Abstract
OBJECTIVE
The aim was to evaluate the ability of liraglutide to augment weight loss and improve insulin resistance, cardiovascular disease (CVD) risk factors, and inflammation in a high-risk population for type 2 diabetes (T2DM) and CVD.
RESEARCH DESIGN AND METHODS
We randomized 68 older individuals (mean age, 58 ± 8 years) with overweight/obesity and prediabetes to this double-blind study of liraglutide 1.8 mg versus placebo for 14 weeks. All subjects were advised to decrease calorie intake by 500 kcal/day. Peripheral insulin resistance was quantified by measuring the steady-state plasma glucose (SSPG) concentration during the insulin suppression test. Traditional CVD risk factors and inflammatory markers also were assessed.
RESULTS
Eleven out of 35 individuals (31%) assigned to liraglutide discontinued the study compared with 6 out of 33 (18%) assigned to placebo (P = 0.26). Subjects who continued to use liraglutide (n = 24) lost twice as much weight as those using placebo (n = 27; 6.8 vs. 3.3 kg; P < 0.001). Liraglutide-treated subjects also had a significant improvement in SSPG concentration (−3.2 vs. 0.2 mmol/L; P < 0.001) and significantly (P ≤ 0.04) greater lowering of systolic blood pressure (−8.1 vs. −2.6 mmHg), fasting glucose (−0.5 vs. 0 mmol/L), and triglyceride (−0.4 vs. −0.1 mmol/L) concentration. Inflammatory markers did not differ between the two groups, but pulse increased after liraglutide treatment (6.4 vs. −0.9 bpm; P = 0.001).
CONCLUSIONS
The addition of liraglutide to calorie restriction significantly augmented weight loss and improved insulin resistance, systolic blood pressure, glucose, and triglyceride concentration in this population at high risk for development of T2DM and CVD.
Approximately one-third of adults in the United States have prediabetes (1,2) and are at risk for type 2 diabetes (T2DM) and cardiovascular disease (CVD) (3). Weight loss has been demonstrated to prevent T2DM (4–7) and to improve CVD risk factors (5) in the prediabetes population. In the Diabetes Prevention Program study, a weight loss goal of 7% was associated with a significant reduction in T2DM incidence (4). However, with intensive guidance, only half of the individuals were able to attain this weight loss goal at 24 weeks, and 38% attained this goal at 3 years. With self-guided weight loss programs, the percentage who can achieve a 7% weight reduction may be <20% (8).
Liraglutide, a glucagon-like peptide 1 (GLP-1) analog, is approved for the treatment of T2DM. In addition to improving glucose tolerance, GLP-1 action has been associated with weight loss in individuals with T2DM (9). Only a few studies have evaluated the effect of GLP-1 action in individuals without diabetes (10–12), and none has focused on individuals with prediabetes.
The purpose of this study was to evaluate the effect of liraglutide treatment compared with matching placebo injections in older (mean age, 58 ± 8 years) overweight/obese individuals with prediabetes—those at highest risk for development of T2DM and CVD. Specifically, we assessed the ability of liraglutide treatment to augment weight loss and to improve insulin resistance, CVD risk factors, and inflammatory markers.
RESEARCH DESIGN AND METHODS
Subjects
Men and women, aged 40–70 years, with BMI of 27–40 kg/m2 with prediabetes were recruited from a single center from December 2009 to December 2012. Volunteers were recruited through online and print advertisements seeking individuals at risk for T2DM. Prediabetes was defined as having elevated fasting glucose (5.6–6.9 mmol/L) or elevated 2-h glucose (7.8–11.0 mmol/L) concentration after a 75-g oral glucose challenge. Individuals also were required to have stable weight (<5% reported change) in the previous 3 months. Key exclusion criteria included T2DM, use of medications that can affect carbohydrate metabolism or promote weight loss, gallstones, history of pancreatitis, medullary carcinoma, family history of medullary carcinoma or multiple endocrine neoplasia type 2, and known cardiac, liver, or kidney disease. Written informed consent was obtained from all individuals. The protocol was approved by the Stanford Institutional Review Board.
Procedures
We conducted a double-blind, randomized, placebo-controlled study of liraglutide. Subjects were block-randomized by sex and BMI (<31 vs. ≥31 kg/m2) to receive liraglutide (n = 35) or matching placebo (n = 33) once per day by subcutaneous injection. The starting dose of medication was 0.6 mg; the dose was titrated by 0.6 mg weekly to a maximum dose of 1.8 mg. The dose was decreased by 0.6-mg increments for intolerable side effects. Subjects were seen by a study personnel and a research dietitian weekly for the first 4 weeks, and then every 2 weeks. They were advised to eat a moderate-carbohydrate diet (43 carbohydrate, 42 fat [<7 saturated fat], and 15% protein) and to decrease total caloric intake by 500 kcal/day. An individualized meal plan was provided, and daily food diaries were reviewed at each visit. Individuals were advised to maintain their baseline physical activity. After 12 weeks, subjects were advised to maintain their weight for 2 weeks before end-of-study testing.
All study visits were conducted in the Stanford Clinical and Translational Research Unit. All blood samples were collected after 12 h of fasting.
Oral glucose tolerance test
A standard 75-g oral glucose tolerance test was performed at screening to confirm diagnosis of prediabetes and to exclude individuals with normal glucose tolerance or diabetes.
Insulin suppression test
Peripheral insulin resistance was directly measured with the modified version (13) of the insulin suppression test at baseline and after 14 weeks of liraglutide or placebo. The values for insulin sensitivity obtained with this approach are highly correlated (r ≥ 0.87) with the euglycemic clamp technique (13,14) and measure peripheral, as opposed to hepatic, insulin resistance. Briefly, after an overnight fast, an intravenous catheter was placed in each of the subject’s arms. One arm was used for the administration of a 180-min infusion of octreotide (0.27 μg/m2/min), insulin (32 mU/m2/min), and glucose (267 mg/m2/min); the other arm was used for collecting blood samples. Blood was drawn at 10-min intervals from 150 to 180 min of the infusion to determine the steady-state plasma glucose (SSPG) and insulin concentrations. Because steady-state insulin concentrations are similar in all subjects, the SSPG concentration provides a direct measure of the ability of insulin to mediate disposal of an infused glucose load; therefore, higher SSPG values indicate greater degree of peripheral insulin resistance.
CVD risk factors
Blood pressure and pulse were measured using a Dinamap automatic blood pressure recorder (GE Healthcare, Tampa, FL). Before measurements, subjects were seated quietly in a chair for 5 min with feet on the floor and one arm supported at heart level. Using an appropriately-sized cuff, three blood pressure and pulse readings were taken at 1-min intervals, and the mean of these readings was used for data analysis. Waist circumference was measured by placing a measuring tape around the waist at the upper point of the iliac crest and determined during minimal inspiration as previously described (15). Lipoprotein concentrations were performed by the core laboratory at Stanford University Medical Center by standardized methods approved by the Centers for Disease Control and Prevention.
Prevalence of the five metabolic syndrome components (16) also was evaluated. The components include the following: waist circumference >102 cm in men or >88 cm in women; blood pressure ≥130/85 mmHg; plasma triglycerides ≥1.695 mmol/L; plasma HDL cholesterol <1.036 mmol/L in men and <1.295 mmol/L in women; and fasting plasma glucose ≥5.6 mmol/L.
Inflammatory markers
Inflammatory markers were measured using ELISA (R&D Systems). They included C-reactive protein, total adiponectin, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1, soluble tumor necrosis factor receptor I, soluble tumor necrosis factor receptor II, and monocyte chemotactic protein-1.
Statistical methods
All statistical analyses were conducted using SPSS (version 20 for Windows; SPSS, Chicago, IL). Data are presented as mean ± SD unless otherwise specified. Only subjects who had end-of-study testing were included in the analyses. Differences between liraglutide and placebo groups were assessed using independent t test or χ2 test. Mean differences within groups were tested using paired t tests. Statistical significance was defined as P < 0.05. Adjustments were not made for multiple testing.
Targeted sample size of 30 subjects in each group provided 90% power to detect a 20% difference (2.2 mmol/L) in SSPG concentration. With 24 subjects per group, there was 82% power to detect a 20% difference. A difference of 20% was chosen because this is the degree of difference in SSPG concentration seen with modest weight loss of 7% (17).
RESULTS
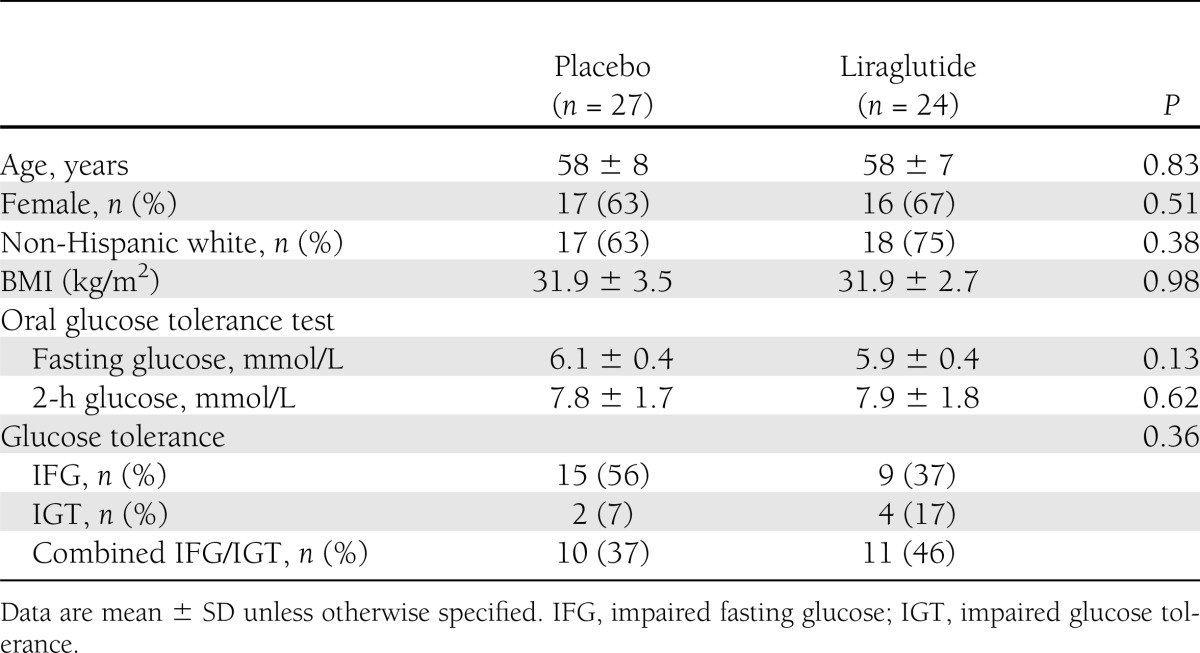
Sixty-nine individuals qualified for the study. One individual withdrew from the study before randomization. Eleven out of 35 individuals (31%) assigned to liraglutide discontinued participation in the study compared with 6 out of 33 (18%) assigned to placebo (P = 0.26). All six individuals using placebo discontinued participation in the study because of personal decision compared with one assigned to liraglutide (P = 0.001). Eight out of 11 individuals using liraglutide discontinued participation in the study because of adverse events compared with none assigned to placebo (P = 0.009). Adverse events included intolerable gastrointestinal side effects (n = 3), injection site reaction (n = 2), pneumonia (n = 1), gallstone (n = 1), and fall (n = 1). Other reasons for discontinuing participation in the study for individuals assigned to liraglutide included protocol deviation (n = 1) and elective back surgery (n = 1). Therefore, 24 individuals randomized to liraglutide and 27 individuals assigned to placebo completed testing at the end of the study and were included in the analyses. Of these individuals, all tolerated the 1.8-mg dose of study medication, except two liraglutide subjects who required a dose reduction down to 1.2 and 0.6 mg, respectively, and one placebo subject who required a dose reduction down to 1.2 mg. As seen in Table 1, baseline characteristics were not different between the two groups. Thus, age (mean, 58 years) and BMI (mean, 31.9 kg/m2) were identical between the two groups.
Table 1.
Baseline characteristics
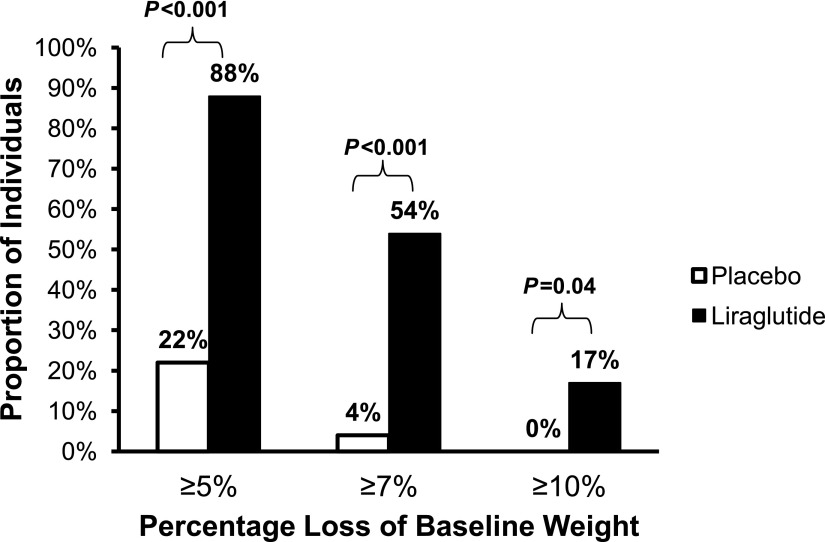
The changes in the two experimental groups are presented in Table 2 and show that individuals randomized to liraglutide lost twice as much weight as those assigned to placebo (6.8 vs. 3.3 kg; P < 0.001). A majority (88%) of liraglutide-treated subjects lost 5% of baseline weight compared with 22% assigned to placebo (Fig. 1). In addition, a loss of 7% of baseline weight occurred significantly more often in those receiving liraglutide (54 vs. 4%), and a loss of 10% of baseline weight was seen only in the liraglutide-treated group (17%).
Table 2.
Changes in CVD risk factors and inflammatory markers
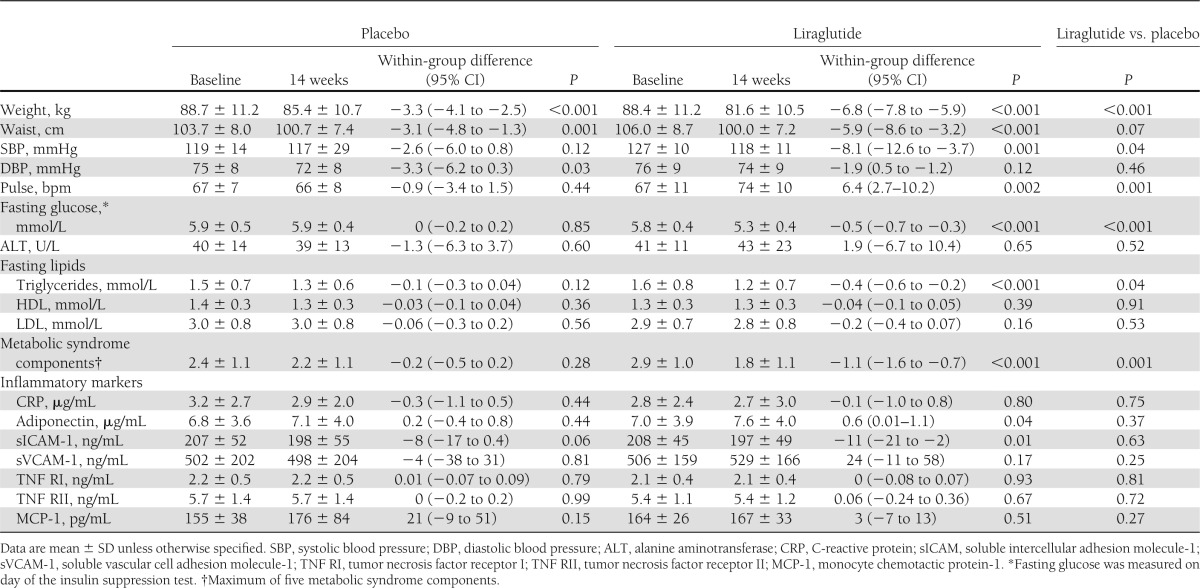
Figure 1.
Proportion of individuals who lost at least 5, 7, and 10% of baseline weight. Liraglutide treatment was associated with greater degree of weight loss compared with placebo.
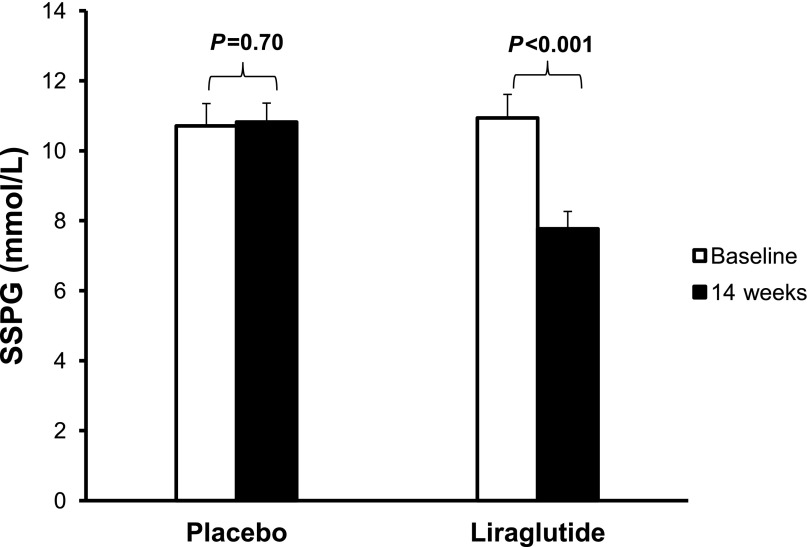
Weight loss after liraglutide treatment was associated with significant improvement in insulin resistance (Fig. 2). SSPG concentration declined by 29% in the liraglutide group compared with no change in the placebo group (P < 0.001 for difference between groups). Changes in steady-state plasma insulin concentrations were not significantly different between groups (P = 0.25 for difference between groups).
Figure 2.
Insulin resistance (SSPG) at baseline and after 14 weeks of liraglutide or placebo treatment. Insulin resistance significantly improved following liraglutide treatment but not placebo.
In addition to augmented weight loss and enhanced insulin sensitivity, the results in Table 2 show that liraglutide treatment was associated with improvement in some CVD risk factors. Liraglutide-treated subjects had significant decreases in systolic blood pressure, fasting glucose, and triglyceride concentration as compared with placebo. In addition, 75% of individuals randomized to liraglutide attained normal fasting glucose (<5.6 mmol/L) compared with 19% assigned to placebo (P < 0.001). As a result, individuals treated with liraglutide but not placebo had a significant decrease in the number of components of the metabolic syndrome (−1.1 vs. −0.2; P = 0.001). There were no within-group changes in inflammatory markers, with the exception of a decrease in soluble intercellular adhesion molecule and an increase in adiponectin associated with liraglutide treatment. However, the between-group differences in these variables were not significant. The only potential adverse change in Table 2 was an increase in pulse seen with liraglutide treatment compared with placebo.
Additional adverse events are detailed in the Supplementary Table. The majority of individuals (79%) treated with liraglutide experienced at least one gastrointestinal side effect compared with 46% treated with placebo (P = 0.02). The most common side effect was nausea, with 67% treated with liraglutide experiencing nausea compared with 26% treated with placebo (P = 0.005).
CONCLUSIONS
The results of our study demonstrated that addition of liraglutide to a calorie-restricted diet can significantly augment weight loss compared with placebo in individuals at high risk for T2DM and CVD. Other clinical benefits in the liraglutide-treated subjects included improvement in insulin sensitivity and a reduction in CVD risk factors. In addition, 75% of individuals treated with liraglutide achieved normal fasting glucose compared with 19% on placebo.
A recent meta-analysis of randomized clinical trials has demonstrated a consistent pattern of modest weight loss and improved glycemic control and cardiometabolic risk factors in patients with T2DM treated with GLP-1 receptor agonists (9). However, only a few studies have evaluated the clinical benefits of liraglutide or other GLP-1 receptor agonists in nondiabetic populations (10–12). In addition, these previous studies differed substantially from ours in the experimental population and results. At the simplest level, none of the three previous studies limited their populations to subjects with prediabetes, and the majority (>60%) of experimental subjects in each of them were obese with normal glucose tolerance. This difference in experimental phenotype may help explain why the improvement in cardiometabolic risk factors was more pronounced in the current study. For example, Astrup et al. (10) described the impact of treatment with several doses of liraglutide compared with placebo and orlistat in obese individuals without diabetes. Although all doses of liraglutide significantly increased weight loss compared with placebo, improvements in CVD risk factors were less pronounced than those observed in our study, with no significant improvement in lipid concentrations between those randomized to liraglutide and placebo. Comparable findings were published by Rosenstock et al. (11) after administration of exenatide to obese subjects without diabetes. Although subjects in their study experienced significantly greater weight loss with exenatide, there was no difference in blood pressure or lipid concentrations compared with placebo. Similarly, Elkind-Hirsch et al. (12) demonstrated that exenatide with or without metformin was associated with greater weight loss as compared with metformin alone in their study of women with polycystic ovarian syndrome. However, as with the two previous studies, the improvements in CVD risk factors were modest.
Finally, none of the previous studies of nondiabetic populations has measured peripheral insulin action quantified by a specific technique. Two of these studies used a surrogate estimate of insulin action, the homeostasis model assessment of insulin resistance (HOMA-IR) (10,12). In contrast to our findings, there was no change in HOMA-IR in one study (10); in the study of women with polycystic ovarian syndrome, HOMA-IR decreased modestly in all experimental groups. As noted, the experimental populations in all three of the previous studies in which GLP-1 receptor agonists were administered to obese nondiabetic subjects differed significantly from ours in the absence of a focus on the effect of this intervention in a group at high risk for development of T2DM and CVD—subjects with prediabetes.
Although it appears clear that the addition of liraglutide to a calorie-restricted diet led to more weight loss and significant improvement in cardiometabolic risk factors as compared with diet alone, it is not clear how much of the beneficial effect of liraglutide is simply a function of the augmented weight loss. For example, the benefits of weight loss in the prediabetes population have been demonstrated in large clinical trials (4–7). In these studies, loss of 5–7% of baseline weight with diet modification and exercise prevented the development of T2DM in individuals with prediabetes. In the Finnish Diabetes Prevention Study, 43% of individuals randomized to the intensive lifestyle intervention achieved the goal weight loss of 5% compared with 13% in the control group. In the Diabetes Prevention Study, 50% of individuals with prediabetes randomized to the intensive lifestyle program were able to lose 7% of their baseline weight at 6 months. In our study, a majority (88%) of the individuals randomized to liraglutide were able to lose 5% of baseline weight compared with 22% of those randomized to placebo. Approximately half of the liraglutide group were able to lose at least 7% of baseline weight compared with 4% in the placebo group. Therefore, addition of liraglutide to a hypocaloric diet was able to provide comparable weight loss to that seen in intensive lifestyle programs used in large clinical trials.
Weight loss associated with liraglutide treatment led to a 29% reduction in peripheral insulin resistance. As discussed, we are not aware of another study that has measured peripheral insulin resistance using a direct method after liraglutide treatment in the prediabetes population. Orskov et al. (18) evaluated the impact of acute infusion of GLP-1 in healthy, young, normal-weight men and found no effect on peripheral insulin sensitivity as measured by the hyperinsulinemic-euglycemic clamp. The degree of improvement in peripheral insulin resistance after liraglutide treatment in the current study was equivalent to previous studies of weight loss of 7–10% baseline weight (17,19). Therefore, liraglutide-associated improvement in peripheral insulin resistance may be predominantly attributable to weight loss rather than an independent effect of GLP-1 action.
In addition to the metabolic benefits associated with liraglutide-assisted weight loss, there may be direct pharmacologic effects of liraglutide that are independent of weight changes. For example, liraglutide treatment can improve glucose tolerance by enhancing glucose-stimulated insulin secretion rate (20). In the current study, liraglutide treatment was associated with a 0.5-mmol/L decrease in fasting glucose concentration. In addition, 75% of those treated with liraglutide had normal fasting glucose at the end of the study compared with 19% treated with placebo. Although difficult to directly compare, the improvement in glycemia in the liraglutide-treated subjects appear better than reported with weight loss alone in the prediabetes population (5,7,21). For example, weight loss goal of 5% has been associated with a decrease in fasting glucose of ≤0.2 mmol/L (5,7), which is half the change in glucose concentration seen with liraglutide in the current study. Ability to normalize glucose, regardless of mechanism, may have an independent effect on decreasing the risk for T2DM (22); therefore, liraglutide seems to provide additional benefits beyond weight loss and improved insulin sensitivity in the prediabetes population.
Another effect of liraglutide that is independent of weight loss is the increase in heart rate. This change also was reported by Astrup et al. (10) in their study of obese individuals without diabetes. The increase in pulse has been noted in studies of patients with T2DM treated with liraglutide (23,24) as well as another GLP-1 receptor agonist, exenatide (25). Although mechanisms are unclear, some have speculated that the increase in pulse may be a compensatory response to the decrease in systolic blood pressure, which also may be greater in magnitude than expected for degree of weight loss (24). One preclinical study also has suggested that GLP-1 action may reduce central parasympathetic outflow to cardiac vagal neurons (26).
The major limitation of our study was that the experimental groups were relatively small. In addition, 31% of individuals randomized to liraglutide discontinued participation in the study. In patients with T2DM, the reported drop-out rates for GLP-1 receptor agonists have been ≤25% (27). However, in two out of three studies of individuals without diabetes, the drop-out rate was similar to that of the current study, 30 (12) and 34% (11). Astrup et al. (10) reported a lower drop-out rate (≤22%) in obese individuals without diabetes randomized to liraglutide. However, they had a run-in period before randomization in which 52 out of 616 (8%) failed. Therefore, tolerance of GLP-1 receptor agonists may vary by population characteristics.
In conclusion, this is the first study to evaluate the effect of GLP-1 administration on weight loss, insulin action, and cardiometabolic risk factors in individuals with prediabetes who are older, overweight/obese, and are at high risk for development of T2DM and CVD. We demonstrate that liraglutide can effectively augment weight loss in this high-risk population. The degree of weight loss was comparable with that seen after intensive lifestyle interventions in large clinical trials of prediabetes. In addition, weight loss associated with liraglutide treatment led to significant improvement in insulin resistance, glucose tolerance, and some CVD risk factors. The increase in pulse was likely a direct pharmacologic effect of liraglutide of unclear significance.
Acknowledgments
The study was funded by the American Diabetes Association (7-09-NOVO-15 to G.R.). Liraglutide and matching placebo were provided by Novo Nordisk. The American Diabetes Association and Novo Nordisk were not involved in the design or conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. No other potential conflicts of interest relevant to this article were reported.
S.H.K. helped design and conducted the research, analyzed the data, and wrote and edited the manuscript. F.A., C.L., A.L., D.A., P.S., K.G., V.T., and H.O. conducted the research. Y.-D.I.C. and Y.V.L. measured the inflammatory markers and edited the manuscript. G.R. designed the study and edited the manuscript. S.H.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
Clinical trial reg. no. NCT01784965, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0354/-/DC1.
References
- 1.Kim SH, Chunawala L, Linde R, Reaven GM. Comparison of the 1997 and 2003 American Diabetes Association classification of impaired fasting glucose: impact on prevalence of impaired fasting glucose, coronary heart disease risk factors, and coronary heart disease in a community-based medical practice. J Am Coll Cardiol 2006;48:293–297 [DOI] [PubMed] [Google Scholar]
- 2.Karve A, Hayward RA. Prevalence, diagnosis, and treatment of impaired fasting glucose and impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care 2010;33:2355–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association AD, American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 6.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Watanabe M, Nishida J, et al. Zensharen Study for Prevention of Lifestyle Diseases Group Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med 2011;171:1352–1360 [DOI] [PubMed] [Google Scholar]
- 8.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792–1798 [DOI] [PubMed] [Google Scholar]
- 9.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astrup A, Rössner S, Van Gaal L, et al. NN8022-1807 Study Group Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 11.Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care 2010;33:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:2670–2678 [DOI] [PubMed] [Google Scholar]
- 13.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes 1981;30:387–392 [DOI] [PubMed] [Google Scholar]
- 14.Knowles JW, Assimes TL, Tsao PS, et al. Measurement of insulin-mediated glucose uptake: Direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism 2013;62:548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services PHS NHANES III anthropometric procedures video. Washington, DC, U.S. Government Printing Office, 1996 [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin T, Carter S, Lamendola C, et al. Effects of moderate variations in macronutrient composition on weight loss and reduction in cardiovascular disease risk in obese, insulin-resistant adults. Am J Clin Nutr 2006;84:813–821 [DOI] [PubMed] [Google Scholar]
- 18.Orskov L, Holst JJ, Møller J, et al. GLP-1 does not not acutely affect insulin sensitivity in healthy man. Diabetologia 1996;39:1227–1232 [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin T, Abbasi F, Kim HS, Lamendola C, Schaaf P, Reaven G. Relationship between insulin resistance, weight loss, and coronary heart disease risk in healthy, obese women. Metabolism 2001;50:795–800 [DOI] [PubMed] [Google Scholar]
- 20.Holst JJ, Toft-Nielsen MB, Orskov C, Nauck M, Willms B. On the effects of glucagon-like peptide-1 on blood glucose regulation in normal and diabetic subjects. Ann N Y Acad Sci 1996;805:729–736 [DOI] [PubMed] [Google Scholar]
- 21.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF, Diabetes Prevention Program Research Group Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marre M, Shaw J, Brändle M, et al. LEAD-1 SU study group Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinman B, Gerich J, Buse JB, et al. LEAD-4 Study Investigators Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010;375:2234–2243 [DOI] [PubMed] [Google Scholar]
- 26.Griffioen KJ, Wan R, Okun E, et al. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res 2011;89:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niswender K, Pi-Sunyer X, Buse J, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab 2013;15:42–54 [DOI] [PubMed] [Google Scholar]