Abstract
OBJECTIVE
Antidepressant use has risen sharply over recent years. Recent concerns that antidepressants may adversely affect glucose metabolism require investigation. Our aim was to assess the risk of type 2 diabetes associated with antidepressants through a systematic review.
RESEARCH DESIGN AND METHODS
Data sources were MEDLINE, Embase, PsycINFO, The Cochrane Library, Web of Science, meeting abstracts of the European Association for the Study of Diabetes, American Diabetes Association, and Diabetes UK, Current Controlled Trials, ClinicalTrials.gov, U.K. Clinical Research Network, scrutiny of bibliographies of retrieved articles, and contact with relevant experts. Relevant studies of antidepressant effects were included. Key outcomes were diabetes incidence and change in blood glucose (fasting and random).
RESULTS
Three systemic reviews and 22 studies met the inclusion criteria. Research designs included 1 case series and 21 observational studies comprising 4 cross-sectional, 5 case-control, and 12 cohort studies. There was evidence that antidepressant use is associated with type 2 diabetes. Causality is not established, but rather, the picture is confused, with some antidepressants linked to worsening glucose control, particularly with higher doses and longer duration, others linked with improved control, and yet more with mixed results. The more recent, larger studies, however, suggest a modest effect. Study quality was variable.
CONCLUSIONS
Although evidence exists that antidepressant use may be an independent risk factor for type 2 diabetes, long-term prospective studies of the effects of individual antidepressants rather than class effects are required. Heightened alertness to potential risks is necessary until these are complete.
Antidepressant medication use has risen sharply over recent years, with 46.7 million prescriptions for antidepressants issued in the U.K. in 2011 compared with 20.1 million in 1999 (1). Recently, there have been concerns that antidepressants may adversely affect glucose metabolism, not least because some antidepressants induce significant weight gain, which may contribute to insulin resistance (2). The noradrenergic nortriptyline and selective serotonin reuptake inhibitors (SSRIs) have been reported to worsen glycemic control in people with diabetes (3,4) whereas tricyclic antidepressants induce hyperglycemia in humans (5) and hyperinsulinemia in mice (6). Because antidepressants may be used in people at higher risk of developing diabetes per se, and disentangling a drug effect from this complex relationship is challenging (7), we therefore aimed to review whether antidepressants are associated with an increased diabetes risk in people without diabetes.
RESEARCH DESIGN AND METHODS
Data sources and searches
The following electronic databases were searched using the Scopus abstract and citation database: The Cochrane Library (Issue 1, 2010), MEDLINE, Embase, Science Citation Index Expanded, Social Sciences Citation Index, Conference Proceedings Citation Index–Science, and PsycINFO. Additional sources were hand searched, including meeting abstracts of the European Association for the Study of Diabetes, American Diabetes Association, Diabetes UK; Psychosocial Aspects of Diabetes study group; Current Controlled Trials, ClinicalTrials.gov, and U.K. Clinical Research Network. The search terms used were antidepressant, antidepressants, impaired fasting glucose, impaired glucose tolerance, and diab*. The reference lists of all included studies were searched manually, and experts in the field were contacted for details of additional relevant studies.
Study selection
The general principles recommended by the Centre for Reviews and Dissemination were followed (8). Eligible studies met the following criteria: adults ≥18 years of age who were prescribed antidepressants and assessed the incidence or prevalence of diabetes or measured blood glucose levels during the study. Any study design was acceptable if comparative data on antidepressant use in the target group were reported in a peer-reviewed journal in the past 25 years. No language restrictions were applied.
Data extraction and quality assessment
Identified abstracts were examined for inclusion by all authors, with full text articles obtained and reviewed independently by all authors. A quality assessment for each included study was performed using tools appropriate to the study design based on the “Crombie” criteria for assessment of cross-sectional studies (9) adapted by Petticrew and Roberts (10) and the Critical Appraisal Skills Program (CASP) for cohort and longitudinal studies (11). Quality was assessed independently by two investigators (K.B. and R.I.G.H.), who also extracted data independently by using a standardized data extraction table.
Data synthesis and analysis
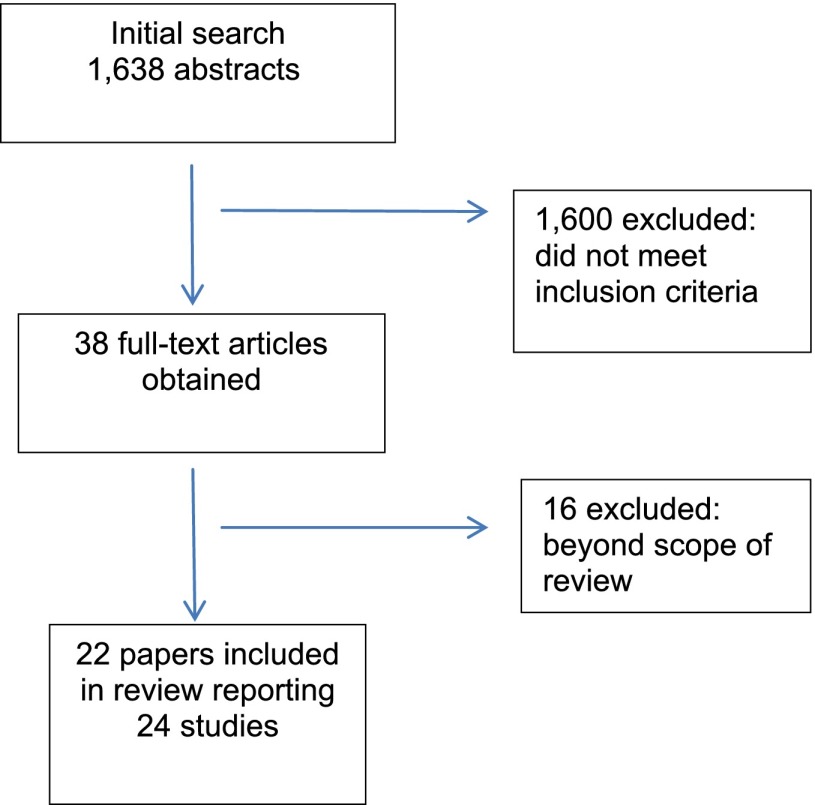
A meta-analysis was not possible owing to study heterogeneity including differences in outcome measures. Studies were, therefore, subjected to a narrative synthesis and critical appraisal (Fig. 1).
Figure 1.
Flow diagram of selection process.
RESULTS
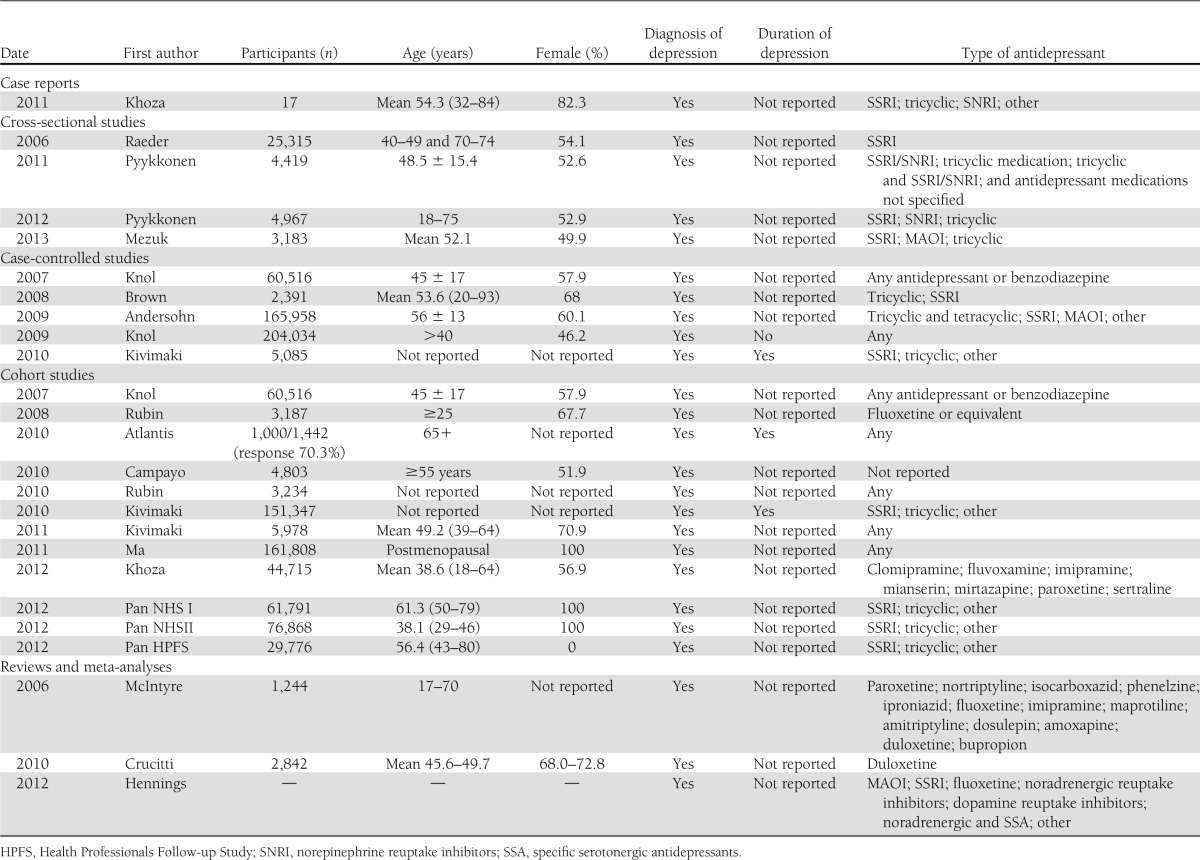
Three systemic reviews and 22 studies met the inclusion criteria. Research designs included 1 case series and 21 observational studies comprising 4 cross-sectional, 5 case-control, and 12 cohort studies (Table 1).
Table 1.
Data extraction from included articles
Case series (n = 1)
A review of 17 case reports of hyperglycemia or hypoglycemia associated with the use of a variety of antidepressants included one patient with worsening hyperglycemia and three patients with incident diabetes including one with of diabetic ketoacidosis (5). The case patients were three women (24, 44, and 84 years of age) and one man (37 years of age) with previously normal glucose. The cases involved three different antidepressants: paroxetine, clomipramine, and mirtazapine (n = 2). Hyperglycemia was found between 3 weeks and 5 months after antidepressant treatment initiation, and the highest recorded blood glucose was 459 mg/dL. The hyperglycemia resolved for all patients, most within a week of discontinuation of treatments.
Observational studies
Cross-sectional (n = 4).
Four cross-sectional studies have examined the association between antidepressant use and the risk of diabetes. Although these studies confirm a relationship between diabetes and depression, this association was not explained by antidepressant use.
Two studies from the Finnish PPP (Prevalence, Prediction and Prevention of Diabetes)-Botnia program examined the relationship between depressive symptoms and glucose metabolism (12,13). In the first study, 4,419 individuals underwent an oral glucose tolerance test and self-reported depressive symptoms (12). Antidepressant medication was recorded in 110 women and 37 men. Although depressive symptoms were associated with insulin resistance, this association was neither augmented nor explained by antidepressant use.
In the second study, which involved 4,967 adults selected at random from the population registry, diabetes was defined by medical history or results of a 75-g oral glucose tolerance test, and depressive symptoms were assessed by using the five-item Mental Health Index (MHI-5) (13). Antidepressant use was reported by 3.7% without established diabetes and cardiovascular disease. Depressive symptoms were associated with elevated 2-h blood glucose, but there was no association with antidepressant use. Furthermore, although antidepressant users had more than 50% increased odds for having the metabolic syndrome, this association was no longer statistically significant (odds ratio [OR] 1.46 [95% CI 0.99–2.14], P = 0.054) after adjustment for confounders. However, antidepressant use was independently associated with increased triglycerides, waist circumference, and systolic blood pressure.
Rates of diabetes and components of the metabolic syndrome were examined in 461 people from the Hordaland Health Survey, Norway, taking SSRIs and compared with 25,315 participants not taking antidepressants (14). Diabetes was diagnosed through self-report, treatment with a diabetes medication or random glucose above 200 mg/dL. They found 2.8% (n = 13) of those taking SSRIs had diabetes compared with 1.8% of those taking no psychotropic medication (adjusted OR 1.43 [95% CI 0.73–2.80], P = 0.293). Antidepressant use was associated with a higher prevalence of obesity and hypercholesterolemia (P ≤ 0.05). Diabetes prevalence was 3.8% in people taking paroxetine, 2.1% in those taking citalopram, and 2.3% in those taking other SSRIs; there was no statistical difference among the groups owing to the low numbers of individuals with diabetes.
The population-based cross-sectional 2005 and 2007 National Health and Nutrition Examination Studies explored the association between clinically identified and undiagnosed prediabetes and type 2 diabetes with depression and antidepressants (15). The study found 8.8% (n = 419) had clinically identified diabetes, 3.5% (n = 126) had clinically identified prediabetes, 3.1% (n = 131) had undiagnosed type 2 diabetes, 38.7% (n = 1,213) had undiagnosed prediabetes, and the remaining 45.8% (n = 1,294) were normoglycemic. Those with clinically identified diabetes reported a greater frequency of health care visits in the past year. Clinically identified diabetes and prediabetes were associated with major depression, even after accounting for health behaviors (adjusted OR 4.26 [95% CI 2.00–9.07], P < 0.001). Undiagnosed diabetes was not associated with depression (crude OR 1.06 [95% CI 0.59–1.89], P = 0.850; adjusted OR 1.35 [95% CI 0.70–2.59], P = 0.375). Clinically identified diabetes and prediabetes were significantly associated with antidepressant use (OR 1.75 [95% CI 1.20–2.54], P = 0.004), but undiagnosed diabetes was not associated with antidepressant use (crude OR 0.78 [95% CI 0.59–1.04], P = 0.094; adjusted OR 0.86 [95% CI 0.66–1.13], P = 0.278). These results persisted when prediabetes was excluded from the analysis.
Case-control studies (n = 5).
In contrast to the cross-sectional studies, five case-control studies since 2008 have demonstrated up to an approximate doubling of diabetes risk in people receiving antidepressants.
In a cohort of 165,958 patients with depression from the U.K. General Practice Research Database who had received at least one new prescription for an antidepressant, the 2,243 people who developed diabetes between 1990 and 2005 were matched with 8,963 healthy individuals (16). Diabetes was diagnosed in people who had received at least one prescription for a diabetes drug, the recording of a diagnosis of diabetes on two separate occasions, or recording of a diagnosis of diabetes and a diabetes-specific test (e.g., glycated hemoglobin) on two separate occasions. Compared with individuals who had not used antidepressants during the past 2 years before the diagnosis of diabetes, recent long-term use (>24 months) of antidepressants in moderate to high daily doses was associated with an increased risk of diabetes (incidence rate ratio 1.84 [95% CI 1.35–2.52]). The magnitude of the risk was similar for long-term use of tricyclic antidepressants (incidence rate ratio 1.77 [95% CI 1.21–2.59]) and SSRIs (incidence rate ratio 2.06 [95% CI 1.20–3.52]). Shorter duration of treatment or lower doses was not associated with an increased risk.
Antidepressant use was examined in 49,593 people with diabetes and a random sample of 154,441 people without diabetes included in a pharmacy database in the Netherlands (17). Antidepressant use was only increased 2 months before and 3 months after initiation of diabetes treatment, with the marked increase in the incidence of antidepressant use in the month after initiation of diabetes treatment, with incidence rate ratio of 2.4 (95% CI 2.0–3.0).
A Finnish occupational study compared 851 people who developed type 2 diabetes between 1 January 2001 and 31 December 2005 with 4,234 individuals who remained diabetes-free during the same period (18). Each diabetes case subject was matched for age-group, sex, socioeconomic position, type of employer and employment contract, and geographic area. Diabetes was defined by a physician-recorded elevated glucose in association with diabetes symptoms or two or more elevated glucose measurements. Antidepressant use was associated with a doubling of diabetes risk in participants with no indication of severe depression (OR 1.93 [95% CI 1.48–2.51]) as well as participants with severe depression (OR 2.65 [95% CI 1.31–5.39]). There was a weaker association between severe depression and incident diabetes among nonusers of antidepressants (OR 1.20 [95% CI 0.64 –2.25]) and users of antidepressants (OR 1.65 [95% CI 1.09–2.48]). These associations remained after adjustment for current physical illness. In a mutually adjusted model, the excess diabetes risk associated with antidepressant use was reduced by 21.0% when depression severity was included in the model. The excess diabetes risk associated with severe depression was attenuated by 68.4% when antidepressant use was included.
In a further analysis of the Finnish Public Sector Study involving 493 individuals who developed type 2 diabetes and 2,450 matched participants without diabetes, antidepressant use was twofold higher among people who developed diabetes (adjusted OR 2.00 [95% CI 1.57–2.55]) (19). Antidepressant use was increased in the 4 years before and in the 4 years after diabetes diagnosis, although there was a temporary peak in antidepressant use during the year of the diabetes diagnosis (OR 2.66 [95% CI 1.94–3.65].
A Canadian case-control study compared different antidepressants. The 2,391 participants had depression and were treated with antidepressant therapy (20). The 1,037 individuals who developed diabetes were compared with 1,354 who did not. Diabetes was diagnosed by physician report or the prescription of an antidiabetes medication. After multivariate adjustment, the concurrent use of SSRIs and tricyclic antidepressants was associated with a significantly increased risk of type 2 diabetes compared with the use of tricyclic antidepressants alone (adjusted OR 1.89 [95% CI 1.35–2.65]). By contrast, there was no difference in the risk of diabetes between those taking SSRIs alone and tricyclic antidepressants alone (adjusted OR 1.05 [95% CI 0.86–1.28]).
Cohort studies (n = 12).
Twelve cohort studies examining the relationship between antidepressants and diabetes have been published since 2008. In general, these show an increased risk of diabetes in those taking antidepressants, with hazard ratios (HRs) up to 3.5. Not all of the studies show a statistically significant increased risk, however, and the most recent larger studies show smaller HRs of <1.6, indicating a weak association.
The effect of antidepressants on the risk of diabetes was examined within the Diabetes Prevention Program (DPP) and its epidemiological follow-up study, the Diabetes Prevention Program Outcomes Study (DPPOS) (21,22). In brief, the DPP randomized 3,234 people at high diabetes risk to an intensive lifestyle intervention (ILS), standard lifestyle advice, and metformin (MET), 850 mg twice daily, or standard lifestyle advice plus a twice-daily MET placebo (PLB). At baseline, 3,187 participants completed the Beck Depression Inventory, and those hospitalized in the previous 6 months with severe depression were excluded, as were those who had used bupropion or any other antidepressant in a daily dose greater than the minimum therapeutic dose for that agent. Mixed-effects modeling compared differences in continuous variables, such as weight, by antidepressant use or depression symptoms. For categorical variables, such as sex, repeated-measures modeling was used to compare differences by antidepressant use or elevated depression symptoms.
Diabetes was diagnosed by an annual oral glucose tolerance test or fasting plasma glucose every 6 months using the 1997 American Diabetes Association criteria. At baseline, 5.7% of participants were taking antidepressants. Intermittent antidepressant use was reported for 7.2% of total person-years and continuous antidepressant use for 3.2% of total person-years. Baseline antidepressant use was strongly associated with diabetes risk for participants in the PLB (HR 2.25 [95% CI 1.38–3.66]) and ILS (HR 3.48 [95% CI 1.93–6.28]) groups but not the MET arm (21). Continuous antidepressant use was also significantly associated with diabetes risk in the PLB (HR 2.60 [95% CI 1.37–4.94] and ILS arms (HR 3.39 [95% CI 1.61–7.13]). Intermittent antidepressant use was only associated with increased diabetes risk in the ILS arm (HR 2.07 [95% CI 1.18–3.62]). The increased risk did not differ between antidepressants. The increased risk did not appear confounded by indication, because elevated depression scores were not associated with increased diabetes risk. A total of 2,665 participants were subsequently enrolled into DPPOS and assessed for diabetes every 6 months for a median of 10.0 years (22). Similar increased risks of diabetes were observed with continuous antidepressant use in the PLB (HR 2.34 [95% CI 1.32–4.15]) and ILS arms (HR 2.48 [95% CI 1.45– 4.22]) but not in the MET arm (HR 0.55 [95% CI 0.25–1.19]), where the risk was significantly lower.
A representative sample of 1,000 noninstitutionalized Australian people over 65 years of age living in Melbourne between 1994 and 2004 were followed up biennially by telephone interview to determine diabetes risk (23). Only 48 of the 110 participants (11%) classified as depressed at baseline were prescribed antidepressants. The Psychogeriatric Assessment Scales depression scale was used to measure depressive symptoms, and incident diabetes was determined by self-report. During the 10-year follow-up, 20 people with depression (74.5 per 1,000 patient-years) and 135 without depression (34.1 per 1,000 patient-years) developed diabetes (HR 2.23 [95% CI 1.40–3.58]). Antidepressant use was associated with a twofold increased diabetes risk (HR 2.02 [95% CI 1.03–3.97], P = 0.041), but after adjustment for significant demographic, lifestyle, functional health, and prevalent chronic disease predictors, including persistent depressive symptoms, the increased HR was no longer statistically significant (HR 1.80 [95% CI 0.91–3.57]). No attempt was made to assess differences between different types of antidepressant, but none of the participants were receiving SSRIs.
A 5-year community-based prospective cohort study in Zaragoza, Spain, examined the relationship between baseline depressive symptoms, antidepressant use, and incident diabetes in 3,521 people aged ≥55 years (24). All participants had a psychiatric interview, and depression was assessed using the Geriatric Mental State Schedule. Diabetes was assessed by self-report. At baseline, 379 people (10.8%) had depression, and 64 (16.9%) were taking antidepressants. There were 163 new cases of diabetes, 25 (6.6%) in those with depression and 138 (4.4%) in those without, giving an overall incidence rate of 13.1 per 1,000 patient-years. The risk of developing diabetes was higher in those with depression (fully adjusted HR 1.65 [95% CI 1.02–2.66]). Antidepressant use was not associated with an increased diabetes risk (HR 1.26 [95% CI 0.63–2.50]).
The Women’s Health Initiative dataset was used to study the effect of elevated depressive symptoms and antidepressant use on the risk of diabetes in 161,808 postmenopausal women followed up for an average of 7.6 years (25). The women were studied annually, and incident diabetes was determined by self-report in the 152,250 women who did not have diabetes at baseline. Depressive symptoms at baseline and year 3 were measured using the Center for Epidemiological Studies Depression Scale (CES-D) six-item form. At baseline, 15.5% of the women were above the CES-D depression cutoff, and 6.9% reported using antidepressants. The cumulative incidence of self-reported diabetes was 6.7%, with an 8.6% rate in women with elevated depressive symptoms. The unadjusted HR for diabetes in women with elevated depressive symptoms was 1.38 (95% CI 1.32–1.45). After adjustment for potential confounders, including age, race/ethnicity, education, smoking status, BMI, recreational physical activity, alcohol intake, dietary energy intake, family history of diabetes, and hormone therapy use, the HR was reduced to 1.13 (95% CI 1.07–1.20). Antidepressant use was associated with an increased risk of diabetes (1.18 [95% CI 1.10–1.28]). Self-reported diabetes incidence rates were highest, at 9.6%, in women with high depressive symptoms and who were also taking antidepressants, compared with 6.3% for those not taking antidepressants and below the CES-D cutoff, 7.6% for those taking antidepressants and below the CES-D cutoff, and 8.4% for those above the CES-D cutoff and not taking antidepressants (P < 0.001). Furthermore diabetes risk was higher in women who reported depressive symptoms and taking antidepressants at baseline and at the 3-year follow-up compared with those who reported depressive symptoms and antidepressant use at one time point.
Data from the Health Professionals Study (1990–2006), the Nurses’ Health Study (1996–2008), and Nurses’ Health Survey II (1993–2005) were pooled to assess the risk of diabetes associated with antidepressant use (26). These studies included 29,776 men and 138,659 women with a total of 1,644,679 person-years of follow-up. Antidepressant use was assessed biennially, and in the latter parts of the study, the types of antidepressant were also recorded. The number of men receiving antidepressants (n = 365) was much lower than women (n = 12,747). Depressive symptoms were assessed in the Nurses Health Studies using the MHI-5. A diagnosis of diabetes was considered confirmed if the participants reported one or more classic symptom plus fasting plasma glucose concentration of ≥140 mg/dL or random plasma glucose of ≥200 mg/dL, at least two elevated plasma glucose values on different occasions, or treatment with hypoglycemic medication. In June 1998, the fasting glucose threshold was lowered to 126 mg/dL in line with the change in diagnostic criteria.
Multivariate analysis included adjustment for age, ethnicity, marital and living status, smoking status, alcohol intake, physical activity, current multivitamin and aspirin use, a family history of diabetes, quintile of dietary score, and major comorbidities. In women, adjustment was also made for menopausal status and use of hormones such as oral contraceptives. There were 1,287 incident cases of type 2 diabetes during 16 years of follow-up in the Health Professionals Study, a further 3,514 during the Nurses’ Health Study, and 1,840 in the Nurses’ Health Survey II. Although baseline antidepressant use did not predict the risk of diabetes, overall use was associated with an increased risk of diabetes in all three cohorts in age-adjusted models (pooled HR 1.68 [95% CI 1.27–2.23]), suggesting that recent antidepressant use might be more relevant to an elevated risk. The average absolute risk difference between women taking antidepressants and nonusers was 2.87 per 1,000 person-years. The association was attenuated after adjustment for diabetes risk factors and histories of high cholesterol and hypertension (unpooled HR 1.30 [95% CI 1.14–1.49]) and was further attenuated by controlling for updated BMI (HR 1.17 [95% CI 1.09–1.25]). The HRs were slightly attenuated with further adjustment for MHI-5 scores in the Nurses Health Studies and became nonsignificant for the Nurses’ Health Study I (HR 1.08 [95% CI 0.97–1.19]). SSRIs and other antidepressants (mainly tricyclic antidepressants) were both associated with an elevated diabetes risk compared with no antidepressant use, with pooled multivariate-adjusted HRs of 1.10 (95% CI 1.00–1.22) and 1.26 (95% CI 1.11–1.42), respectively, but the risk did not appear to differ between antidepressant type.
A retrospective cohort analysis using the Texas Medicaid prescription claims database studied 35,552 adults receiving antidepressants and 9,163 treated with benzodiazepines between 1 January 2002 and 31 December 2009 (27). Diabetes was diagnosed when an individual began treatment with antidiabetes medication, and 2,943 people (6.6%) developed type 2 diabetes. After adjustment for age, sex, medication adherence, medication persistence, number of diabetogenic medications, Chronic Disease Score, and year of cohort entry, people receiving antidepressants had a 58% increased risk of developing diabetes (adjusted HR 1.558 [95% CI 1.401–1.734]) compared with those receiving benzodiazepines. The association was seen with tricyclic antidepressants (HR 1.759 [95% CI 1.517–2.040]), serotonin–norepinephrine reuptake inhibitors (HR 1.566 [95% CI 1.351–1.816]), SSRIs (HR 1.481 [95% CI 1.318–1.665]), and other antidepressants (HR 1.376 [95% CI 1.198–1.581]). All of the 9,197 antidepressant users, defined as ≥200 daily doses a year, from the primary retrospective analysis of an occupational cohort of 151,347 employees in Finland were followed up prospectively for ≥1 year and compared with 45,658 control participants from the same database matched for age-group, sex, socioeconomic position, type of employment contract, type of employer, and geographic area (18). Based on a mean follow-up of 4.75 years, the absolute risk of incident diabetes was 1.8% for antidepressant users and 1.1% for matched nonusers. Diabetes risk was higher in those who had taken more doses, supporting a dose-response association (200–399 daily doses: 1.7%, ≥400 daily doses: 2.3%). Compared with nonusers, diabetes risk was increased 53% in people taking 200–399 daily doses (HR 1.53 [95% CI 1.25–1.87]) and doubled in those taking ≥400 daily doses (HR 2.00 [95% CI 1.51–2.66]), respectively.
The relationship among antidepressant use, glucose levels, and diabetes status was explored in 5,978 civil servants in the Whitehall II study during an 18-year period (28). Most participants were white European men. Diabetes was recorded by self-report of a physician diagnosis, use of antidiabetes medication, or after a 75-g oral glucose tolerance test, which were undertaken approximately every 5–6 years. During the study, there were 294 incident cases of diabetes as a result of physician diagnosis and 346 screen-detected cases of diabetes. Antidepressant use was self-reported in 94 individuals (1.6%) at baseline, and overall, 419 (7.0%) reported using antidepressants at some point in the study. Depressive symptoms were assessed with CES-D. A strong association between baseline antidepressant use and incident physician-diagnosed diabetes was observed (adjusted OR 3.10 [95% CI 1.66–5.78]), but there was no association with incident study screen-detected diabetes (adjusted OR 1.24 [95% CI 0.54–2.87], P = 0.62) with no sex differences. Furthermore, after exclusion of those with physician-diagnosed diabetes, antidepressant use was not associated with a change in fasting or 2-h blood glucose. The study found evidence of reverse causality: among those who were not taking antidepressants at baseline, the proportion who began treatment with antidepressants was higher in those with physician-diagnosed diabetes (adjusted OR 1.72 [95% CI 1.02–2.88]).
In a Dutch pharmacy database study, 60,516 individuals were followed up from their first prescription for an antidepressant or benzodiazepine until end of registration or a first prescription for an antidiabetes drug (29). Although the crude diabetes incidence rate was increased in people taking antidepressants, after adjustment for age, sex, and chronic diseases, compared with people taking no psychotropic medication, the HRs for the development of diabetes were 1.05 (95% CI 0.88–1.26) for antidepressant users, 1.21 (95% CI 1.02–1.43) for benzodiazepine users, and 1.37 (95% CI 1.12–1.68) for users of antidepressants and benzodiazepine.
Systematic reviews (n = 3).
A recently published meta-analysis of 66 experimental studies dating back to 1960 included 42 studies of people without diabetes (30). Most studies involved small numbers and were of short duration. All included studies were assessed in terms of the specific pharmacodynamics properties of the antidepressants and the pathophysiological changes in depression and impaired glucose homeostasis.
Relevant studies were categorized according to medication type and effect. Four studies explored unselective and irreversible monoamine oxidase inhibitors (MAOIs), including isocarboxazid, iproniazid, and phenelzine. Eight studies looked at unselective monoaminergic reuptake inhibitors (amitriptyline, imipramine), and one studied the effect of other predominantly or selective noradrenergic reuptake inhibitors (nortriptyline, maprotiline, mianserin). Twelve studied the effect of serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine), with a further 11 exploring the effect of SSRIs, and five the effect of fluoxetine. A further six examined other antidepressants, including bupropion, mirtazapine, and tianeptine. The review concluded that antidepressants could be divided into three groups: hydrazine-type MAOIs and SSRIs were associated with improved glycemic control, whereas noradrenergic substances and possibly also dual acting antidepressants were associated with worsened glycemic control. There is an uncertain effect with bupropion, mirtazapine, and other newer agents.
In a second article, antidepressant effects on glucose-insulin homeostasis were reviewed and mechanisms of actions explored, with mixed results (31). Preclinical and clinical investigations were both included; however, only experimental clinical investigations are included here. The study populations were variable, with participant numbers being small in most studies (n = 6, 12, 13, 16, 33, 43, 48, 59, 143, 422 and 449 participants) and poorly defined baseline risk factors. Serotonergic antidepressants, such as fluoxetine, reduced hyperglycemia, normalized glucose homeostasis, and increased insulin sensitivity, whereas some noradrenergic antidepressants, such as desipramine, exerted the opposite effects. Dual-mechanism antidepressants, such as duloxetine and venlafaxine, did not appear to disrupt glucose homeostasis dynamics, whereas nonselective hydrazine MAOIs, such as phenelzine, were associated with hypoglycemia and an increased glucose disposal rate. The heterogeneity of included populations may well have obscured any effect of antidepressants on glucose metabolism.
Crucitti et al. (32) examined short- and long-term effects of duloxetine (20–120 mg/day) on glycemic control in patients with diagnoses other than diabetic peripheral neuropathic pain. Seven short-term studies (9–27 weeks) and two long-term (41 and 52 weeks) studies were included. In the short-term, no statistically significant differences were observed in baseline-to-end point changes in fasting plasma glucose or HbA1c in duloxetine-treated patients compared with those treated with placebo. In the long-term, a statistically significant increase in HbA1c was found in patients with chronic lower back pain treated with duloxetine in the 41-week open-label extension study, but this difference was not observed in the 52-week double-blind placebo-controlled study in patients with recurring major depressive disorder. Neither long-term study showed significant changes in fasting plasma glucose.
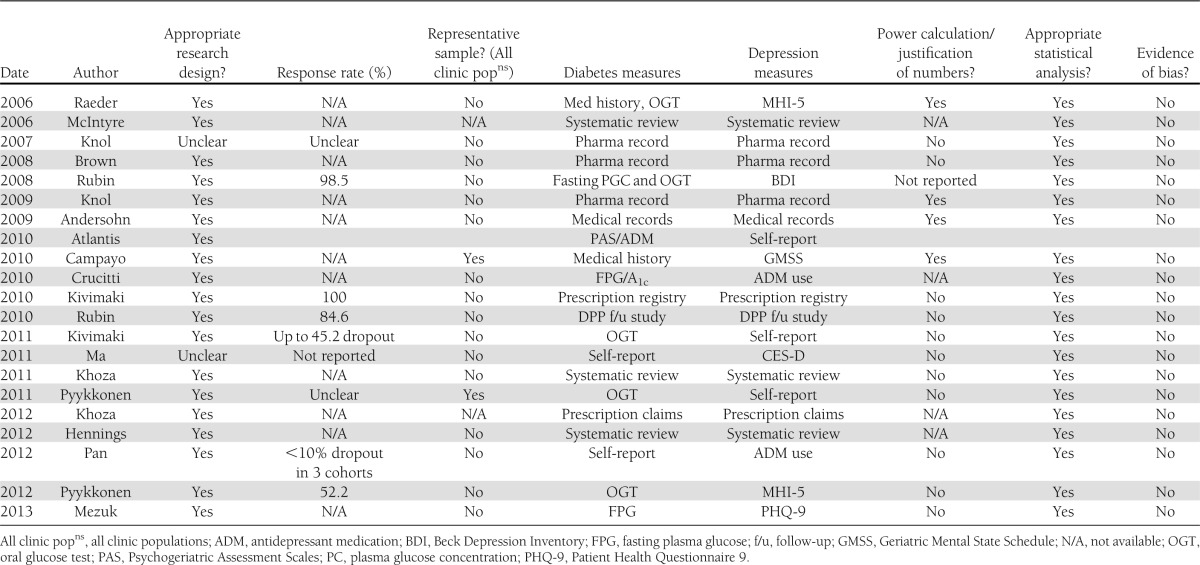
Quality of included studies
The quality of studies was variable, with shortcomings including self-report of diabetes, lack of adjustment of traditional diabetes risk factors, and little account of confounding variables (Table 2). Comparison is further hindered across studies by different measures of depression diagnoses, different measures of diabetes diagnoses, participant numbers, and variable assessment time-points.
Table 2.
Quality assurance tables
Evidence summary
This review has found evidence that some antidepressants affect glucose metabolism and that antidepressant use may be an independent risk factor for diabetes. However, the most recent studies that have included large numbers of people receiving antidepressants, such as the Nurses’ Health Study, suggest that any risk is small.
Case reports have found that certain antidepressants have been associated with the development of diabetes, which returns to normal after treatment discontinuation. Cross-sectional studies have not demonstrated an increase in diabetes prevalence, independent from depressive symptoms; however, there are changes in metabolic and body composition measurements that are associated with diabetes. Case-control studies have reported an approximate doubling of diabetes rates in those taking antidepressants, with higher rates seen with higher doses, longer duration, or as combinations. Some cohort studies have also found an increase in the incidence of diabetes in those taking antidepressants, although the most recent larger studies have shown a much lower risk than in the first studies published in 2008. There is evidence of potential confounding, because rates of undiagnosed diabetes were not increased in the one study that compared diagnosed and undiagnosed diabetes. A further study provided evidence of a dose-response, with a higher rate in those taking higher doses.
There was evidence of reverse causality in one study that reported that among those who were not taking antidepressants at baseline, the proportion that began treatment with antidepressants was higher in those with a physician diagnosis of diabetes. Experimental studies have shown that different antidepressants affect glucose metabolism in different ways, but certainly some, including noradrenergic substances, have adverse effects.
CONCLUSIONS
The evidence suggests a link between antidepressant use and diabetes, but causality is not established. The strength of association in the larger most recent cohort studies is weak, which increases the chance that the finding occurs through residual confounding. However, it is likely that most serious adverse drugs effects have weak association only because a high risk of a serious side effect would have prevented a drug reaching the market. There is inconsistency among findings from different study types regarding increased diabetes risk, although more consistency is found within the cohort and experimental studies that provide the strongest evidence.
To conclude that diabetes is a consequence of antidepressant use, the drug must be prescribed before the onset of diabetes. Although this occurs in the case histories, cohort, and experimental studies, in other studies, diabetes preceded antidepressant use, with evidence of reverse causality. There is evidence of a biological gradient (i.e., increasing exposure associated with increasing diabetes risk), because several studies have shown that diabetes is more common in those using antidepressants in a higher dose or for longer duration, or both. This may, however, be confounded with worse severity of depression, rather than antidepressants, increasing diabetes risk.
There are several plausible reasons why antidepressants may be associated with an increased diabetes risk. Several antidepressants are associated with significant weight gain, which in turn increases insulin resistance and the risk of diabetes (2,33). However, several studies still observed an increased risk of diabetes after adjustment for changes in body weight, implying that other mechanisms are involved. This is consistent with previous findings that other psychiatric drugs, for example, antipsychotics, may affect glucose metabolism by altering insulin resistance or secretion directly.
Increased antidepressant use is occurring concurrently with increasing diabetes prevalence; thus, any cause-and-effect interpretation does not conflict with generally known facts of the natural history and biology of the disease. Experimental studies provide some evidence of such a cause-and-effect relationship, but many of these are small, with differences between different drugs. These differences provide challenges for the interpretation of the many observational studies that do not separate antidepressant types. However, the observational studies that have attempted to differentiate between different drugs have not found consistent differences in risk.
Applying the Bradford Hill criteria (34) to determine causality, we find that some are fulfilled whereas others are not. The strength of association is weak and there is lack of consistency or specificity, but there is evidence for temporality for some cases of diabetes, a biological gradient, and a plausible explanation. Furthermore, the causative link between antidepressants and diabetes is coherent with our understanding of diabetes, and there are analogies with other drugs. We therefore conclude that there may be a causative link between antidepressants and diabetes but that this risk is probably low and the majority of patients receiving antidepressants will not develop diabetes as a result of their medication.
Study design variability prevents meaningful meta-analyses. Differing populations in age, baseline risk factors, and lifestyle, such as BMI and smoking status, prevent appropriate comparison. The nature of prescribed medications and patient exposure to them in duration, dose, and continuous versus intermittent use is further complicated by questions of adherence to medication regimens.
Future studies are required to answer this unresolved issue. Although some studies have examined different classes of antidepressants and found that SSRIs and other antidepressant medications have a similar association with diabetes risk, it seems essential that epidemiological studies differentiate between antidepressants rather than considering them as a whole. It will also be important to consider whether combination antidepressant treatment has additive effects. For such an epidemiological study to be adequately powered, it would be necessarily large scale. An assessment of glucose metabolism should be included in any future randomized controlled trials of antidepressants with a minimum dataset for reporting adverse metabolic consequences of treatment. Interactions with other drugs are also needed in view of the findings of the DPP study that antidepressant use with MET reduced the risk of diabetes.
In conclusion, from the evidence reviewed, there is a link between antidepressant use and diabetes, but causality is not established. Long-term prospective studies are required to assess this relationship further, but in the interim, caution is advised and a heightened alertness to the potential risk of diabetes is necessary, not least because of the large numbers of antidepressants that are prescribed.
Acknowledgments
This work was funded by the University of Southampton.
No potential conflicts of interest relevant to this article were reported.
The study was conceived and designed by all authors. K.B. undertook the literature search. K.B., R.C.P., and R.I.G.H. reviewed the abstracts and full articles. K.B. and R.I.G.H. undertook the quality analysis and wrote the first draft. All authors contributed to discussion and critically reviewed the final manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0560/-/DC1.
References
- 1.National Health Executive. Available from: http://www.nationalhealthexecutive.com/Health-Care-News/antidepressant Accessed 5 February 2013
- 2.Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry 2010;71:1259–1272 [DOI] [PubMed] [Google Scholar]
- 3.Isotani H, Kameoka K. Hypoglycemia associated with maprotiline in a patient with type 1 diabetes. Diabetes Care 1999;22:862–863 [DOI] [PubMed] [Google Scholar]
- 4.Sansone RA, Sansone LA. Driving on antidepressants: cruising for a crash? Psychiatry (Edgmont) 2009;6:13–16 [PMC free article] [PubMed] [Google Scholar]
- 5.Khoza S, Barner JC. Glucose dysregulation associated with antidepressant agents: an analysis of 17 published case reports. Int J Clin Pharmacol 2011;33:484–492 [DOI] [PubMed] [Google Scholar]
- 6.Erenmemisoglu A, Ozdogan UK, Saraymen R, Tutus A. Effect of some antidepressants on glycaemia and insulin levels of normoglycaemic and alloxan-induced hyperglycaemic mice. J Pharm Pharmacol 1999;51:741–743 [DOI] [PubMed] [Google Scholar]
- 7.Barnard KD, Skinner TC, Peveler R. The The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabet Med 2006;23:445–448 [DOI] [PubMed] [Google Scholar]
- 8.Centre for Reviews and Dissemination Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York, University of York, 2009 [Google Scholar]
- 9.Crombie I. The Pocket Guide to Critical Appraisal. London, BMJ Publishing Group, 1996 [Google Scholar]
- 10.Petticrew M, Roberts H. Systematic Reviews in the Social Sciences: A Practical Guide. Oxford, Blackwell, 2006 [Google Scholar]
- 11.Sheldon TA. Making evidence synthesis more useful for management and policy-making. J Health Serv Res Policy 2005;10(Suppl. 1):1–5 [DOI] [PubMed] [Google Scholar]
- 12.Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, Isomaa B. Depressive symptoms, antidepressant medication use, and insulin resistance: the PPP-Botnia Study. Diabetes Care 2011;34:2545–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, Isomaa B. Association between depressive symptoms and metabolic syndrome is not explained by antidepressant medication: results from the PPP-Botnia Study. Ann Med 2012;44:279–288 [DOI] [PubMed] [Google Scholar]
- 14.Raeder MB, Bjelland I, Emil Vollset S, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry 2006;67:1974–1982 [DOI] [PubMed] [Google Scholar]
- 15.Mezuk B, Johnson-Lawrence V, Lee H, et al. Is ignorance bliss? Depression, antidepressants, and the diagnosis of prediabetes and type 2 diabetes. Health Psychol 2013;32:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry 2009;166:591–598 [DOI] [PubMed] [Google Scholar]
- 17.Knol MJ, Geerlings MI, Grobbee DE, Egberts AC, Heerdink ER. Antidepressant use before and after initiation of diabetes mellitus treatment. Diabetologia 2009;52:425–432 [DOI] [PubMed] [Google Scholar]
- 18.Kivimäki M, Hamer M, Batty GD, et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care 2010;33:2611–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kivimäki M, Tabák AG, Lawlor DA, et al. Antidepressant use before and after the diagnosis of type 2 diabetes: a longitudinal modeling study. Diabetes Care 2010;33:1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown LC, Majumdar SR, Johnson JA. Type of antidepressant therapy and risk of type 2 diabetes in people with depression. Diabetes Res Clin Pract 2008;79:61–67 [DOI] [PubMed] [Google Scholar]
- 21.Rubin RR, Ma Y, Marrero DG, et al. Diabetes Prevention Program Research Group Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care 2008;31:420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin RR, Ma Y, Peyrot M, et al. Diabetes Prevention Program Research Group Antidepressant medicine use and risk of developing diabetes during the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care 2010;33:2549–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atlantis E, Browning C, Sims J, Kendig H. Diabetes incidence associated with depression and antidepressants in the Melbourne Longitudinal Studies on Healthy Ageing (MELSHA). Int J Geriatr Psychiatry 2010;25:688–696 [DOI] [PubMed] [Google Scholar]
- 24.Campayo A, de Jonge P, Roy JF, et al. ZARADEMP Project Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry 2010;167:580–588 [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Balasubramanian R, Pagoto SL, et al. Elevated depressive symptoms, antidepressant use, and diabetes in a large multiethnic national sample of postmenopausal women. Diabetes Care 2011;34:2390–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan A, Sun Q, Okereke OI, et al. Use of antidepressant medication and risk of type 2 diabetes: results from three cohorts of US adults. Diabetologia 2012;55:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoza S, Barner JC, Bohman TM, Rascati K, Lawson K, Wilson JP. Use of antidepressant agents and the risk of type 2 diabetes. Eur J Clin Pharmacol 2012;68:1295–1302 [DOI] [PubMed] [Google Scholar]
- 28.Kivimäki M, Batty GD, Jokela M, et al. Antidepressant medication use and risk of hyperglycemia and diabetes mellitus: a noncausal association? Biol Psychiatry 2011;70:978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knol MJ, Geerlings MI, Egberts AC, Gorter KJ, Grobbee DE, Heerdink ER. No increased incidence of diabetes in antidepressant users. Int Clin Psychopharmacol 2007;22:382–386 [DOI] [PubMed] [Google Scholar]
- 30.Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des 2012;18:5900–5919 [DOI] [PubMed] [Google Scholar]
- 31.McIntyre RS, Soczynska JK, Konarski JZ, Kennedy SH. The effect of antidepressants on glucose homeostasis and insulin sensitivity: synthesis and mechanisms. Expert Opin Drug Saf 2006;5:157–168 [DOI] [PubMed] [Google Scholar]
- 32.Crucitti A, Zhang Q, Nilsson M, Brecht S, Yang CR, Wernicke J. Duloxetine treatment and glycemic controls in patients with diagnoses other than diabetic peripheral neuropathic pain: a meta-analysis. Curr Med Res Opin 2010;26:2579–2588 [DOI] [PubMed] [Google Scholar]
- 33.Holt RI, Peveler RC. Association between antipsychotic drugs and diabetes. Diabetes Obes Metab 2006;8:125–135 [DOI] [PubMed] [Google Scholar]
- 34.Bradford Hill A. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300 [PMC free article] [PubMed] [Google Scholar]