Abstract
OBJECTIVE
Diabetes treatment should be effective and cost-effective. HbA1c-associated complications are costly. Would patient-centered care be more (cost-) effective if it was targeted to patients within specific HbA1c ranges?
RESEARCH DESIGN AND METHODS
This prospective, cluster-randomized, controlled trial involved 13 hospitals (clusters) in the Netherlands and 506 patients with type 2 diabetes randomized to patient-centered (n = 237) or usual care (controls) (n = 269). Primary outcomes were change in HbA1c and quality-adjusted life years (QALYs); costs and incremental costs (USD) after 1 year were secondary outcomes. We applied nonparametric bootstrapping and probabilistic modeling over a lifetime using a validated Dutch model. The baseline HbA1c strata were <7.0% (53 mmol/mol), 7.0–8.5%, and >8.5% (69 mmol/mol).
RESULTS
Patient-centered care was most effective and cost-effective in those with baseline HbA1c >8.5% (69 mmol/mol). After 1 year, the HbA1c reduction was 0.83% (95% CI 0.81–0.84%) (6.7 mmol/mol [6.5–6.8]), and the incremental cost-effectiveness ratio (ICER) was 261 USD (235–288) per QALY. Over a lifetime, 0.54 QALYs (0.30–0.78) were gained at a cost of 3,482 USD (2,706–4,258); ICER 6,443 USD/QALY (3,199–9,686). For baseline HbA1c 7.0–8.5% (53–69 mmol/mol), 0.24 QALY (0.07–0.41) was gained at a cost of 4,731 USD (4,259–5,205); ICER 20,086 USD (5,979–34,193). Care was not cost-effective for patients at a baseline HbA1c <7.0% (53 mmol/mol).
CONCLUSIONS
Patient-centered care is more valuable when targeted to patients with HbA1c >8.5% (69 mmol/mol), confirming clinical intuition. The findings support treatment in those with baseline HbA1c 7–8.5% (53–69 mmol/mol) and demonstrate little to no benefit among those with HbA1c <7% (53 mmol/mol). Further studies should assess different HbA1c strata and additional risk profiles to account for heterogeneity among patients.
Type 2 diabetes causes an enormous economic burden in almost every country. Diabetes treatment must be both effective and efficient (1–7). In 2011, diabetes affected at least 366 million people or 5% of the world’s population (8% of adults) and was responsible for 4.6 million deaths (8). The prevalence of diabetes is expected to increase to 552 million in 2030 (8). In 2011, diabetes care consumed at least 465 billion USD, accounting for 11% of health care expenditures in adults 20–79 years of age (8). The main cause of the high-cost burden of diabetes is its acute and chronic complications, leading to a 3–13-fold increase in costs per patient (9–14). The occurrence of complications is related to nonmodifiable risk factors such as age, sex, and socioeconomic status. It is also related to modifiable risk factors, such as BMI and waist circumference, as well as risk factors not directly related to diabetes, such as comorbidities including depression. HbA1c is widely monitored as a measure of the risk of complications and as a target for intervention. HbA1c may also be important in defining a more comprehensive risk-based approach to diabetes management.
We have previously explored patient-centered care as a treatment strategy for type 2 diabetes (15–17). Earlier, we conducted a cluster-randomized, controlled clinical trial that compared patient-centered care versus professional-directed and usual care (control) in 13 hospitals (clusters). Patient-centered care had very acceptable incremental cost-effectiveness ratios (ICERs) as compared with professional-directed and usual care. These findings stimulated additional studies of self-management promotion, which is presently regarded as an essential and potentially very cost-effective approach to diabetes management (18–20).
Unfortunately, most, if not all, studies focus on the average patient, whereas individual characteristics relate to the risk of developing complications (21–23), the effectiveness of treatment (23), and health care costs (24–26). More effective and efficient diabetes care might be achieved by focusing patient-centered strategies on patients with specific risk profiles. Such approaches have infrequently been described (27,28).
Therefore, we analyzed data from our trial using individual patient data to compare patient-centered with usual care. We stratified patients by baseline HbA1c and measured HbA1c and costs at 1 year, and quality-adjusted life years (QALYs) and health care costs over a lifetime. We tested the hypothesis that a policy of patient-centered care, provided to patients in higher baseline HbA1c strata, would result in significantly better outcomes and more efficient health care.
RESEARCH DESIGN AND METHODS
Population and intervention (see CONSORT flow diagram online)
We conducted a prospective cluster-randomized trial, aiming at 18 hospitals. Four did not participate and one dropped out for financial reasons. Eligible hospitals were situated across the Netherlands and met predefined eligibility criteria in terms of numbers of beds and diabetes specialist nurses. The 13 hospitals were representative of the 120 general hospitals in the Netherlands and delivered ambulatory secondary care. There was no systematic contamination due to geographical differences. The characteristics of the 13 hospitals that participated and the 5 that did not did not differ substantially. There was a small difference in mean HbA1c (SD) between participating and nonparticipating hospitals (7.8 [1.2] vs. 8.0 [1.4], respectively) (Table 1). In the 13 participating hospitals, internists recruited the first 150 patients with type 1 and type 2 diabetes who attended a diabetes clinic, excluding patients who were pregnant or had a poor life expectancy due to other diseases. Enrollment took place between November 1999 and March 2000. Exclusion criteria included participation in another study or being an academic hospital as we sought to study real-life day-to-day clinical care using a low-impact observational approach. After several pilot studies and preintervention baseline patient measurements, each hospital was randomized (without restrictions) to one of three intervention arms, allocating patients with type 2 diabetes into patient-centered, professional-directed, and usual care arms (see CONSORT flow diagram in Supplementary Data online). Allocation was performed by a noninvolved person, a so-called third party, outside the research group, and allocation results were concealed from the investigators until the start of the intervention. The allocation ratio was 4:4:5. Internists and patients allocated to the intervention group were aware of the allocated arm. The unit of randomization equaled the unit of analysis and was depicted as a continuous (the percentage of people benefitting) rather than as a dichotomous (success or failure) outcome. For practical reasons, and as the outcome was nonsubjective, the study was not blinded. The study design was clustered since the intervention strategy could only be implemented by a provider team with a group of patients in a single hospital outpatient setting. Without clustering by hospital, serious contamination at the hospital, patient, and provider level would have taken place. Ex ante, we made no modifications to the trial design or protocol in response to changing circumstances or allocation results.
Table 1.
Baseline characteristics
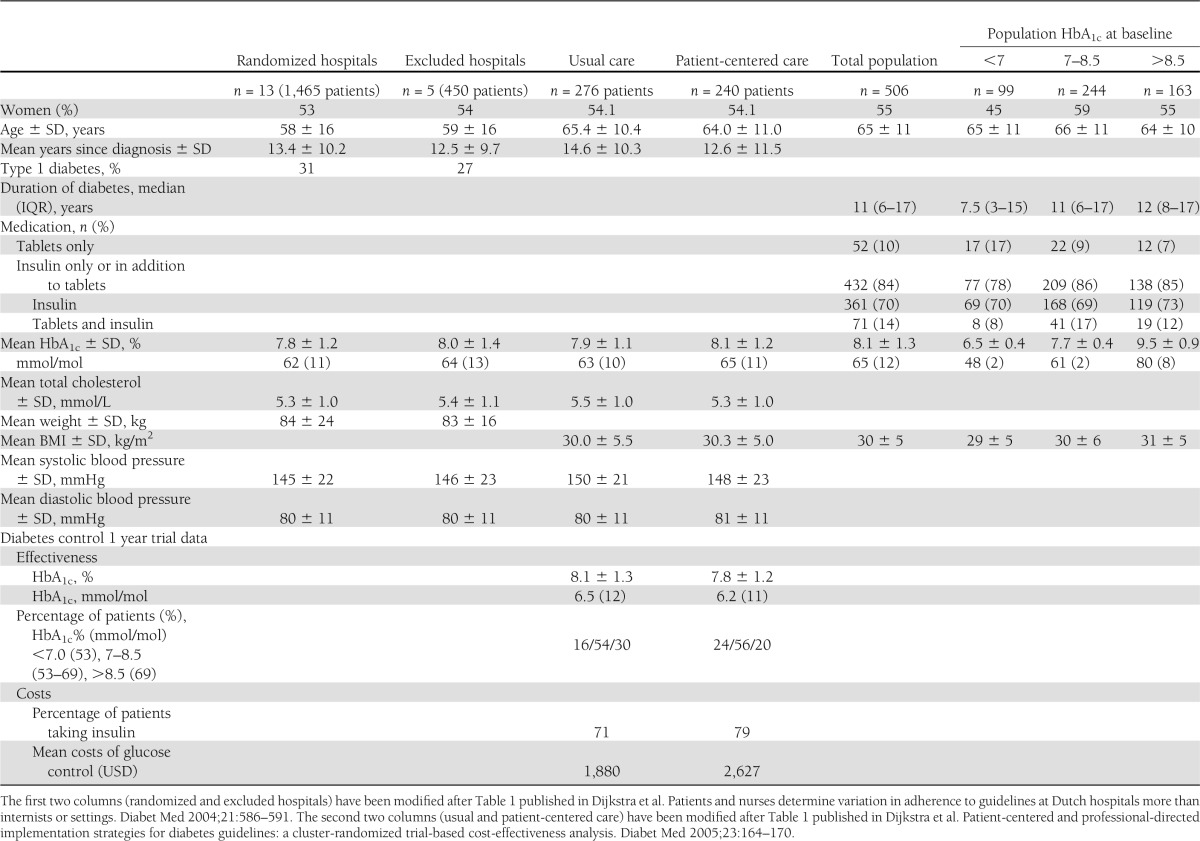
This article compares two trial arms (see CONSORT flow diagram online) of randomly assigned clusters, the patient-centered arm with n = 240 patients (n = 237 with available HbA1c data at baseline) and the usual care arm with 276 patients (n = 269 with available HbA1c data at baseline). Both subgroups are comparable with respect to baseline patient characteristics (Table 1). In the patient-centered care clusters, patients were not only seen by their internal medicine doctors and diabetes team as in usual care but additionally received detailed diabetes passports based on national guidelines that aim to educate and record results of medical examinations in order to promote shared disease management. Educational meetings for patients were organized in all of the hospitals where the diabetes passports were introduced. Physicians, diabetes specialist nurses, and dietitians attended these meetings with an opinion leader and received personal feedback with benchmarks on baseline data, adherence to key guidelines, and the use of the diabetes passports. Barriers and facilitators were discussed. Internists received personal feedback on clinical performance after 6 months as well as on the use of the diabetes passports. Leaflets and waiting room posters were also distributed. Usual care consisted of visits every 3 months to a specialized nurse and/or internist according to national evidence-based guidelines (CBO Banda Heereveen 1998, ISBN 90-6910-217-X). The standard protocol was rechecked, re-explained, re-emphasized, and followed up in the hospitals involved.
Using individual patient data, we stratified all patients into three groups according to baseline HbA1c (<7% [53 mmol/mol], 7–8.5%, >8.5% [69 mmol/mol]) (Table 1) and examined the effectiveness and cost-effectiveness of patient-centered care in each stratum. The analyses described in this article were not part of the original analyses of the cluster randomized control trial and were therefore performed as secondary analyses.
The institutional review board (Medical Ethics Committee of University Medical Center Nijmegen) and the Committee for Scientific Research with Human Subjects (CWOM 9810–0208) approved of the study. All patients gave written informed consent. The trial has been assigned the ISRCTN number ISRCTN3581744 at the Commissie Mensgebonden Onderzoek (CMO), with the title The Diabetes Guidelines Implementation in Hospitals Study. The full protocol can be requested from the CMO and the authors; at the time, an online trial registry was not in place.
During and after the study, there were no departures from the initial study protocol. There were no changes to eligibility criteria, interventions, examinations, data collection, methods of analysis, and outcomes. The initial study had HbA1c as the primary outcome measure for the sample size calculations. A mean HbA1c of 7.9% (63 mmol/mol) was specified that could drop 0.5% (3.1 mmol/mol) after the intervention. α was set at 0.05 and β at 0.20. Sample sizes for cluster-randomized trials were inflated to adjust for clustering. The intracluster correlation coefficient was set at ρ = 0.01. Given a potential of four hospitals per arm and a 70% response rate, the sample size needed was 150 patients (with a single medical record) per arm. The power is further indicated by the confidence interval. Potential inconsistencies in laboratory outcomes in pre- and postmeasurements were checked by the Dutch Foundation for Quality Assessment in Clinical Laboratories, in which all hospitals participate. Calibration of HbA1c was performed according to the guidelines of the National Glycohemoglobin Standardization Program. No interim analyses were warranted or performed. Using the health care perspective, our analyses of trial effect and cost include health-related outcomes and health care costs related to the individual patients for the 1-year duration of the trial and during a simulated patient lifetime. Individual patient outcome and cost data from the trial follow-up were entered into an existing national diabetes model multiple times. This model has been used and described in previous studies, including one that estimated the long-term costs of diabetes and cardiovascular complications and hospitalizations (15).
Health effects (HbA1c), costs, and cost-effectiveness over 1 year
The end points regarding the impact of stratification over 1 year were the effectiveness of HbA1c reduction, costs, and ICERs. The latter were obtained from nonparametric bootstrapping and estimated mean (95% CI). Each of these simulations used 1,000 bootstrap samples drawn from the original dataset containing the individual patient records. Direct costs per patient were estimated and standardized by multiplying each resource use component by the unit cost and summing the results at baseline and after 1 year for the main cost drivers: costs of medication (unit costs: insulin, –497 USD; tablets, –223 USD), costs of glucose monitoring (236 USD for glucose testing once every 6 weeks), and costs of implementation strategies (3.7 USD per patient) (29).
Health gain, medical costs, and cost-effectiveness over a lifetime
The primary end points with respect to efficacy over a lifetime were effectiveness, QALYs (assessing the long-term complications and the excess cardiovascular morbidity and mortality associated with diabetes), as well as costs, based on the estimated events and prevalence of complications. These were estimated by extrapolating and bootstrapping individual patient data in a probabilistic cost-effectiveness analysis with 10,000 iterations using a per intervention arm validated probabilistic Markov diabetes model (10,30–33). Progression of diabetes complications was based on the formula β^(HbA1c/10) (10,31,34). We adjusted for the natural increase in HbA1c over time, ageing of patients, and the age-related increase in complication risk, accounting for uncertainties by including distributions in values of input variables, including HbA1c at the end of the trial and mortality risk (10,31). We only discounted costs (3%) and did not discount QALYs (32,33). Costs and health outcomes of the probabilistic analyses are presented as point estimates with 95% CIs.
Statistical analysis
The primary and secondary outcomes by HbA1c strata were compared using ANOVA for continuous normally distributed variables (mean and SD, such as HbA1c and age), the Kruskal-Wallis test for continuous nonnormally distributed variables (median or interquartile range), like duration of diabetes, as well as the χ2 test for categorical variables (numbers, sex, etc.). All tests were two tailed, and the limit of statistical significance was defined as P < 0.05. An intention-to-treat analysis was performed in this study. We used SPSS version 11.0 (SPSS Inc., Chicago, IL) and Excel version 9.0 (Microsoft, Seattle, WA).
RESULTS
Participant flow, for each arm and for each stratum is provided in the CONSORT 2010 flow diagram online. The trial was completed after 1 year of follow-up as planned. There was no reason to stop or end prematurely. Baseline characteristics of the participating and nonparticipating hospitals were similar as were the baseline characteristics of subjects in the two arms, patient-centered and usual care, apart from HbA1c (Table 1). Baseline characteristics of subjects in the three strata were also comparable, apart from longer duration of diabetes and more insulin use in the highest HbA1c stratum (Table 1).
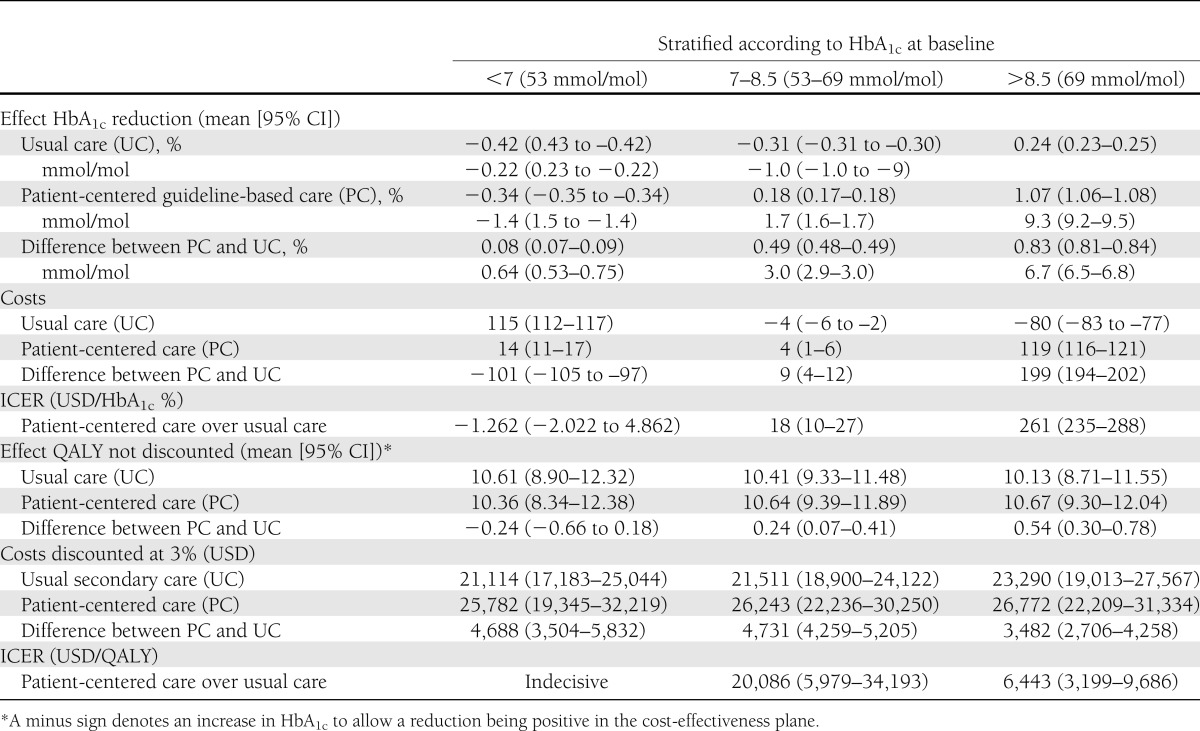
A summary of the continuous outcomes in each trial arm according to stratum (HbA1c reduction, QALYs, and costs) as well as the effect size representing their contrast (differences between patient-centered and usual care and the ICERs) and their 95% CIs are presented in Table 2.
Table 2.
HbA1c reduction and extra costs for patient-centered and usual care after the 1st year, and QALYs and extra costs over a lifetime
Health effects (HbA1c), costs, and cost-effectiveness at 1 year
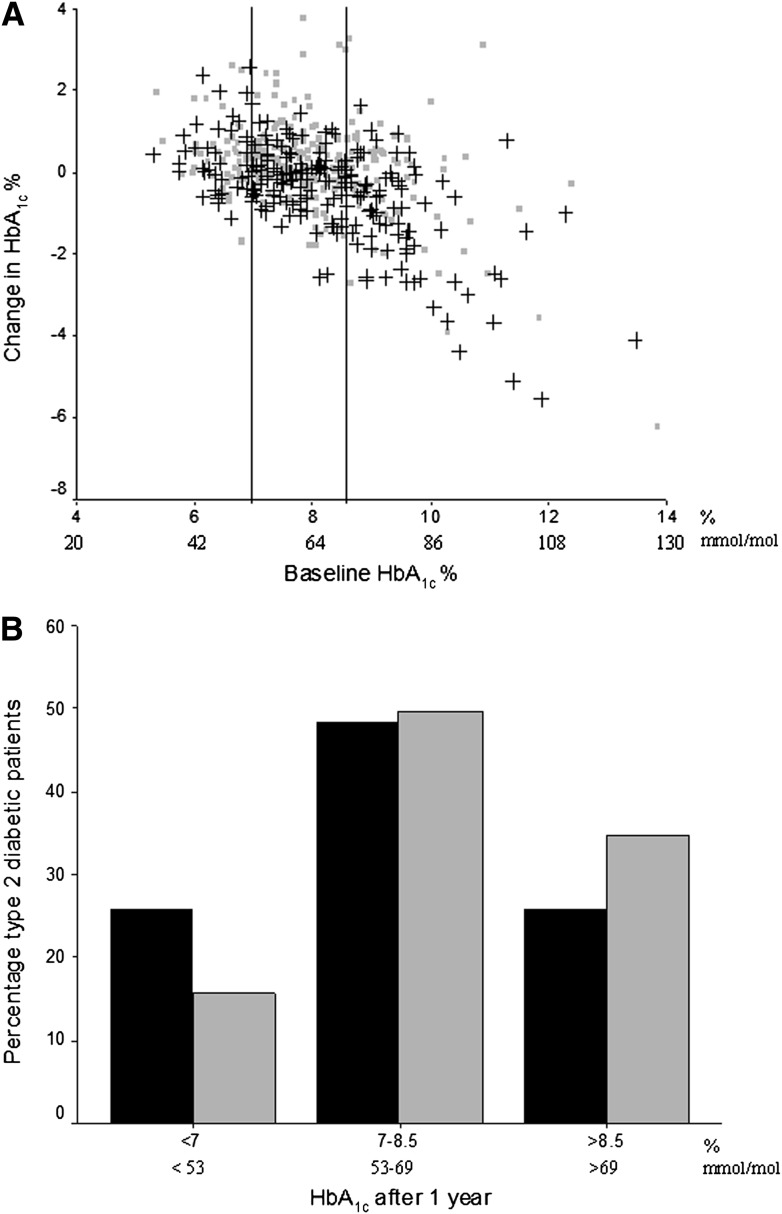
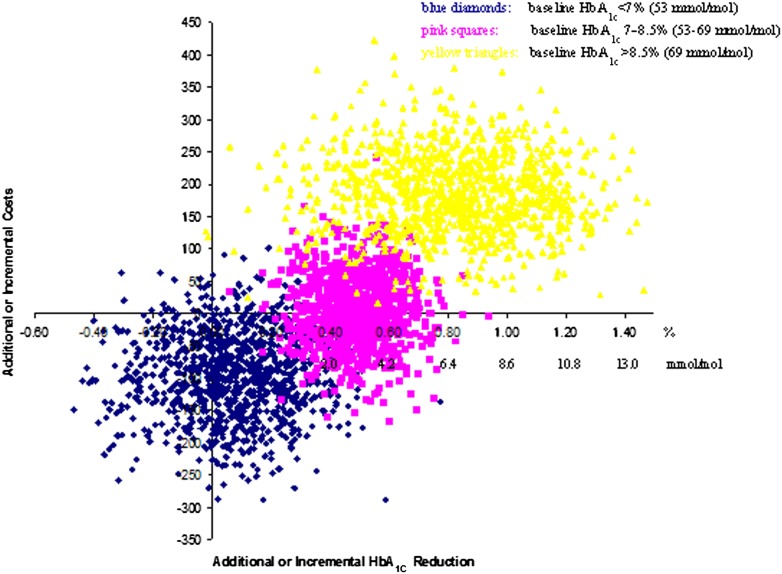
Change and distribution of HbA1c are depicted in Fig. 1. Over 1 year, the ICER for patient-centered care was highest in the highest HbA1c stratum (Table 2). In general, the ICERs were quite low. Bootstrapping the results of the individual patients and plotting the gain in a cost-effectiveness plane confirmed this (Fig. 2). The scatter plots at lower baseline HbA1c were in the two lower quadrants and with higher HbA1c at baseline in the upper right quadrant. Hence, for the highest stratum (baseline HbA1c >8.5 [69 mmol/mol]), patient-centered care showed a reduction in HbA1c at higher costs (dots above the x-axis). For patients with baseline HbA1c = 7–8.5% (53–69 mmol/mol), patient-centered care showed an HbA1c reduction and was cost saving in 45% of cases. For patients with a baseline HbA1c <7% (53 mmol/mol), the health effects were uncertain as points were divided over the left and right sides of the y-axis. With 64% of the points falling below the x-axis, there is a reasonable chance that patient-centered care would be dominant or cheaper than usual care.
Figure 1.
A: Change in HbA1c at 1 year, according to HbA1c at baseline. Change in HbA1c % in the 506 patients with type 2 diabetes after 1 year of patient-centered (black crosses, n = 237) or usual care (rectangles, n = 269) according to baseline HbA1c %. Vertical black lines represent the different strata: HbA1c <7 (53 mmol/mol), 7–8.5, or >8.5% (69 mmol/mol). B: HbA1c distribution of 506 type 2 diabetic patients at 1-year follow-up. HbA1c distribution of the 506 patients with type 2 diabetes according to HbA1c strata after having received patient-centered (black bars, n = 237) or usual care (gray bars, n = 269) for 1 year.
Figure 2.
Cost-effectiveness plane of patient-centered over usual care. Results for incremental 1-year cost-effectiveness of patient-centered vs. usual care, according to strata of HbA1c % at baseline in 506 patients. Distribution of the cost-effectiveness plane: HbA1c <7% (53 mmol/mol) shows 29% in the lower left quadrant and 64% in the dominant lower right. HbA1c = 7–8.5% (53–69 mmol/mol) shows 45% in the dominant lower right quadrant and 56% in the upper right quadrant. HbA1c >8.5% (69 mmol/mol) always results in health gains and shows no cost savings.
Lifetime extrapolation of costs and effects
The difference in total lifetime QALYs between patient-centered care and usual care varied according to the baseline HbA1c stratum (Table 2), and the difference was positively associated with HbA1c. The gain achievable from patient-centered care was greatest in patients with an HbA1c >8.5% (0.54) (69 mmol/mol) (0.36) and lowest in patients with an HbA1c <7% (53 mmol/mol). In both arms, costs were higher, the higher the baseline HbA1c, and the difference was lowest in the highest stratum. Hence, the ICER of patient-centered over usual care was most favorable in patients with HbA1c >8.5% (69 mmol/mol) (6,443 USD/QALY). The higher cost-effectiveness ratio of 20,086 USD/QALY measured in the second stratum (7< HbA1c <8.5 [53–69 mmol/mol]) was below prevailing thresholds used to decide whether or not an intervention is cost-effective (e.g., 50,000 USD for the U.S.) (35). The lowest stratum (HbA1c <7 [53 mmol/mol]) showed uncertain health gains and an unfavorable ICER.
The cluster design did not change the outcomes of the analyses. The intracluster-correlation for reduction in HbA1c and other long-term parameters was low and varied, except for HbA1c <7% (53 mmol/mol) for life years and QALYs. The latter was 0.06.
Analyses were only performed according to predefined protocol. No adverse events, harms, or unintended events were reported.
CONCLUSIONS
Stratification is an important tool to optimize effectiveness and efficiency. Patient-centered care is more effective when targeted at a subgroup defined by higher baseline HbA1c. Over a lifetime, patient-centered care is particularly effective and a “better buy” for patients with baseline HbA1c >8.5% (69 mmol/mol) and does not provide value for patients with baseline HbA1c <7% (53 mmol/mol). This suggests that patient-centered care should focus on patients with a baseline HbA1c >8.5% (69 mmol/mol), be considered for those with HbA1c = 7.0–8.5% (53–69 mmol/mol), and not be implemented in those with baseline HbA1c <7% (53 mmol/mol).
This article transforms intuition into evidence and quantifies the benefits of targeting the patient-centered care intervention by baseline HbA1c. Exploring additional criteria for stratification, as well as additional interventions aimed at the high-risk patient groups, seems warranted.
Our study is among the first to stratify patients with type 2 diabetes according to baseline risk in order to optimize lifetime benefits and lower costs. Our results are consistent with the recent literature on cost-effectiveness of interventions in people at high risk for diabetes and stratified analyses in other diseases (18,34,36–40). A recent Cochrane review suggests a benefit of individual education on glycemic control when compared with usual care in a subgroup of those with a baseline HbA1c >8% (64 mmol/mol) in an at least 6-month follow-up (41). We extend these findings over a lifetime and show that such benefits persist.
Several limitations should be acknowledged. Further studies should replicate and refine these analyses and include other risk profiles to account for heterogeneity among patients. This would also provide a more comprehensive picture of the additional key risk factors impacting the development of complications. Also, further studies should include primary care settings since treatment of chronic diseases like type 2 diabetes tends to occur in primary care settings. In addition, longer follow-up will be needed. We assumed that the level of improvement seen after 1 year would be maintained over a lifetime (as shown in the UK Prospective Diabetes Study [UKPDS]). This is especially relevant for the stratum with HbA1c >8.5% (69 mmol/mol). Another potential limitation could relate to the generalizability of our findings. Although it is likely that our findings apply to other European and North American hospital settings, since the prevalence, characteristics, treatment strategies, and costs of type 2 diabetes are similar (37), the intensity of care might vary. Finally, more complex models might be needed that include side effects and disutilities related to insulin and oral medication use and other health care costs (related to patient admissions, primary care, or specialist visits).
Further insight can be achieved by replication of the present approach in larger completed studies hypothesizing gradients or threshold levels below which patient-centered care is not cost-effective and above which it is cost saving. Moreover, a study using a priori stratification would provide valuable confirmatory evidence for the findings of our exploratory study. Conceptually, the terminology and emphasis of patient-centered care has evolved over the years. At the time of our study, it referred to care in which the patient through the use of self-monitoring was more involved in decision making than those enrolled in usual care. The current concept of patient-centered care is one where the patient plays a much more active role.
For now, our results have several implications. When faced with the question of whether intervention A is effective and cost-effective relative to intervention B, the answer may be “it depends” instead of an unequivocal “yes” or “no,” when referring to the average patient. Targeting treatments at specific risk groups may result in better outcomes and better use of resources. Targeting those with HbA1c >8.5% (69 mmol/mol), those who are most in need, is preferable to targeting those who have little to gain. Especially in low- and middle-income countries, targeted implementation might reduce health care expenditures (3).
Future research should confirm our findings in primary care and investigate risk profiles other than HbA1c. These might include BMI or waist circumference or cardiovascular risk factors that predict cardiovascular events.
Targeting interventions to the highest risk population may allow resources to be better used, costs to be reduced, and negative side effects to be reduced by avoiding unnecessary use of medications. Focusing on HbA1c and examining a variety of HbA1c reduction strategies is valuable for patients, health care organizations, and the economy.
Acknowledgments
This trial was funded by a grant from the Netherlands Ministry of Health, Welfare, and Sport.
No potential conflicts of interest relevant to this article were reported.
The sponsor had no involvement in the design, execution, and report of this study.
A.S.S. collected, researched, and discussed the data and wrote, reviewed, and edited the manuscript. W.H.H. and J.W.J. contributed to discussion and reviewed and edited the manuscript. W.K.R. researched the data, contributed to the discussion, and reviewed and edited the manuscript. R.F.D. collected and researched the data, contributed to the discussion, and reviewed and edited the manuscript. L.W.N. collected and researched the data, contributed to the discussion, and reviewed and edited the manuscript. L.W.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all patients and their doctors and other health staff at the affiliated institutions and elsewhere, W.S.H. van Rijn-d’Hane and C. van Rijn (Leiden University Medical Center, Leiden, the Netherlands) for navigating with effectiveness and cost-effectiveness through generations, and Dr. Willem Jan Meerding and Ewout W. Steyerberg (Center for Medical Decision Making, Department of Public Health, Erasmus University Rotterdam, Rotterdam, the Netherlands) for suggestions and statistical approval. The authors thank the reviewers for their valuable suggestions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1865/-/DC1.
Clinical trial reg. no. ISRCTN35851744, www.isrctn.org.
References
- 1.Li R, Zhang P, Barker LE, Chowdhury FM, Zhang X. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010;33:1872–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Diabetes Cost-effectiveness Group Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–2551 [DOI] [PubMed] [Google Scholar]
- 3.Barceló A, Aedo C, Rajpathak S, Robles S. The cost of diabetes in Latin America and the Caribbean. Bull World Health Organ 2003;81:19–27 [PMC free article] [PubMed] [Google Scholar]
- 4.Tarride JE, Hopkins R, Blackhouse G, et al. A review of methods used in long-term cost-effectiveness models of diabetes mellitus treatment. Pharmacoeconomics 2010;28:255–277 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Prevention of Diabetes Mellitus. Report of a WHO Study Group Geneva, World Health Org., 1994 (Tech. Rep. Ser., no. 844) [PubMed]
- 6.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005;365:1333–1346 [DOI] [PubMed] [Google Scholar]
- 7.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–787 [DOI] [PubMed] [Google Scholar]
- 8.International Diabetes Federation. IDF Diabetes Atlas, Fifth Edition [Internet]. Available from http://www.idf.org/diabetesatlas/5e/the-global-burden Accessed 5 June 2013
- 9.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Wagner EH. Patient-level estimates of the cost of complications in diabetes in a managed-care population. Pharmacoeconomics 1999;16:285–295 [DOI] [PubMed] [Google Scholar]
- 10.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997;20:735–744 [DOI] [PubMed] [Google Scholar]
- 11.Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care 1998;21:1167–1172 [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 13.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 14.van der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: The Hoorn Study. Diabetes Care 2009;32:2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleveringa FG, Welsing PM, van den Donk M, et al. Cost-effectiveness of the diabetes care protocol, a multifaceted computerized decision support diabetes management intervention that reduces cardiovascular risk. Diabetes Care 2010;33:258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schouten LM, Niessen LW, van de Pas JW, Grol RP, Hulscher ME. Cost-effectiveness of a quality improvement collaborative focusing on patients with diabetes. Med Care 2010;48:884–891 [DOI] [PubMed] [Google Scholar]
- 17.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med 2004;140:689–699 [DOI] [PubMed] [Google Scholar]
- 18.Mensing C, Boucher J, Cypress M, et al. National standards for diabetes self-management education. Diabetes Care 2006;29(Suppl. 1):S78–S85 [PubMed] [Google Scholar]
- 19.Gillett M, Dallosso HM, Dixon S, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost-effectiveness analysis. BMJ 2010;341:c4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobden DS, Niessen LW, Barr CE, Rutten FF, Redekop WK. Relationships among self-management, patient perceptions of care, and health economic outcomes for decision-making and clinical practice in type 2 diabetes. Value Health 2010;13:138–147 [DOI] [PubMed]
- 21.van ‘t Riet E, Rijkelijkhuizen JM, Alssema M, et al. HbA1c is an independent predictor of non-fatal cardiovascular disease in a Caucasian population without diabetes: a 10-year follow-up of the Hoorn Study. Eur J Prev Cardiol 2012;19:23–31 [DOI] [PubMed] [Google Scholar]
- 22.Ginde AA, Cagliero E, Nathan DM, Camargo CA., Jr Value of risk stratification to increase the predictive validity of HbA1c in screening for undiagnosed diabetes in the US population. J Gen Intern Med 2008;23:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothwell PM. Can overall results of clinical trials be applied to all patients? Lancet 1995;345:1616–1619 [DOI] [PubMed]
- 25.Coyle D. Determining the optimal combinations of mutually exclusive interventions: a response to Hutubessy and colleagues. Health Econ 2003;12:159–162; discussion 163–164 [DOI] [PubMed] [Google Scholar]
- 26.Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461–475 [DOI] [PubMed] [Google Scholar]
- 27.Cao MM, Tong NW. Stratifying and tailoring HbA1c control targets for adults with type 2 diabetes: interpretation of the consensus proposed by the Chinese Society of Endocrinology. J Diabetes 2011;3:201–207 [DOI] [PubMed] [Google Scholar]
- 28.Lauritzen T, Sandbaek A, Skriver MV, Borch-Johnsen K. HbA1c and cardiovascular risk score identify people who may benefit from preventive interventions: a 7 year follow-up of a high-risk screening programme for diabetes in primary care (ADDITION), Denmark. Diabetologia 2011;54:1318–1326 [DOI] [PubMed] [Google Scholar]
- 29.Oostenbrink JB, Koopmanschap MA, Rutten FF. Standardisation of costs: the Dutch Manual for Costing in economic evaluations. Pharmacoeconomics 2002;20:443–454 [DOI] [PubMed] [Google Scholar]
- 30.Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolffenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care 2002;25:458–463 [DOI] [PubMed] [Google Scholar]
- 31.Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care 1997;20:725–734 [DOI] [PubMed] [Google Scholar]
- 32.Gravelle H, Brouwer W, Niessen L, Postma M, Rutten F. Discounting in economic evaluations: stepping forward towards optimal decision rules. Health Econ 2007;16:307–317 [DOI] [PubMed] [Google Scholar]
- 33.Brouwer WB, Niessen LW, Postma MJ, Rutten FF. Need for differential discounting of costs and health effects in cost effectiveness analyses. BMJ 2005;331:446–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray A, Raikou M, McGuire A, et al. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41). United Kingdom Prospective Diabetes Study Group. BMI 2000;320:1373–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 2003;163:1637–1641 [DOI] [PubMed] [Google Scholar]
- 36.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251–264 [DOI] [PubMed] [Google Scholar]
- 37.CDC Diabetes Cost-effectiveness Group Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–2551 [DOI] [PubMed] [Google Scholar]
- 38.Huang ES, Shook M, Jin L, Chin MH, Meltzer DO. The impact of patient preferences on the cost-effectiveness of intensive glucose control in older patients with new-onset diabetes. Diabetes Care 2006;29:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark LT. Issues in minority health: atherosclerosis and coronary heart disease in African Americans. Med Clin North Am 2005;89:977–1001,994 [DOI] [PubMed]
- 40.Hauber A, Gale EA. The market in diabetes. Diabetologia 2006;49:247–252 [DOI] [PubMed] [Google Scholar]
- 41.Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009;21:CD005268. [DOI] [PMC free article] [PubMed] [Google Scholar]