Abstract
OBJECTIVE
Low cardiorespiratory fitness (CRF) is an independent risk factor for cardiovascular disease (CVD), especially in individuals with type 2 diabetes. Age-predicted, sex-stratified, and maximal MET cut points have been developed to determine the risk of CVD events and mortality in low CRF categories. We examined the proportion of Health Benefits of Aerobic and Resistance Training in Individuals With Type 2 Diabetes (HART-D) participants above these cut points before and after 9 months of aerobic training (AT), resistance training (RT), or a combination of both (ATRT).
RESEARCH DESIGN AND METHODS
Participants from the HART-D study (n = 196) who were randomly assigned to exercise training (AT, RT, or ATRT) or to a nonexercise control group between April 2007 and August 2009 were used in this ancillary study. Cut points were previously established for age-predicted METs (>100% and >85%, mean and increased CVD risk, respectively), age- and sex-stratified METs (Aerobic Center Longitudinal Study), and clinically discernible METs (men >8.0, women >6.5).
RESULTS
Baseline prevalence of participants above these cut points was similar for all intervention groups (P > 0.50) and ranged from 11.9% (>100% age predicted) to 55.1% (>85% age predicted). Baseline prevalence and age-, sex-, and race/ethnic group–adjusted percentage of participants above each cut point increased significantly after AT and ATRT (P < 0.05 for all).
CONCLUSIONS
Structured exercise training, especially the AT component, was associated with a greater number of participants moving above established cut points indicative of low CRF. These results have public health and clinical implications for the growing number of patients with type 2 diabetes at high risk for CVD.
A critical cardiovascular disease (CVD) risk factor for patients with type 2 diabetes is cardiorespiratory fitness (CRF), and considerable evidence suggests a powerful role of CRF for predicting all-cause and CVD mortality (1). A meta-analysis demonstrated that for every 1-unit increase in maximal estimated METs, all-cause mortality and combined CVD and coronary heart disease (CHD) events were reduced by 13 and 15%, respectively (1). These results may be particularly applicable to patients with type 2 diabetes, who have a marked increased risk of CVD and all-cause mortality and who commonly present with low CRF (2–4). Specifically, a steep inverse relationship exists between both physical activity and CRF and mortality in patients with type 2 diabetes, which appears to be independent of BMI (5,6).
Further evidence supports the importance of aerobic exercise for the treatment of patients with type 2 diabetes, including improvements in glycemic control, body composition, CHD risk factors, and vascular as well as ventricular function (7). The American Heart Association issued guidelines for aerobic exercise in patients with type 2 diabetes to improve glycemic control and multiple CVD risk factors, including low CRF (7). Patients with type 2 diabetes may also incur additional benefits to glycemic control, body composition, and CVD risk factors by combining aerobic training (AT) and resistance training (RT) (7–9). As such, many organizations recommend the combination of AT and RT (ATRT) in all adults, including those with type 2 diabetes (7,10–13).
The Health Benefits of Aerobic and Resistance Training in Individuals With Type 2 Diabetes (HART-D) study was a randomized trial that compared AT, RT, and ATRT on hemoglobin A1C (HbA1c) in sedentary men and women with type 2 diabetes (8). Secondary outcomes reported in the main results article were changes in CRF, including Vo2max and maximal estimated METs. Although group changes in CRF have been previously reported in HART-D, we believe that a categorical analysis of the CRF data that uses clinical cut points representative of significant CVD risk is warranted. Accordingly, several researchers have developed age-predicted, sex-stratified, maximal estimated MET cut points to determine the risk of CVD events and mortality in low CRF categories (2,14–17). However, few studies have investigated the prevalence of low CRF in individuals with type 2 diabetes as determined by the percentage of participants in the low CRF categories. To our knowledge, no data exist on the extent to which individuals with type 2 diabetes traverse from low CRF classifications to higher CRF categories after AT, let alone the additional benefits of ATRT. In the current study, we assessed the impact of AT, RT, and ATRT in patients with type 2 diabetes on changes in CRF in relation to cut points indicative of increased CVD risk.
RESEARCH DESIGN AND METHODS
The HART-D study was approved by the Pennington Biomedical Research Center institutional review board annually for continued analyses, and written consent was obtained from all participants before study screening. The main outcomes for the HART-D study, including changes in CRF (maximal METs and respiratory gases [Vo2max]) across intervention groups, were published previously (8). In the HART-D study, 262 individuals with type 2 diabetes were randomly assigned to participate in one of four intervention groups. To evaluate the efficacy of the intervention to change an individual’s age- and sex-stratified CRF category, only compliant participants (≥70% of sessions completed) with complete baseline and follow-up data were evaluated in this ancillary analysis (n = 196). Participants in HART-D were sedentary (aerobic exercise <20 min <3 days per week and no RT) men and women 30–75 years of age with type 2 diabetes (HbA1c 6.5–11.0% inclusive) and a BMI ≤48.0 kg/m2, fasting triglyceride level <500 mg/dL, and blood pressure <160/100 mmHg. Volunteers were excluded for presence or medical history of stroke, advanced neuropathy or retinopathy, or other serious medical condition contraindicated for exercise or that may prevent adherence to the study protocol.
Study design
A full report of the methods of the exercise protocol and training data was published in the primary outcomes article (8). Volunteers who met the inclusion and exclusion criteria were randomized to 9 months of either AT, RT, or ATRT or to a nonexercise control group. The data monitoring and safety board discontinued randomization to the control group during the study after a significant number of participants (∼17%) had an increase in HbA1c >1.0%, resulting in an unequal number of participants in the control group. We maintained separate intervention and assessment teams, and clinical testing and intervention laboratories were housed in separate buildings. Participants were reminded frequently not to discuss their group assignment with blinded assessment staff.
Intervention
All participants met with a certified diabetes educator each month to track medication and health history changes, and all exercise sessions were constantly monitored by a trained study staff. The control group was offered weekly stretching and relaxation classes. Participants randomized to the control group were asked to maintain their normal daily physical activity level throughout the intervention. We confirmed physical activity levels with step counters (8). The stretching and relaxation classes were optional and considered light-intensity physical activity inadequate to influence CRF or produce significant increases in strength.
The AT and ATRT groups participated in treadmill walking 3–5 days per week at a moderate to vigorous intensity (mean ± SD peak oxygen uptake; Vo2peak 65.4 ± 14.6%). The aerobic exercise dose in the AT group was prescribed at 12 kcal/kg body weight per week (KKW). We estimated this dose of aerobic exercise to be equivalent to ∼150 min of physical activity per week. Participants were weighed weekly to calculate the prescribed weekly aerobic exercise dose, and caloric energy expenditure rate was estimated by standard equations published by the American College of Sports Medicine (18). The time required per session was calculated by dividing the weekly dose by the estimated caloric expenditure rate and the total number of sessions completed that week. The aerobic exercise dose was lowered to 10 KKW in the ATRT group to accommodate the RT component and ensure equal time commitment across all exercise groups. During weeks 12 and 24, the exercise dose was reduced by one-third to provide a recuperation week.
The RT group completed 3 days of strength training exercises per week comprising two sets of four upper-body exercises (bench press, seated row, shoulder press, and lat pull down), three sets of three lower-body exercises (leg press, extension, and flexion), and two sets of abdominal crunches and back extensions. Each set consisted of 10–12 repetitions, and the amount of weight lifted was progressively increased when the participant was able to complete 12 repetitions on the final set of an exercise in two consecutive RT sessions. Participants in the ATRT group completed two sessions of RT each week comprising one set of 10–12 repetitions for all nine resistance exercises.
Measurements
After a 10-h fast, HbA1c was assessed by venous blood sample on a Beckman Coulter DxC 600 Pro (Fullerton, CA) chemistry analyzer. Body weight was measured on an electronic scale (GSE Scale Systems, Novi, MI), and height was assessed on a calibrated stadiometer. BMI was calculated as weight (in kilograms) divided by height (in meters squared).
An electrocardiographic-monitored maximal CRF test was completed before and after 9 months of intervention following a medical history review and physical examination. Participants taking β-blockers (antiadrenergic) were required to take their medications as prescribed before testing. Exercise testing was conducted on a treadmill (TMX425; Trackmaster, Newton, KS), with respiratory gases analyzed with a Truemax 2400 Metabolic Cart (ParvoMedics, Salt Lake City, UT). During the exercise test, participants were allowed to self-select a comfortable, yet brisk pace and walked for 2 min at a level grade. The treadmill grade then increased by 2% every 2 min until volitional fatigue. The same speed was used during postintervention testing. Vo2peak from respiratory gases is reported relative to body weight (mL⋅kg−1⋅min−1), and maximal estimated oxygen uptake was calculated with American College of Sports Medicine equations (18) at self-selected speed, maximal grade, and body weight and converted to maximal estimated METs by dividing by 3.5 mL⋅kg−1⋅min−1.
Cut points for CVD risk
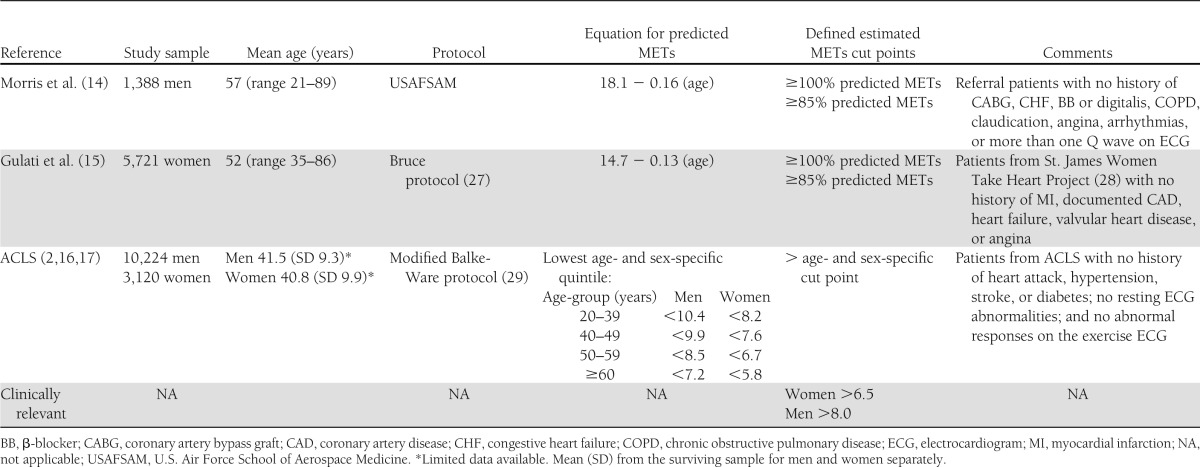
Cut points for CVD risk categories are from published resources (Table 1) (2,14–17). Morris et al. (14) and Gulati et al. (15) equations were used to compare the maximal estimated METs from the current sample to normative data for men and women, respectively, above and below 100% (normal) and 85% (increased CVD risk) of the age-predicted maximal METs. Age- and sex-stratified fitness groups from the Aerobics Center Longitudinal Study (ACLS) (low fitness vs. moderate and high fitness) were also used to demonstrate changes in maximal estimated METs in the HART-D study relative to cut points for increased CVD (2,16,17). Furthermore, we used clinically relevant maximal MET values of 8.0 and 6.5 for men and women, respectively, to compare changes in maximal estimated METs across cut points that are easily discernible.
Table 1.
Cut points for CVD risk according to age- and sex-specific predicted METs
Statistical analyses
All data were analyzed with JMP version 9.0.2 (SAS Institute, Inc., Cary, NC) statistical software. Baseline demographic and anthropometric data were analyzed across intervention groups by one-way ANOVA for continuous variables and χ2 test for categorical variables. The actual change and prevalence for a significant change in Vo2peak (>0, 1.75, and 3.5 mL⋅kg−1⋅min−1) and maximal estimated METs (>0, 0.5, and 1.0) during the treadmill test were analyzed with general linear models adjusting for baseline value, age, sex, and race/ethnic group. Baseline prevalence for above age-predicted maximal MET cut points, ACLS fitness cut points, and clinically relevant cut points were analyzed with χ2 likelihood ratio tests. Follow-up prevalence for above age-predicted MET cut points, ACLS fitness cut points, and clinically relevant cut points were examined across intervention groups with general linear models adjusting for baseline prevalence, age, sex, and race/ethnic group. ANCOVA was used to examine the interaction between baseline CVD risk, as defined as above or below the clinically relevant cut points, and intervention group on the absolute and percent change in maximal estimated METs after adjusting for age, sex, and race/ethnic group. Student t post hoc analyses were used to determine between-intervention group and cut point category differences. Baseline demographic and anthropometric data are presented as mean ± SD or n (%), and baseline and follow-up prevalence data that met the particular criterion for that variable are presented as n (%). P < 0.05 was considered significant for all analyses.
RESULTS
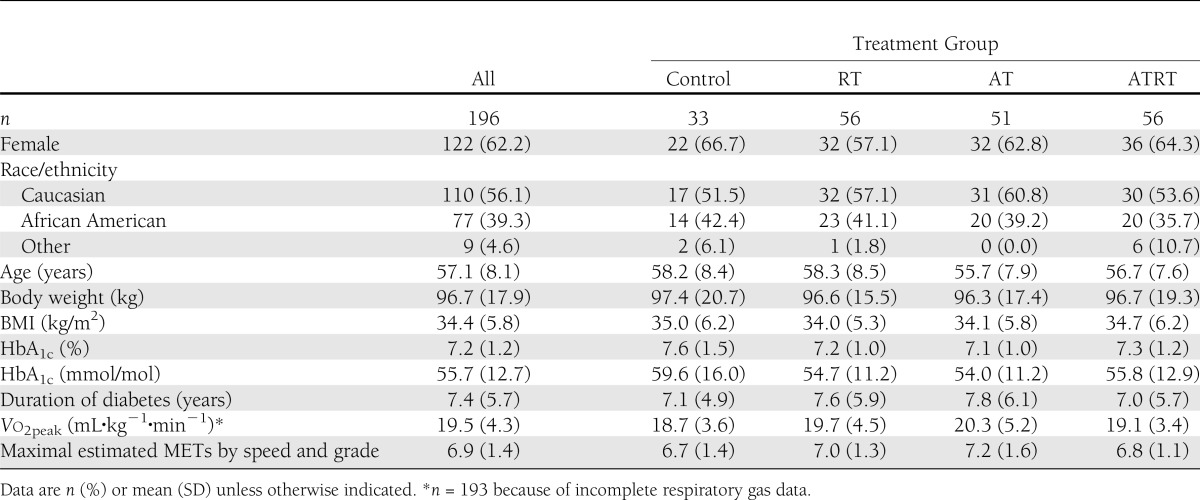
Baseline demographic and anthropometric data are presented in Table 2. Women comprised 62.2% of the participants, and 56.1% of participants were of Caucasian descent. Average age was 57.1 ± 8.1 years, BMI, 34.4 ± 5.8 kg/m2, and HbA1c, 7.2 ± 1.2% (55.7 ± 12.7 mmol/mol). Baseline Vo2peak and maximal estimated METs were 19.5 ± 4.3 mL⋅kg−1⋅min−1 and 6.9 ± 1.4, respectively. Baseline characteristics were similar across exercise intervention groups (P > 0.10 for all).
Table 2.
Baseline participant characteristics
At baseline and follow-up, Vo2peak was highly correlated with maximal estimated METs (both visits r = 0.88, P < 0.001, n = 193). However, the association between the change scores for Vo2peak and maximal estimated METs was more variable (r = 0.57, P < 0.001).
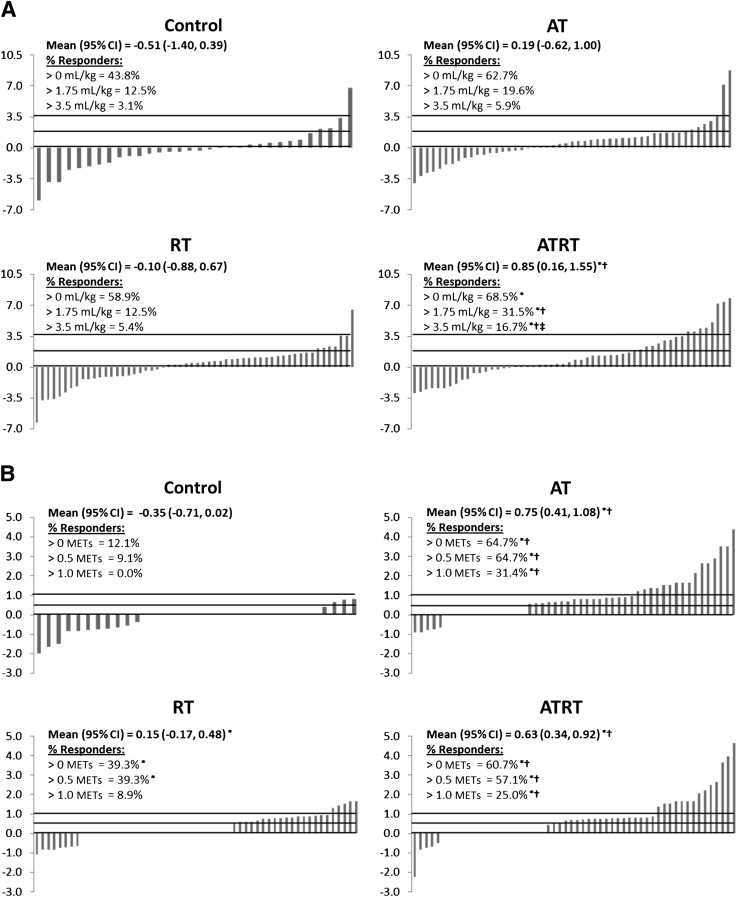
The analyses for absolute change and prevalence of improvements in measured Vo2peak and maximal estimated METs are shown in Fig. 1. The change in Vo2peak (Fig. 1A) was significantly greater after ATRT than in the control and RT groups (both P < 0.05). Likewise, a greater prevalence of participants with an increase in measured Vo2peak >0, 1.75, and 3.5 mL⋅kg−1⋅min−1 (equivalent to 0, 0.5, and 1.0 METs, respectively) was observed after ATRT compared with the control group (all P > 0.05). A greater prevalence of participants with an increase >1.75 and 3.5 mL⋅kg−1⋅min−1 was also observed after ATRT compared with RT alone and >3.5 mL⋅kg−1⋅min−1 after ATRT compared with AT alone (P < 0.05). The increase in maximal estimated METs (Fig. 1B) was greater after both AT and ATRT compared with the control and RT groups (all P < 0.05) and greater after RT compared with the control group (P < 0.05). Similar results were observed when examining the prevalence of participants with an increase in maximal estimated METs >0 and 0.5 to the absolute change in maximal estimated METs. However, the proportion of participants who achieved an increase in maximal estimated METs >1.0 was only greater in the AT and ATRT groups compared with the control and RT groups (all P < 0.05).
Figure 1.
Waterfall diagram demonstrating the change in Vo2peak (mL⋅kg−1⋅min−1) (A) and maximal estimated METs (B). Each bar represents a participant and the corresponding change in Vo2peak or maximal estimated MET value. The % responders for each panel is the number of participants who had a change in Vo2peak or maximal estimated METs >0 divided by the total number of participants in the intervention group multiplied by 100. The lines on each graph indicate a change in fitness >0, 0.5, and 1.0 METs or equivalent change in Vo2peak. Continuous data are mean (95% CI), and categorical analyses are percentage of participants who met the indicated criteria for changes in fitness. General linear models adjusting for baseline value, age, sex, and race/ethnic group were used to determine significant between-intervention group differences. n = 193 for respiratory data because of incomplete respiratory gas data. *P < 0.05 vs. control; †P < 0.05 vs. RT; ‡P < 0.05 vs. AT.
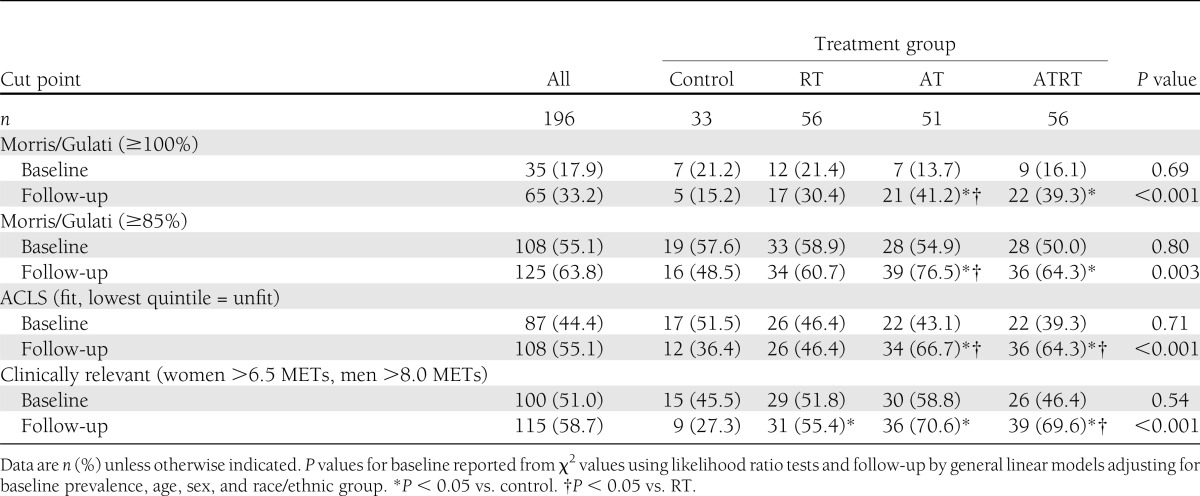
The prevalence of participants meeting age-predicted maximal METs (≥100%), ≥85% of age-predicted maximal METs, ACLS moderate and high CRF levels, and clinically relevant cut points at baseline and follow-up across intervention groups are shown in Table 3. The average prevalence between cut points at baseline ranged from 17.9% by Morris/Gulati sex-specific age-predicted equations to 55.1% by Morris/Gulati sex-specific age-predicted cut points for participants with increased risk of CVD (≥85%). The prevalence of maximal estimated METs above each of the four cut points was similar across intervention groups at baseline (all P ≥ 0.50). At follow-up, the proportion of participants with maximal estimated METs higher than the age-predicted Morris/Gulati cut points (≥100% and ≥85%) was greater in the AT group than in the control and RT groups (both P < 0.05) and greater in the ATRT group than in the control group (P < 0.05). The proportion of participants with maximal estimated METs greater than the age- and sex-stratified ACLS cut points was greater in the AT and ATRT groups than in the control and RT groups (all P < 0.05). The response for the clinically relevant cut points was somewhat different at follow-up, with a greater proportion of participants having maximal estimated MET values higher than the sex-specific cut points in all intervention groups compared with the control group (all P < 0.05) and after ATRT compared with the RT group (P < 0.05).
Table 3.
Participants above specified cut points for maximal estimated METs
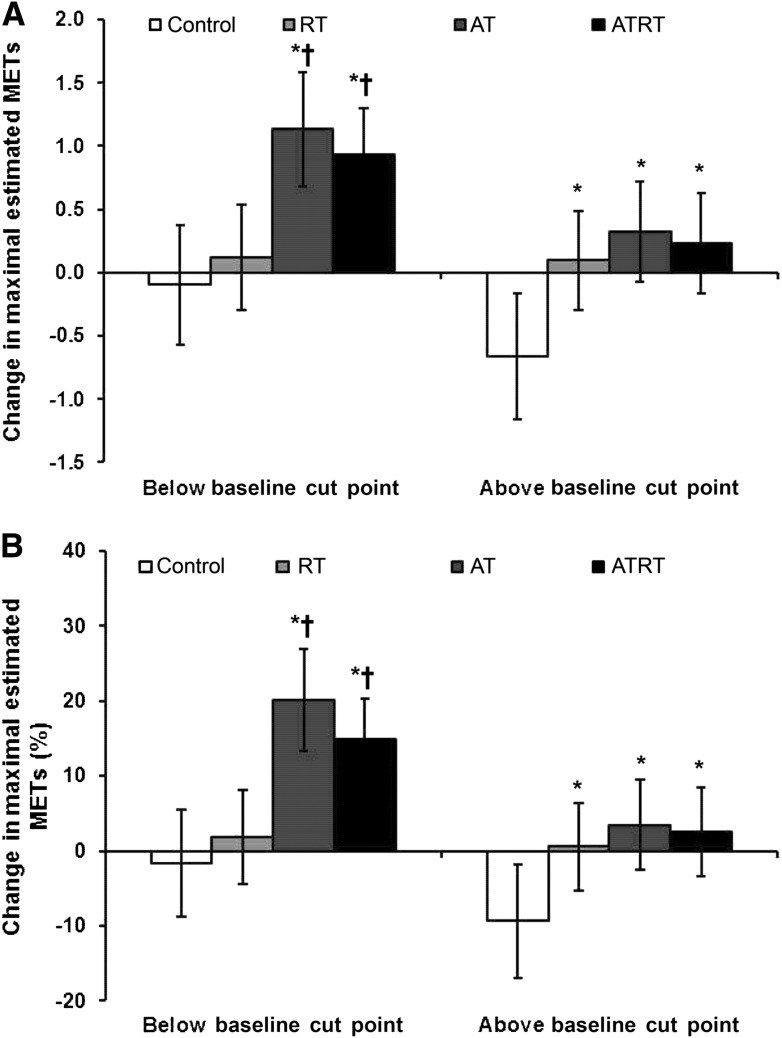
The effect of the interaction between baseline CRF (above and below the clinically relevant cut points) and intervention group on the increase in maximal estimated METs is shown in Fig. 2. In this analysis, AT and ATRT increased maximal estimated METs in participants below the clinically relevant cut points compared with the control and RT groups (all P ≤ 0.0015) (Fig. 2A). Participants in all exercise intervention groups categorized as above the clinically relevant cut points had increased maximal estimated METs compared with the control group (all P ≤ 0.01). Similar results to the absolute change in maximal estimated METs were observed when change was expressed as a percentage of the baseline MET value (Fig. 2B). A lower response was observed within an intervention group for participants starting above baseline clinically relevant cut points in the AT and ATRT groups for absolute change (P < 0.03 and P = 0.005, respectively) and percent change (P < 0.001 and P = 0.001, respectively) in maximal estimated METs compared with those below the cut point at baseline.
Figure 2.
Change (A) and percent change (B) in maximal estimated METs and across intervention groups stratified by baseline clinically relevant cut points after adjusting for age, sex, and race/ethnic group. Above baseline cut point, >6.5 for women and >8.0 for men; below baseline cut point, ≤6.5 for women and ≤8.0 for men. *P < 0.05 vs. control; †P < 0.05 vs. RT.
CONCLUSIONS
The primary outcome of the current study demonstrates that AT alone or in combination with RT was particularly effective at improving CRF in sedentary individuals defined as having low CRF by established clinical cut points, with a significant number of participants with type 2 diabetes traversing out of low CRF categories. The data suggest that RT can be substituted for part of the prescribed aerobic exercise dose to obtain improvements in CRF (aerobic dose of 12 and 10 KKW for AT and ATRT, respectively) or that a threshold response for improvements in CRF occurred before 10 KKW. Irrespective of the indications for RT, these data reinforce the importance of AT for the improvement of CRF in individuals with type 2 diabetes.
Numerous studies have emphasized the importance of CRF for future risks of mortality and CVD events, including a meta-analysis of 33 studies where researchers observed that each 1-MET increase in CRF was equivalent to a 13% reduction in all-cause mortality and a 15% reduction in CVD and CHD events (1,19). A study by Lee et al. (20) also demonstrated that for every 1-MET CRF increase, all-cause and CVD morality decreased by 15 and 19%, respectively, independently of BMI (21). The results from the current study suggest that after AT or ATRT, ∼25–30% of the participants were able to increase their maximal estimated METs by >1.0, which is associated with a reduction in CVD risk of 15–20%.
To support these findings and tie the cut point results to the improvements in METs, we examined the number of participants who both significantly increased their maximal estimated METs by 0.5 or 1.0 and increased CRF above the Morris/Gulati (≥85%), ACLS, and clinically relevant cut points. For all these cut points, 100% of the participants increased their CRF by >0.5 METs, and ∼65% had an increase >1 MET. These findings suggest that 1) traversing out of the low CRF group is achievable and associated with what should be a meaningful reduction in CVD risk and 2) Morris/Gulati (≥85%), ACLS, and clinically relevant cut points are all equally capable of identifying the majority of participants who responded positively to the exercise stimulus.
Physical inactivity and low levels of CRF are independent predictors of all-cause mortality, increasing the risk by 1.7-fold (CI 1.2–2.3) compared with physically active men and by 2.1-fold (1.5–2.9) compared with fit men (5). Similarly, at every level of BMI, the risk of all-cause mortality in men with diabetes increased by 4.5-, 2.8-, and 1.6-fold, respectively, in those in the lowest three quartiles of CRF compared with men in the highest quartile (6). Likewise, CVD-related mortality in men with diabetes was associated with low CRF for normal weight, overweight, and class I obese patients (22). In a study of men with diabetes followed for 16 years, all-cause, CVD, and CHD mortality were markedly reduced in those with moderate and high levels of CRF compared to those with low CRF (23). These studies strongly support the powerful role of CRF in patients with type 2 diabetes. On the basis of these data, the improvements in CRF following exercise, especially exercise with a significant AT stimulus as demonstrated in the current study, are noteworthy and consistent with potential significant reductions in overall CVD risk in sedentary patients with type 2 diabetes. As further demonstrated in Fig. 2, aerobic exercise is necessary in individuals with type 2 diabetes and low CRF to improve maximal fitness as well as to maintain fitness in those with higher levels of baseline CRF. This dichotomous response provides hope for the least fit individuals with type 2 diabetes to improve their global CVD risk.
Current recommendations from the American Heart Association (7), American Diabetes Association (11,12), and American College of Sports Medicine (13) strongly support the need for regular exercise in patients with type 2 diabetes. Although the importance of AT is emphasized, these organizations, along with the 2008 Federal Physical Activity Guidelines (10), recommend RT in combination with AT. Together, AT and RT have the potential to improve muscular strength, glycemic control, and overall CVD prognosis and survival (24,25). To our knowledge, this randomized trial is the first in individuals with type 2 diabetes to directly assess aerobic exercise prescriptions that are consistent with the 2008 Physical Activity Guidelines. Although the primary outcomes report from HART-D demonstrated a significant impact of RT when combined with AT on HbA1c levels (8), RT had only a minimal impact on overall CRF levels. We cannot determine the exact impact of RT on CRF in this study, although the results suggest that RT may be a substitute for at least part of the AT dose required to significantly improve CRF at reduced doses of aerobic exercise training.
A strength of the HART-D study is that it was designed as an efficacy study, using tightly controlled, randomly assigned exercise regimens and with all training completed in the laboratory with extensive monitoring (8). However, the ideal circumstances also represent a potential relative limitation because the results may not extend to real-world situations. The participants were diverse in age, sex, ethnicity, medication usage, and comorbidities, making the findings generalizable to a population of sedentary patients with type 2 diabetes. Of importance, both the AT and the RT exercise doses are easily obtainable and were well tolerated by this cohort, which has potentially important clinical implications for refining exercise recommendations for patients with type 2 diabetes. Finally, we assessed CRF in the present analysis by maximal estimated METs as opposed to Vo2peak (26). Estimating maximal METs has some limitations compared with those assessed by cardiopulmonary assessment. However, we chose to use maximal estimated METs because most exercise stress tests performed in the clinical setting routinely use maximal estimated METs, making it potentially more applicable to practitioners. Additionally, this method has been well validated to assess CRF and predict prognosis in epidemiological studies (1), including those in patients with type 2 diabetes (5,6,22,23). The present data demonstrate excellent correlations between Vo2peak and maximal estimated METs at baseline, although this relationship was only modest after the training intervention. Finally, although the CRF cut points used in this analysis have significant clinical validity and prognostic implications, they were not specifically developed for a population with type 2 diabetes.
In conclusion, among a sedentary cohort with type 2 diabetes, a formal exercise training program, especially the AT component, was associated with significant improvements in overall CRF, which should be associated with significant reductions in CVD and CHD risk. Considering the very high CVD and CHD risk for patients with type 2 diabetes and the critical role of increasing CRF in reducing risks in these patients, CRF should be monitored and tracked together with HbA1c. International efforts are needed to urge clinicians to promote the importance of improving levels of CRF through exercise training (AT or ATRT) in the treatment of patients with type 2 diabetes.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (DK-068298).
No potential conflicts of interest relevant to this article were reported.
N.M.J., D.L.S., C.J.L., C.P.E., S.N.B., and T.S.C. contributed to the critical review of the manuscript for important intellectual content. N.M.J., D.L.S., C.J.L., S.N.B., and T.S.C. contributed to the study concept and design. N.J.M., D.L.S., C.J.L., and T.S.C. analyzed and interpreted the data and drafted the manuscript. N.M.J., C.P.E., S.N.B., and T.S.C. contributed administrative, technical, and material support. N.M.J., C.P.E., and T.S.C. contributed to the acquisition of data. N.M.J. and T.S.C. contributed to the study supervision and conducted the statistical analyses. S.N.B. and T.S.C. obtained funding. N.M.J. and T.S.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all HART-D participants and the Pennington Biomedical Research Center staff members who contributed to the main HART-D outcome trial for their hard work and commitment.
Footnotes
Clinical trial reg. no. NCT00458133, www.clinicaltrials.gov.
References
- 1.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 2009;301:2024–2035 [DOI] [PubMed] [Google Scholar]
- 2.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol 2007;165:1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampert JB, Blair SN, Barlow CE, Kohl HW., 3rd Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol 1996;6:452–457 [DOI] [PubMed] [Google Scholar]
- 4.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care 2005;28:391–397 [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 6.Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004;27:83–88 [DOI] [PubMed] [Google Scholar]
- 7.Marwick TH, Hordern MD, Miller T, et al. Council on Clinical Cardiology, American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee. Council on Cardiovascular Disease in the Young. Council on Cardiovascular Nursing. Council on Nutrition, Physical Activity, and Metabolism. Interdisciplinary Council on Quality of Care and Outcomes Research Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:3244–3262 [DOI] [PubMed] [Google Scholar]
- 8.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:357–369 [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans Rockville, MD, U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion, 2008 [Google Scholar]
- 11.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:1433–1438 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 13.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol 1993;22:175–182 [DOI] [PubMed] [Google Scholar]
- 15.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med 2005;353:468–475 [DOI] [PubMed] [Google Scholar]
- 16.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens 2007;20:608–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989;262:2395–2401 [DOI] [PubMed] [Google Scholar]
- 18.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia, Lippincott, Williams & Williams, 2006 [Google Scholar]
- 19.Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation 2011;123:1377–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol 2012;59:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DC, Sui X, Artero EG, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the Aerobics Center Longitudinal Study. Circulation 2011;124:2483–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Church TS, LaMonte MJ, Barlow CE, Blair SN. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med 2005;165:2114–2120 [DOI] [PubMed] [Google Scholar]
- 23.Lyerly GW, Sui X, Church TS, Lavie CJ, Hand GA, Blair SN. Maximal exercise electrocardiography responses and coronary heart disease mortality among men with diabetes mellitus. Circulation 2008;117:2734–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artero EG, Lee DC, Lavie CJ, et al. Effects of muscular strength and cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev 2012;32:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artero EG, Lee DC, Ruiz JR, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol 2011;57:1831–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavie CJ, Milani RV. Metabolic equivalent (MET) inflation—not the MET we used to know. J Cardiopulm Rehabil Prev 2007;27:149–150 [DOI] [PubMed] [Google Scholar]
- 27.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res 1971;3:323–332 [PubMed] [Google Scholar]
- 28.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation 2003;108:1554–1559 [DOI] [PubMed] [Google Scholar]
- 29.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J 1959;10:675–688 [PubMed] [Google Scholar]