Abstract
OBJECTIVE
We sought to determine whether food insecurity is associated with worse glycemic, cholesterol, and blood pressure control in adults with diabetes.
RESEARCH DESIGN AND METHODS
We conducted a cross-sectional analysis of data from participants of the 1999–2008 National Health and Nutrition Examination Survey. All adults with diabetes (type 1 or type 2) by self-report or diabetes medication use were included. Food insecurity was measured by the Adult Food Security Survey Module. The outcomes of interest were proportion of patients with HbA1c >9.0% (75 mmol/mol), LDL cholesterol >100 mg/dL, and systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg. We used multivariable logistic regression for analysis.
RESULTS
Among the 2,557 adults with diabetes in our sample, a higher proportion of those with food insecurity (27.0 vs. 13.3%, P < 0.001) had an HbA1c >9.0% (75 mmol/mol). After adjustment for age, sex, educational attainment, household income, insurance status and type, smoking status, BMI, duration of diabetes, diabetes medication use and type, and presence of a usual source of care, food insecurity remained significantly associated with poor glycemic control (odds ratio [OR] 1.53 [95% CI 1.07–2.19]). Food insecurity was also associated with poor LDL control before (68.8 vs. 49.8, P = 0.002) and after (1.86 [1.01–3.44]) adjustment. Food insecurity was not associated with blood pressure control.
CONCLUSIONS
Food insecurity is significantly associated with poor metabolic control in adults with diabetes. Interventions that address food security as well as clinical factors may be needed to successfully manage chronic disease in vulnerable adults.
Diabetes is a common condition in the adult population (1). Failure to achieve recommended levels of cardiometabolic parameters such as HbA1c, LDL cholesterol, and blood pressure is associated with significant morbidity and mortality (1). Socioeconomically disadvantaged patients have increased risk of diabetes-related morbidity (2) and mortality (3), prompting a search for specific actionable factors that drive these disparities in diabetes outcomes.
One potentially modifiable risk factor for adverse diabetes outcomes among socially disadvantaged populations is food insecurity, which is defined as “limited or uncertain availability of nutritionally adequate and safe foods or limited or uncertain ability to acquire acceptable foods in socially acceptable ways” (4). Thus, food insecurity represents a state of uncertainty as to whether enough food will be available for the household. It may include changes in eating habits, such as substituting high-calorie, lower-cost food for healthier but more expensive choices (5), or forgoing meals altogether due to lack of resources. In 2011, ∼18 million American households were food insecure (6). Although related to household income, food insecurity exists in households with incomes far above the federal poverty line, whereas many in poverty remain food secure (6).
Previous work has demonstrated an association between food insecurity and the prevalence of diabetes (7). Prior studies in safety-net clinics (8,9) have suggested that food insecurity may be associated with worse glycemic control but did not address control of lipids or hypertension. Furthermore, because of the setting of these studies, the generalizability of their results to adults outside of the safety net is unclear. A population-based study of all adults with diabetes could address these issues; such a study has not been conducted. To address these gaps in evidence, we examined the association between food insecurity and measures of cardiometabolic control in a national sample of adults with diabetes.
RESEARCH DESIGN AND METHODS
Data source and study sample
We analyzed pooled cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) cycles. NHANES is a series of large, cross-sectional surveys conducted by the National Center for Health Statistics (NCHS) for the Centers for Disease Control and Prevention (CDC) in community-dwelling participants designed to generate estimates of population health (10). Since 1999, NHANES has been conducted in 2-year survey “cycles.” NHANES interviewers administer a questionnaire in randomly selected participant homes, in English or Spanish or with an interpreter (10). Participants then travel to a mobile examination center (MEC), where physical examinations and nonfasting blood work are performed (10). A smaller, random subsample submits fasting blood work (10). Full details of NHANES methods have been previously described (11).
Our study includes all adult NHANES participants (≥20 years of age) with diabetes (type 1 or type 2) from 1999 through 2008, the most recent study year with available food security data. Because of the relatively small number of patients who receive fasting blood work each cycle (10), this pooling of data was necessary to ascertain a sufficient number of cases to permit robust adjustment for confounding. In accordance with prior studies (12–14) and methodology used in CDC reports (15), participants were considered to have diabetes if they answered “yes” to the question, “Other than during pregnancy, have you ever been told by a doctor or health care professional that you have diabetes or sugar diabetes?” Because prior studies have noted that some participants who are under treatment for diabetes do not report their diagnosis (16), we also considered a participant to have diabetes if he or she was taking diabetes medications, such as a sulfonylurea, insulin, or an incretin mimetic. Metformin alone was not considered evidence of diabetes because it is commonly used for nondiabetes indications.
The Partners HealthCare Human Research Committee exempted this study from institutional review board review.
Measures
Food insecurity.
Food insecurity was assessed using the 10-item Adult Food Security Survey Module within NHANES. These items are used by the U.S. Department of Agriculture (USDA) to report national rates of food security (4). Using the established scoring system (6), responses on the food security items were converted to either food secure (zero to two affirmative responses, categorized as “food secure” or “marginally food secure”) or food insecure (three or more affirmative responses, “low food security” or “very low food security” categories). The items used to determine food security status remained unchanged throughout the study period. The Food Security Survey Module was validated as part of the Food Security Measurement Project, conducted by the USDA Food and Nutrition Service using data from the U.S. Census Bureau’s Current Population Survey (4). It is now considered “the government’s primary measure of this dimension of the well-being of the U.S. population” (4). Because NHANES administers the Food Security Survey Module in accordance with recommendations from the USDA, the results are highly reproducible, leading to statistics that are “directly comparable to published national statistics” (17).
Outcomes
We wanted to fully describe the relationship between food insecurity and the spectrum of cardiovascular control, so we considered indicators of poor glycemic, poor cholesterol, and poor blood pressure control.
Poor glycemic control was defined as HbA1c >9.0% (75 mmol/mol). We chose this level because reducing the proportion of diabetes participants with an HbA1c >9.0% (75 mmol/mol) is a Healthy People 2020 goal (18), since “an HbA1c level of 9% (75 mmol/mol) constitutes a clearly modifiable, high level of risk that few, if any, persons with diabetes should be exposed to” (15). Moreover, 9.0% (75 mmol/mol) was considered to represent out-of-control glycemia for all diabetic patients throughout the study period. In contrast, lower HbA1c targets, such as 7.0% (53 mmol/mol), are controversial, are not intended to be applied to all patients (1), and have not been consistently recommended throughout the 10-year study period.
Poor cholesterol control was defined as having an LDL >100 mg/dL, because this is the only Healthy People 2020 LDL target for patients with diabetes (18). This was also consistent with recommendations for diabetic patients throughout the study period (19). Poor blood pressure control was defined as a mean systolic blood pressure (SBP) >140 mmHg or mean diastolic blood pressure (DBP) >90 mmHg, averaged over up to four readings obtained by MEC staff (10). This is higher than a blood pressure target of <130/80 mmHg, which has at times been recommended (19). However, the former was a more consistent goal throughout the study period.
Demographic and socioeconomic variables
We considered several sociodemographic factors that might confound the association between food insecurity and metabolic control. Demographic variables included age (as a continuous variable), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and mixed race/other). Educational attainment was categorized into less than high school, high school diploma, or greater than high school. Income was expressed as percentage of federal poverty level, which accounts for household size and for inflation over the 10-year study period. Insurance status was categorized as no insurance, private insurance, Medicare, and non-Medicare public insurance, including Medicaid.
Clinical variables
We also considered clinical variables such as BMI (weight in kilograms divided by height in meters squared, measured in the MEC), smoking status (current/former/never), duration of diabetes (current age minus reported age at diabetes diagnosis), and having a usual place of care. For our glycemic control analysis, we ascertained diabetes medication use, according to the medications recorded by the NHANES interviewer. For our blood pressure and LDL analysis, we additionally ascertained antihypertensive medication use and statin use, respectively.
Statistical analysis
We first performed descriptive statistics on demographic and clinical factors associated with glycemic control. Differences were tested for significance with χ2 tests for categorical values and Student t tests for continuous variables. We used multivariable logistic regression analysis to assess the independent association between food insecurity and poor diabetes control, controlling for the sociodemographic and clinical variables described above. To account for secular trends over the duration of the study period, we also adjusted for survey year. In order to help clinicians identify patient populations at higher risk of food insecurity, we performed descriptive statistics and univariate logistic regression to identify the factors associated with food insecurity.
Using a similar approach, we performed crude and multivariable logistic regression to determine the associations between food insecurity and poor LDL control, and between food insecurity and poor blood pressure control. A P value of <0.05 on a χ2 test, Student t test, or Satterthwaite adjusted F test was taken to indicate statistical significance. Because only a subset of NHANES participants undergo fasting blood work, our analysis of LDL control includes only those participants for whom a fasting LDL value is reported.
Analyses were performed using SAS (version 9.3, Cary, NC) and SAS-callable SUDAAN (version 10.0.1, Research Triangle Park, NC) to account for the complex multistage survey design. As recommended by the NCHS (11), appropriate weights were used in our analysis: MEC weights for glycemic and blood pressure results and fasting laboratory weights for LDL results (11). As recommended by the NCHS (20), we excluded 1999–2001 food security questionnaire data when calculating population estimates.
RESULTS
There were 2,726 adults examined in the MEC who met our definition of diabetes. We excluded 169 participants who did not have an HbA1c value, which left a sample of 2,557 for analysis of HbA1c and blood pressure control. For our analysis of LDL control, 932 NHANES participants were included.
Over 12% of adults with diabetes (n = 371) in our sample were food insecure, representing ∼2 million Americans. Sixteen percent (n = 414) of adults with diabetes had an HbA1c >9.0% (75 mmol/mol), representing >2.1 million Americans. Of these, 22% were food insecure, representing 480,000 Americans. Among the subset who also had a fasting blood draw, 53% (n = 493) had LDL >100 mg/dL. This represents 7.1 million adults with diabetes who have out-of-control LDL cholesterol, of whom 1.1 million are food insecure. Table 1 characterizes adults with diabetes overall and by metabolic control. Adults with food insecurity comprised a significantly higher proportion of those with poor glycemic (27.0 vs. 13.3%, P < 0.001) and LDL control (68.8 vs. 49.8%, P = 0.002) but not of those with poor blood pressure control (Table 1).
Table 1.
Sample characteristics of adults with diabetes overall and by metabolic control
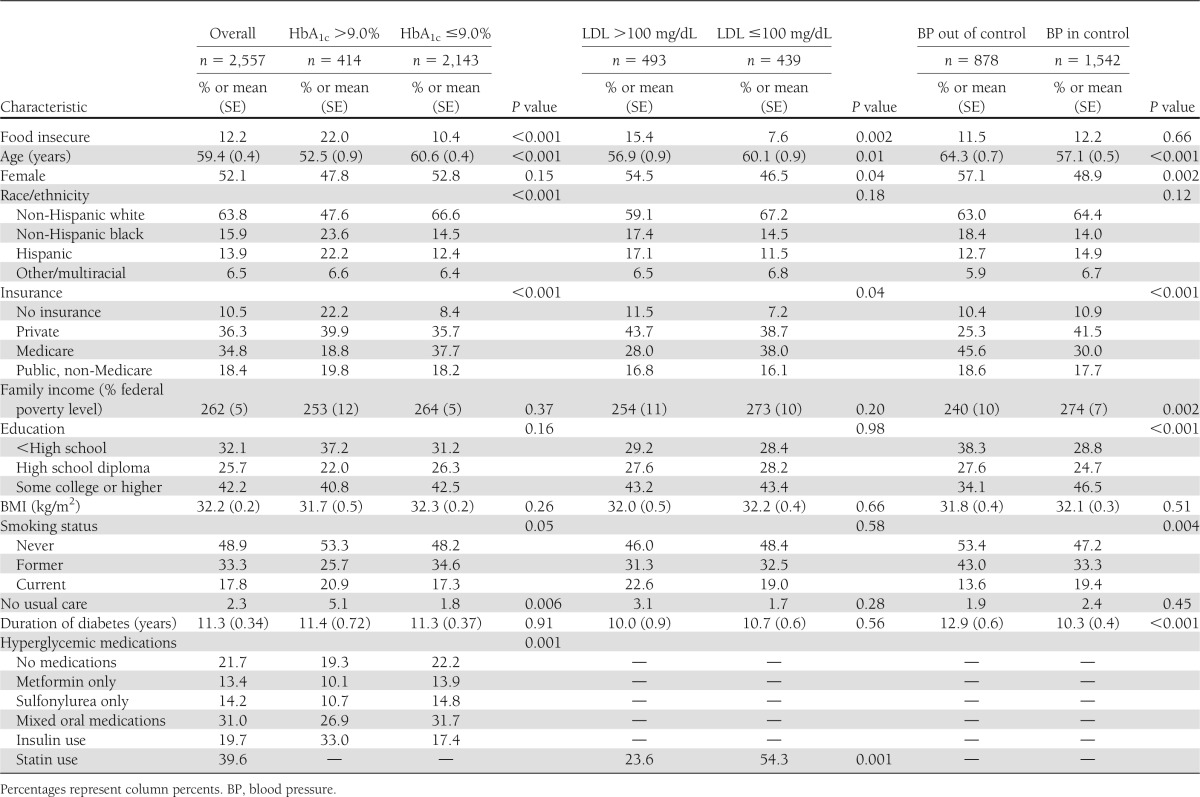
Table 2 presents the relationship between food insecurity and metabolic control after consideration of other potential confounders. After adjustment for sociodemographic factors, smoking status, BMI, duration of diabetes, diabetes medication use, and presence of a usual source of care, participants with food insecurity remained significantly more likely to have poor glycemic control (odds ratio [OR] 1.53 [95% CI 1.07–2.19]). Participants with food insecurity were also more likely to have an LDL >100 mg/dL after adjustment (1.86 [1.01–3.44]) for sociodemographic factors, smoking status, BMI, duration of diabetes, statin use, and presence of a usual source of care. There was no evidence of an association between food insecurity and the proportion of participants with SBP >140 mmHg or DBP >90 mmHg before (31.8% in food insecure vs. 32.9% in food secure, P = 0.75) or after adjustment (OR 1.10 [0.75–1.61]). Table 2 presents the full results of our adjusted models for poor glycemic, LDL cholesterol, and blood pressure control.
Table 2.
Factors associated with poor glycemic, LDL, and blood pressure control after adjustment
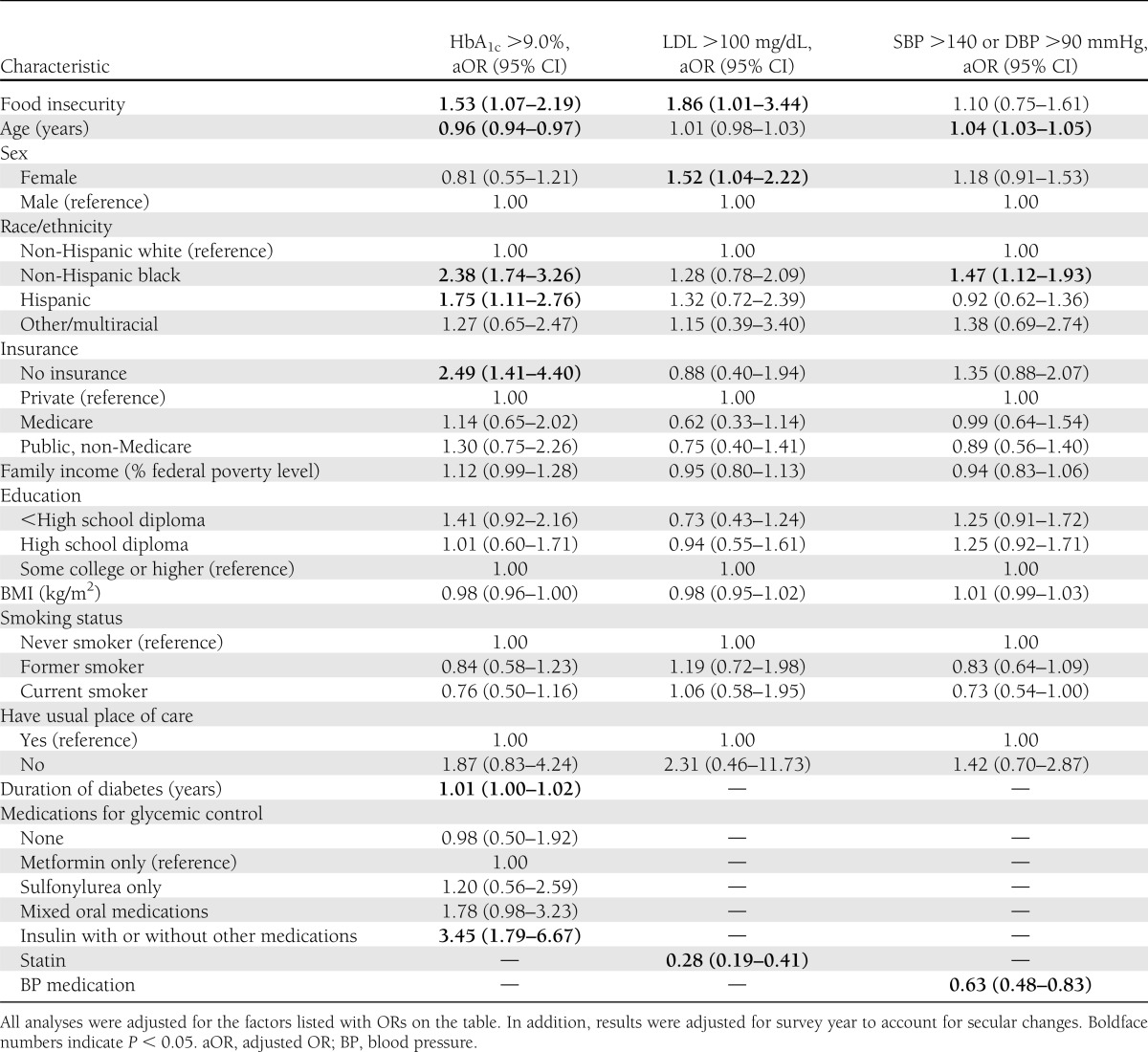
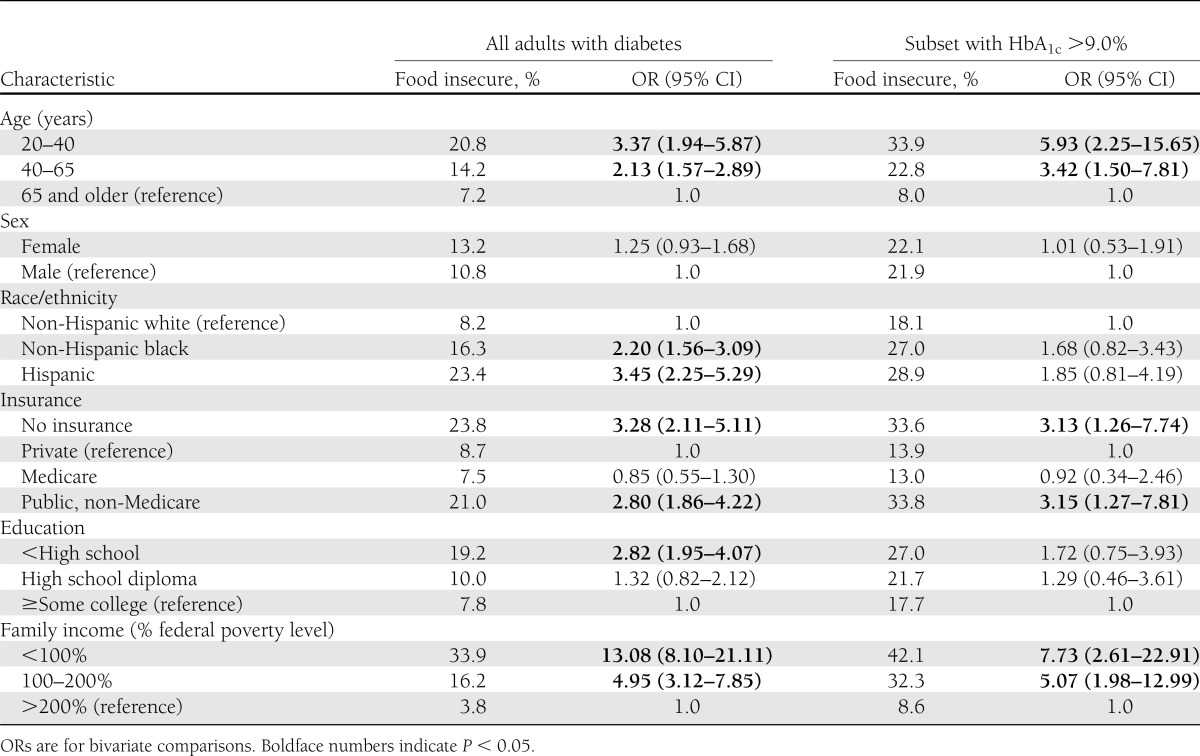
Table 3 presents the factors associated with food insecurity among adults with diabetes overall and among those with poor glycemic control. Those with younger age, less education or income, race/ethnicity other than non-Hispanic white, and either no or public insurance other than Medicare were significantly more likely to report food insecurity. Younger age, no insurance, public insurance other than Medicare, and low income were also commonly associated with food insecurity in those with HbA1c >9.0% (75 mmol/mol).
Table 3.
Risk of food insecurity by selected characteristics
CONCLUSIONS
In this nationally representative sample of U.S. adults with diabetes, food insecurity was associated with poor glycemic and cholesterol control even after adjusting for numerous demographic, socioeconomic, and clinical factors. However, we found no evidence of an association between food security and blood pressure control. These data suggest that >2.1 million adults with diabetes have inadequate glycemic control, and of these, nearly 25% are food insecure. LDL cholesterol reduction may be more important than glycemic control for cardiovascular disease outcomes in diabetic patients (21). Because of this, our observation that nearly 70% of food-insecure participants with diabetes had poorly controlled cholesterol may be particularly relevant.
These findings are consistent with and extend those from prior reports. Previous clinic-based studies noted worse glycemic control among food-insecure diabetic patients in safety-net clinics (8,9), but did not evaluate lipid control. Additionally, no population-based study had previously examined glycemic, lipid, and blood pressure control among all adults with diabetes. A prior study (22) restricted to younger, low-income participants did report an association with glycemic control but did not evaluate lipid or blood pressure control in participants with diabetes. Furthermore, because the methodology of this study would have excluded over two-thirds of our sample, it was not clear that the glycemic control findings would be generalizable to a nationally representative sample. Finally, because there was no adjustment for important variables such as insurance status, medications, BMI, or having a usual source of care, it was not known whether these factors confounded the observed association. By providing robustly controlled estimates from nationally representative data, we have demonstrated that food insecurity is associated with poor glycemic and cholesterol control in a broad population of adults with diabetes, independent of several important potential confounders.
Our observation that food insecurity worsens glycemia and LDL cholesterol levels, but not blood pressure, suggests distinct physiological processes for these cardiometabolic parameters. This finding is consistent with the results of a factor analysis from the Framingham cohort that noted that insulin resistance, higher blood glucose, and adverse serum lipid levels cluster together and often track differently from blood pressure (23). Although this does suggest a possible pathophysiologic pathway, further research will be required to more finely delineate mechanisms that underlie these associations.
Beyond this possible pathophysiology, however, food insecurity likely increases risk for poor metabolic control in two other ways (5). First, food insecurity can lead to the substitution of low-cost, calorically dense food, such as processed carbohydrates and fats, for higher-cost, less calorically dense foods, such as whole grains and fresh fruits and vegetables. Secondly, the circumstances of food insecurity force competing choices that may direct resources away from successful self-management of diabetes.
Our work has important public health implications because of the substantial morbidity and mortality associated with poor metabolic control in diabetes. Nearly 480,000 patients with out-of-control hyperglycemia are food insecure, as are nearly 1.1 million of those with out-of-control lipid levels. Our findings have clinical implications as well. Because extreme hyperglycemia is very sensitive to diet (24,25), it may be difficult to improve hyperglycemia in these patients without addressing food insecurity. However, the circumstances of material deprivation, which produce food insecurity, are not readily amenable to intervention via the traditional provider-patient encounter. To address these circumstances, we need to expand clinical care to include population-based health care strategies that identify and intervene in the case of those at high risk of morbidity due to social circumstances. Programs like the South Side Diabetes Project (25), which addresses community factors such as the built environment and food availability, may point toward interventions that are particularly important for vulnerable patients.
We identified several participant characteristics that are associated with food insecurity, some of which are more readily accessible to clinicians than others. For example, low income is closely associated with food insecurity, but clinicians may not have access to this data. In contrast, factors such as patient educational attainment or insurance status, which is often available from administrative data, could be used to target screening for food insecurity identification when it is not practical to assess food security in the entire population. More than one in four of participants with diabetes and poor glycemic control under 65 years of age were food insecure, as were one-third of those with no or public insurance. Despite the increased risk in these groups, however, it is important to note that almost 15% of participants with private insurance and almost one in five out-of-glycemic-control participants with some college education were food insecure.
Although no studies, to our knowledge, have demonstrated improved glycemic or cholesterol control with food insecurity interventions, patients identified in this way can nevertheless be assisted through established food security promotion programs such as the Supplemental National Assistance Program (SNAP, formerly the Food Stamp Program) and local food banks. Because food banks may promote food sources associated with worse diabetes management, such as refined carbohydrates and fats (26), nutritional counseling explicitly incorporating advice recognizing the unique circumstances of diabetic patients with food insecurity should be a cornerstone of disease management in this population.
This study does have limitations. Food security is a household-level variable, whereas disease control is measured at an individual level. Although this study used a robust set of controls for confounding, unmeasured confounders, such as neighborhood effects, could not be controlled for. Physical activity, which has been associated with diabetes (27), underwent a change in assessment during the study period and thus could not be included in our models. However, because prior studies in NHANES reported that food insecurity is not associated with physical activity (7) and because, in conceptual models of food insecurity (5), physical inactivity can be considered a mediator of poor health outcomes, we do not believe physical inactivity to be a confounder of our results. Given these limitations, and because we analyzed cross-sectional data, this study cannot establish that food insecurity causes poor glycemic and LDL cholesterol control. Nevertheless, our study does suggest that food insecurity indicates a group at high risk for poor disease control. Furthermore, these limitations are balanced by several strengths. The study sample comprised a large, nationally representative set of participants with robust interview, examination, and laboratory data. Food insecurity was measured using the “gold standard” assessment tool. Other self-reported items were collected from consistent, validated instruments, and examination measures were performed by trained personnel in controlled settings. Laboratory results were all standardized. By combining 10 years of data, we ascertained enough cases to adjust for demographic, socioeconomic, and clinical variables. We used a conservative analytic strategy that included factors, such as BMI, that might plausibly be on the causal pathway between food insecurity and metabolic control (28) and a robust set of controls, yet still detected a significantly increased risk for poor metabolic control in food-insecure participants.
In summary, food insecurity is significantly associated with poor metabolic control in adults with diabetes. With increasing interest in advanced population management, we should consider interventions that address social circumstances such as food insecurity to help successfully manage chronic disease in vulnerable patients.
Acknowledgments
S.A.B. was supported by an institutional National Research Service Award (T32HP10251), the Ryoichi Sasakawa Fellowship Fund, and the General Medicine Division at Massachusetts General Hospital. T.P.B. receives funding from the National Institute on Drug Abuse of the National Institutes of Health (K23-DA-034008). D.J.W. receives funding from the National Institutes of Health (R03-DK-090196). C.C.W. is supported by a midcareer mentorship award from the National Institutes of Health (K24-DK-087932).
No potential conflicts of interest relevant to this article were reported.
S.A.B. conceptualized the study, performed data analysis, and authored the manuscript. T.P.B. and D.J.W. assisted in the design of the study and revised the manuscript for critical content. K.W.H. assisted in design of the study and with data analysis and reviewed the manuscript. C.C.W. assisted in the design of the study and coauthored the manuscript. S.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Disparities Report [article online], 2011. Available from http://www.ahrq.gov/research/findings/nhqrdr/nhdr11/chap2a.html#diabetes Accessed 26 February 2013
- 3.Saydah S, Lochner K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep 2010;125:377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security, Revised 2000. Alexandria, VA, U.S. Department of Agriculture, 2000 [Google Scholar]
- 5.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med 2010;363:6–9 [DOI] [PubMed] [Google Scholar]
- 6.Coleman-Jensen A, Nord M, Andrews M, Carlson S. Statistical Supplement to Household Food Security in the United States in 2011 Alexandria, VA, U.S. Department of Agriculture, 2012 (publ. no. AP-058) [Google Scholar]
- 7.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999-2002. J Gen Intern Med 2007;22:1018–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seligman HK, Jacobs EA, Lopez A, Sarkar U, Tschann J, Fernandez A. Food insecurity and hypoglycemia among safety net patients with diabetes. Arch Intern Med 2011;171:1204–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care 2012;35:233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC National Health and Nutrition Examination Survey Data. Hyattsville, MD, National Center for Health Statistics, 2012 [Google Scholar]
- 11.Continuous NHANES tutorial. [article online], 2012. Available from http://www.cdc.gov/nchs/tutorials/Nhanes/index_continuous.htm Accessed 8 November 2012
- 12.Heliövaara M, Aromaa A, Klaukka T, Knekt P, Joukamaa M, Impivaara O. Reliability and validity of interview data on chronic diseases. The Mini-Finland Health Survey. J Clin Epidemiol 1993;46:181–191 [DOI] [PubMed] [Google Scholar]
- 13.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav 1997;38:21–37 [PubMed] [Google Scholar]
- 14.Kehoe R, Wu SY, Leske MC, Chylack LT., Jr Comparing self-reported and physician-reported medical history. Am J Epidemiol 1994;139:813–818 [DOI] [PubMed] [Google Scholar]
- 15.Ali MK, McKeever Bullard K, Imperatore G, Barker L, Gregg EW, Centers for Disease Control and Prevention (CDC) Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes—National Health and Nutrition Examination Survey, United States, 2007-2010. MMWR Morb Mortal Wkly Rep 2012;61(Suppl.):32–37 [PubMed] [Google Scholar]
- 16.Wee CC, Hamel MB, Huang A, Davis RB, Mittleman MA, McCarthy EP. Obesity and undiagnosed diabetes in the U.S. Diabetes Care 2008;31:1813–1815 [DOI] [PMC free article] [PubMed]
- 17.Food Security in the U.S., survey tools [article online], 2013. Available from http://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/survey-tools.aspx#.UWOAAIVyHqs Accessed 8 April 2013
- 18.Healthy People 2020 [article online], 2012. Available from http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=8 Accessed 20 November 2012
- 19.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food security questionnaire analytic notes [article online], 2012. Available from http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/FSQ.htm#Analytic_Notes Accessed 28 February 2013
- 21.Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J Gen Intern Med 2013;28:691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr 2010;140:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson PW, Meigs JB. Cardiometabolic risk: a Framingham perspective. Int J Obes (Lond) 2008;32(Suppl. 2):S17–S20 [DOI] [PubMed] [Google Scholar]
- 24.Coppell KJ, Kataoka M, Williams SM, Chisholm AW, Vorgers SM, Mann JI. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment—Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ 2010;341:c3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peek ME, Wilkes AE, Roberson TS, et al. Early lessons from an initiative on Chicago’s South Side to reduce disparities in diabetes care and outcomes. Health Aff (Millwood) 2012;31:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handforth B, Hennink M, Schwartz MB. A qualitative study of nutrition-based initiatives at selected food banks in the feeding America network. J Acad Nutr Diet 2013;113:411–415 [DOI] [PubMed] [Google Scholar]
- 27.Nelson KM, Reiber G, Boyko EJ, NHANES III Diet and exercise among adults with type 2 diabetes: findings from the third national health and nutrition examination survey (NHANES III). Diabetes Care 2002;25:1722–1728 [DOI] [PubMed] [Google Scholar]
- 28.Pan L, Sherry B, Njai R, Blanck HM. Food insecurity is associated with obesity among US adults in 12 states. J Acad Nutr Diet 2012;112:1403–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]


