.W.P. and N.L. developed the ELISA kits. S.Y.H. analyzed the dataset. G.J.C., H.C.H., H.J.Y., and T.G.H. collected data. S.M.K., S.H.B., K.S.P., and B.-S.Y. reviewed and edited the manuscript. K.M.C. designed the study. K.M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01688622, www.clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0178/-/DC1.
References
- 1.Frühbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol 2008;456:1–22 [DOI] [PubMed] [Google Scholar]
- 2.Schäffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab 2012;23:194–204 [DOI] [PubMed] [Google Scholar]
- 3.Kopp A, Bala M, Buechler C, et al. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010;151:5267–5278 [DOI] [PubMed] [Google Scholar]
- 4.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 2010;285:39691–39701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp A, Bala M, Weigert J, et al. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine 2010;49:51–57 [DOI] [PubMed] [Google Scholar]
- 6.Choi KM, Hwang SY, Hong HC, et al. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes 2012;61:2932–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefanyk LE, Dyck DJ. The interaction between adipokines, diet and exercise on muscle insulin sensitivity. Curr Opin Clin Nutr Metab Care 2010;13:255–259 [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Choi JH, Ryu HS, et al. C1q tumor necrosis factor alpha-related protein isoform 5 is increased in mitochondrial DNA-depleted myocytes and activates AMP-activated protein kinase. J Biol Chem 2009;284:27780–27789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia 2005;48:1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TN, Yang SJ, Yoo HJ, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33:885–892 [DOI] [PubMed] [Google Scholar]
- 11.Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010;33:1497–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 13.Kim SM, Lee J, Ryu OH, et al. Serum osteoprotegerin levels are associated with inflammation and pulse wave velocity. Clin Endocrinol (Oxf) 2005;63:594–598 [DOI] [PubMed] [Google Scholar]
- 14.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J 2011;35:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis KE, Scherer PE. Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J 2008;416:e7–e9 [DOI] [PubMed] [Google Scholar]
- 16.Compton SA, Cheatham B. CTRP-3: blocking a toll booth to obesity-related inflammation. Endocrinology 2010;151:5095–5097 [DOI] [PubMed] [Google Scholar]
- 17.Hofmann C, Chen N, Obermeier F, et al. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 2011;17:2462–2471 [DOI] [PubMed] [Google Scholar]
- 18.Schmid A, Kopp A, Hanses F, Bala M, Müller M, Schäffler A. The novel adipokine C1q/TNF-related protein-3 is expressed in human adipocytes and regulated by metabolic and infection-related parameters. Exp Clin Endocrinol Diabetes 2012;120:611–617 [DOI] [PubMed] [Google Scholar]
- 19.Weigert J, Neumeier M, Schäffler A, et al. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett 2005;579:5565–5570 [DOI] [PubMed] [Google Scholar]
- 20.Wölfing B, Buechler C, Weigert J, et al. Effects of the new C1q/TNF-related protein (CTRP-3) “cartonectin” on the adipocytic secretion of adipokines. Obesity (Silver Spring) 2008;16:1481–1486 [DOI] [PubMed] [Google Scholar]
- 21.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 2008;416:161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med 2009;6(Suppl. 1):60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böttner A, Kratzsch J, Müller G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 2004;89:4053–4061 [DOI] [PubMed] [Google Scholar]
- 24.Kos K, Wong S, Tan BK, et al. Human RBP4 adipose tissue expression is gender specific and influenced by leptin. Clin Endocrinol (Oxf) 2011;74:197–205 [DOI] [PubMed] [Google Scholar]
- 25.Kaess BM, Enserro DM, McManus DD, et al. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham Heart Study. J Clin Endocrinol Metab 2012;97:E1943–E1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berggren JR, Hulver MW, Houmard JA. Fat as an endocrine organ: influence of exercise. J Appl Physiol 2005;99:757–764 [DOI] [PubMed] [Google Scholar]
- 27.Graham TE, Yang Q, Blüher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 28.Choi KM, Kim JH, Cho GJ, Baik SH, Park HS, Kim SM. Effect of exercise training on plasma visfatin and eotaxin levels. Eur J Endocrinol 2007;157:437–442 [DOI] [PubMed] [Google Scholar]
- 29.Choi KM, Kim TN, Yoo HJ, et al. Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clin Endocrinol (Oxf) 2009;70:569–574 [DOI] [PubMed] [Google Scholar]
- 30.Chalupova L, Zakovska A, Adamcova K. Development of a novel enzyme-linked immunosorbent assay (ELISA) for measurement of serum CTRP1: a pilot study: measurement of serum CTRP1 in healthy donors and patients with metabolic syndrome. Clin Biochem 2013;46:73–78 [DOI] [PubMed] [Google Scholar]
- 31.Lim S, Choi SH, Koo BK, et al. Effects of aerobic exercise training on C1q tumor necrosis factor α-related protein isoform 5 (myonectin): association with insulin resistance and mitochondrial DNA density in women. J Clin Endocrinol Metab 2012;97:E88–E93 [DOI] [PubMed] [Google Scholar]