Abstract
OBJECTIVE
We examined the predictors and risks associated with pre-existing versus new-onset diabetes mellitus (DM) after initiation of chronic dialysis therapy in end-stage renal disease (ESRD) patients.
RESEARCH DESIGN AND METHODS
In the Taiwan National Health Insurance Research Database, we examined records of ESRD patients who initiated dialysis between 1999 and 2005. Patients were followed until death, transplant, dialysis withdrawal, or 31 December 2008. Predictors of new-onset DM and mortality were calculated using Cox models.
RESULTS
A total of 51,487 incident dialysis patients were examined in this study, including 25,321 patients with pre-existing DM, 3,346 with new-onset DM, and 22,820 without DM at any time. Patients’ age (mean ± SD) was 61.8 ± 11.5, 61.6 ± 13.7, and 56.5 ± 16.6 years in pre-existing, new-onset DM, and without DM groups, respectively. The cumulative incidence rate of new-onset DM was 4% at 1 year and 21% at 9 years. Dialysis modality was not a risk factor for new-onset DM (peritoneal dialysis to hemodialysis hazard ratio [HR] of new-onset DM, 0.94 [95% CI 0.83–1.06]). Pre-existing DM was associated with 80% higher death risk (HR 1.81 [95% CI 1.75–1.87]), whereas the new-onset DM was associated with 10% increased death risk (HR 1.10 [95% CI 1.03–1.17]).
CONCLUSIONS
Whereas dialysis modality does not appear to associate with new-onset DM, both pre-existing and new-onset DM are related to higher long-term mortality in maintenance dialysis patients.
The increasing prevalence of diabetes mellitus (DM) is a global health issue in the obese and aging (1). Chronic kidney disease is an important complication of DM. Diabetic nephropathy, the leading cause of end-stage renal disease (ESRD) (2), accounts for ∼40% of patients on maintenance dialysis (3).
Many studies (2,4) report an association between pre-existing DM at the initiation of dialysis and a poor outcome in ESRD patients undergoing dialysis. However, few published studies have focused on postdialysis new-onset DM (4–7). Glucose is one of the contents of hemodialysates (8) and peritoneal dialysates (9). Peritoneal dialysis (PD) patients, who received 24-h continuous high-glucose–concentration peritoneal dialysates, can develop hyperglycemia and transient hyperinsulinism (10). Woodward et al. (6), examining the U.S. Renal Data System, showed the incidence of new-onset DM to be ∼6% per year in dialysis patients. In Asia, Chinese patients in Hong Kong have been observed to have a high prevalence of hyperglycemia with a daily exchange of 1.5% glucose dialysate (7). Some epidemiological studies of glycemic load in relation to incident DM report inconsistent results. For example, although high intake of foods with high glycemic load has been found to increase the risk of type 2 DM in Chinese (11), Mosdøl et al. (12) did not find such an association in the Whitehall II study. Nevertheless, one meta-analysis of prospective cohort studies enrolling 13 trials concluded that there was a positive association between glycemic load and type 2 DM (13). Pure glucose has the highest glycemic index, but few long-term follow-up studies have investigated the glucose load and the risk of DM, especially in patients with ESRD. In addition, it has been demonstrated that increased plasma glucose levels are an independent risk factor for mortality among dialysis patients, even a minor degree of hyperglycemia (7). It has also been reported that the cumulative advanced atherosclerotic change in DM could be responsible for the increased further cardiovascular mortality thereafter.
The worldwide number of ESRD patients undergoing dialysis has grown significantly in recent decades. The incidence and prevalence rates of ESRD are high in Taiwan (14). However, studies on new-onset DM are scarce, especially studies with epidemiological data from a national cohort of Asians with ESRD on maintenance dialysis. Therefore, this study investigates whether there is an association between dialysis modality and new-onset DM and whether new-onset DM is a risk factor for long-term mortality. To find out, we used a large dataset from the Taiwan National Health Insurance Research Database (NHIRD) from 1999 to 2008 to evaluate the epidemiology, incidence, and mortality of new-onset DM in ESRD patients undergoing dialysis.
RESEARCH DESIGN AND METHODS
Database
The National Health Insurance (NHI) program has provided compulsory universal health insurance in Taiwan since 1995. With the exception of prison inmates, all citizens are enrolled in the program. All contracted medical institutions must submit standard computerized claim documents for medical expenses. Patients with ESRD are eligible for any type of renal replacement therapy free of any charge. All chronic dialysis patients are covered by NHI.
Data were obtained from the NHIRD (Bureau of National Health Insurance; www.doh.gov.tw/statistic/index.htm [in Chinese]; http://www.doh.gov.tw/EN2006/index_EN.aspx [in English]) and released for research by the Taiwan National Health Research Institute. The NHIRD covers nearly all (99%) inpatient and outpatient medical benefit claims for Taiwan’s 23 million residents, is one of the largest and most comprehensive databases in the world, and has been used extensively in various studies. Patient identification numbers, sex, birthday, dates of admission and discharge, medical institutions providing the services, the ICD-9-CM diagnostic and procedure codes (up to five each), and outcomes are encrypted. This study tapped the NHIRD for ambulatory care claims, all inpatient claims, and the updated registry for beneficiaries from 1998 to 2008 for this study. All datasets can be interlinked.
Patient selection and definition
For this longitudinal cohort study, we selected incident ESRD patients on maintenance dialysis who began renal replacement therapy between 1 January 1999 and 31 December 2005 from outpatient claims (n = 51,794) (Supplementary Fig. 1). ESRD patients on maintenance dialysis were defined as having undergone dialysis for >90 days. Patients who had undergone renal transplantation before beginning dialysis were excluded (n = 307). Patients were followed up from the first reported date of dialysis to the date of death, end of dialysis, or 31 December 2008. In total, 51,487 incident dialysis patients were analyzed in this study. We first examined the patients with DM diagnosed before the initiation of dialysis (pre-existing DM group, n = 25,321). Next, we identified the patients with new-onset DM during the follow-up period from a subset of patients who did not have pre-existing DM (n = 26,166). New-onset DM after the initiation of dialysis was defined as DM diagnosed at least 3 months after dialysis began (new-onset DM group, n = 3,346). The remaining members of the no pre-existing DM, those without new-onset DM during follow-up period, were assigned to the non-DM group (n = 22,820).
Ascertaining the demographic and comorbid variables
We linked to the diagnostic codes through the inpatient and outpatient claims databases of the NHI. Cases of DM and those with comorbidities were identified according to one of the following definitions: 1) diagnostic codes in outpatient visits if the patient had an initial diagnosis at any time the year leading up to beginning of dialysis and then experienced one or more additional diagnoses within the subsequent 12 months, and the first and last outpatient visit within 1 year must had to be >30 days apart to avoid accidental inclusion of miscoded patients; or 2) diagnostic codes in hospitalization databases at least one time within the year leading up to start of dialysis. This method of identifying these comorbidities has been used extensively in various studies of Taiwan NHIRD, and many articles have been published (15–18). This study included not only the cumulative incidence of new-onset DM, but also date of death, patient demographics, and baseline comorbidities. ICD codes are provided in Supplementary Table 1.
Statistical analyses
Baseline characteristics between groups (pre-existing DM, new-onset DM, and without DM) were compared. Age was entered as a categorical variable (<45, 45–64, and ≥65 years). Significance was set at P < 0.05. The cumulative proportion of patients with new-onset DM and of survivors after the initiation of dialysis was calculated using the Kaplan-Meier method. The log-rank test was used to analyze significance. Cox proportional hazards models were used to identify the risk factors of new-onset DM and mortality after the initiation of dialysis. Hazard ratios (HRs) and 95% CIs were derived from Cox proportional hazards models. Cox models met the assumption of proportionality of risks. The purposeful selection process begins by a univariate analysis of each variable. Any variable having a significant univariate test was selected as a candidate for the multivariate analysis. The independent associations were examined using multivariate analysis. All statistical operations were performed using the Statistical Package for Social Sciences for Windows 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Demographics and clinical characteristics
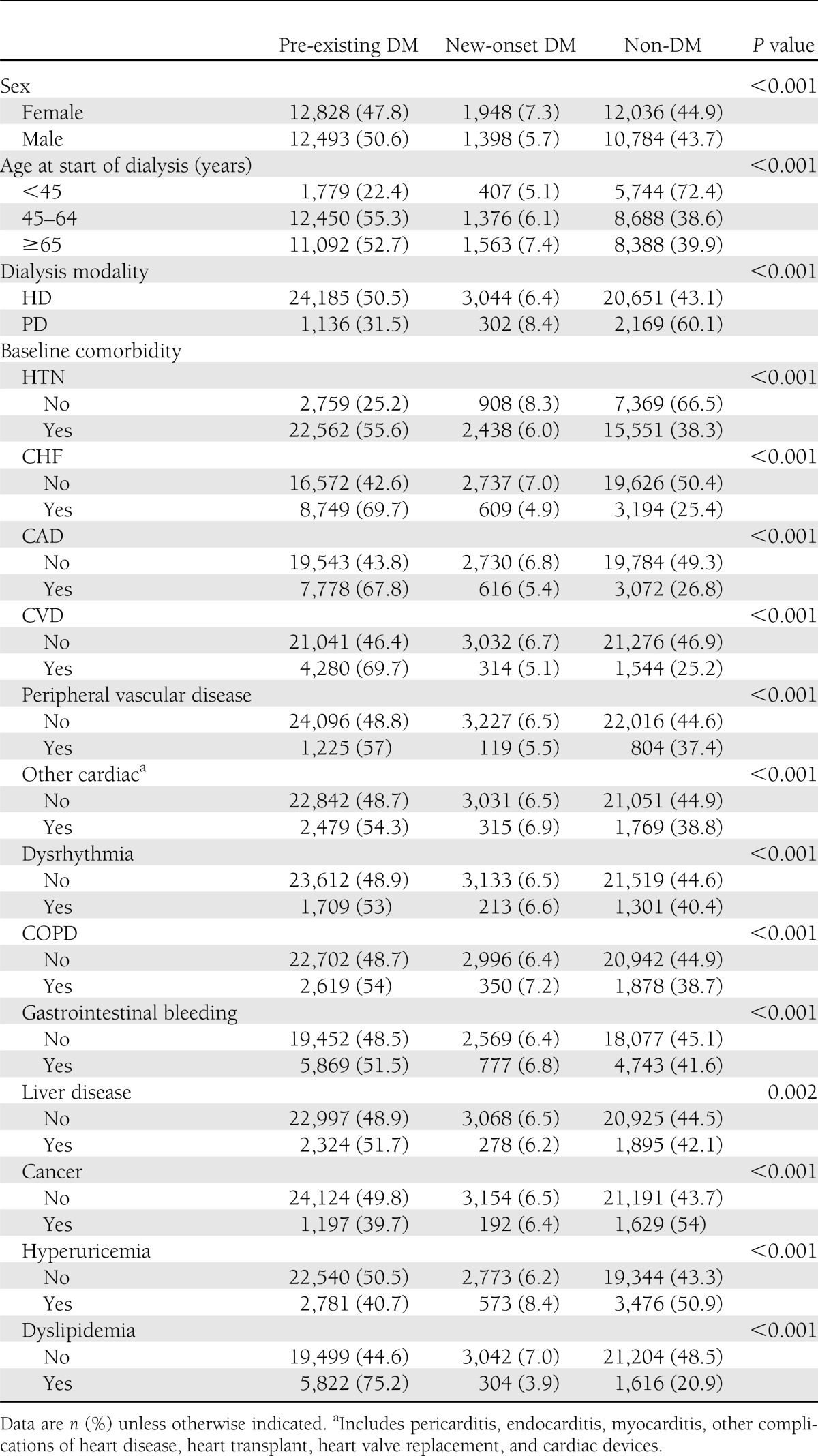
A total of 51,487 incident dialysis patients were enrolled in this study. Of these patients, 25,321 had pre-existing DM, 3,346 had new-onset DM, and the other 22,820 did not have DM throughout the study period (Table 1). There were 147 cases of type 1 DM among 25,321 pre-existing DM patients, but no type 1 DM among 3,346 new-onset DM patients. A total of 7% of female and 6% of male patients had new-onset DM (P < 0.001). Only 5% of those <45 years old had new-onset DM, but 7% of those ≥65 years old did (P < 0.001). In Taiwanese dialysis patients, 93% patients received hemodialysis (HD), and only 7% patients received PD. A total of 51 and 6% of the HD patients had pre-existing DM and new-onset DM, respectively, whereas only 32 and 8% of the PD patients had pre-existing DM had new-onset DM, respectively (P < 0.001).
Table 1.
Patient characteristics and association with pre-existing DM (n = 25,321), new-onset DM (n = 3,346), and non-DM (n = 22,820) in ESRD dialysis patients
Cumulative incidence and risk factors for new-onset DM
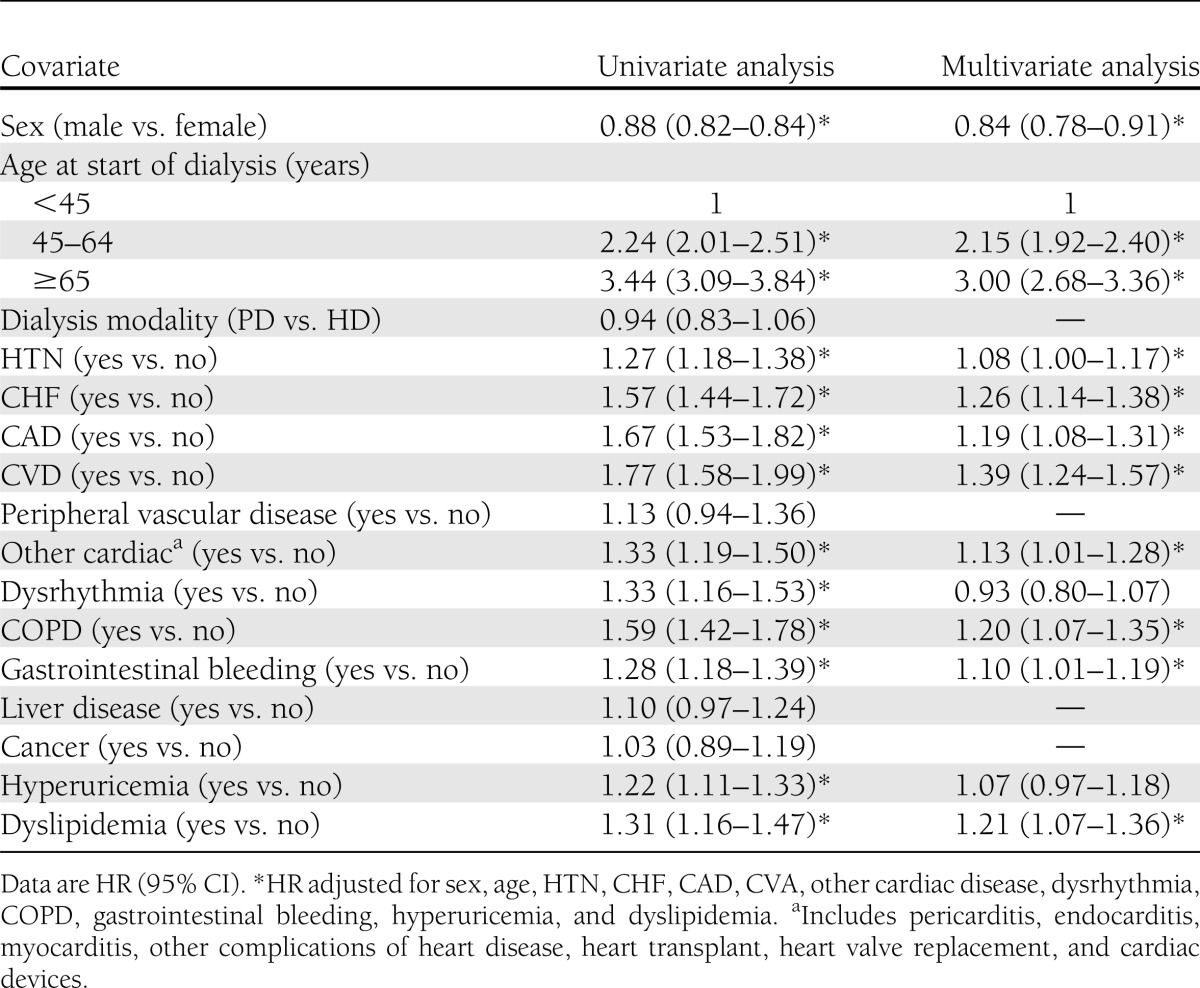
The cumulative incidence rate of new-onset DM were 4% at 1 year, 9% at 3 years, 14% at 5 years, and 21% at 9 years (Supplementary Fig. 2A). Being female, being older, and having baseline comorbidities were independent risk factors for new-onset DM in dialysis patients (Table 2). There was no significant difference between the modalities of HD and PD with regard to new-onset DM (Supplementary Fig. 2B). Patients ≥65 years old had nearly a threefold increase in new-onset DM compared with those <45 years old (HR 3.00 [95% CI 2.68–3.60]). Additionally, factors increasing the likelihood that new-onset DM would develop included hypertension (HTN) (HR 1.08, 95% CI 1.00–1.17), congestive heart failure (CHF) (HR 1.26, 95% CI 1.14–1.38), coronary artery disease (CAD) (HR 1.19 [95% CI 1.08–1.31]), cerebrovascular accident (CVA) (HR 1.39 [95% CI 1.24–1.57]), and chronic obstructive pulmonary disease (COPD) (HR 1.20 [95% CI 1.07–1.35]).
Table 2.
Risk factors for new-onset DM after initiation of dialysis in ESRD non–pre-existing DM dialysis patients (n = 26,166)
Cumulative survival rate and risk factors for all-cause mortality
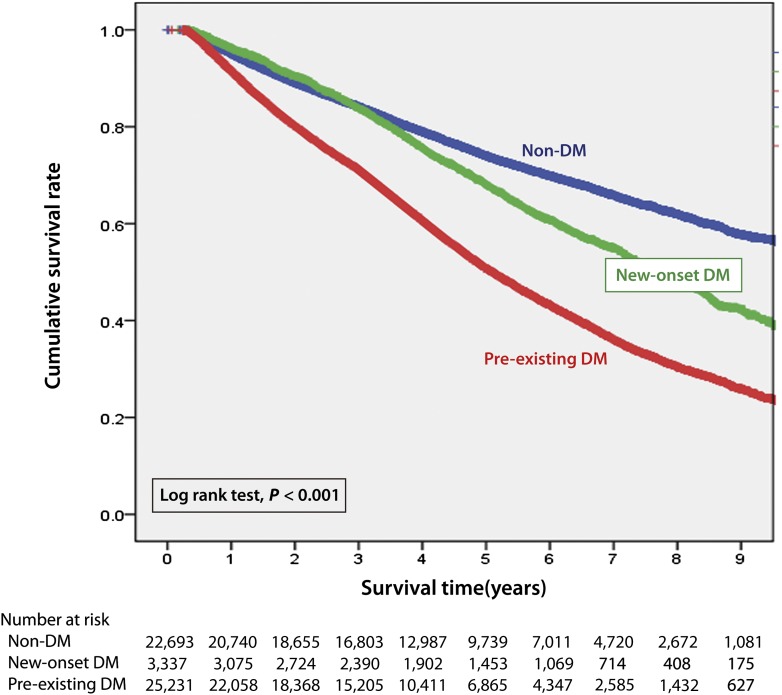
The Kaplan-Meier survival curves for patients in the pre-existing DM, new-onset DM, and non-DM groups are shown in Fig. 1. The cumulative survival rate of the pre-existing DM group was 92% at 1 year, 51% at 5 years, and 26% at 9 years. The cumulative survival rate of the new-onset DM group was 96% at 1 year, 68% at 5 years, and 42% at 9 years. The cumulative survival rate of the non-DM group was 95% at 1 year, 74% at 5 years, and 58% at 9 years. The differences in survival among these three groups were significant (log-rank: P < 0.001). We have further analyzed the survival rate after new-onset DM was diagnosed (Supplementary Fig. 3) and found the mean duration between new-onset DM diagnosed and death was 6.10 ± 1.01 years.
Figure 1.
Crude overall survival curves after initiation of dialysis stratified by pre-existing DM (n = 25,321), new-onset DM (n = 3,346), and non-DM (n = 22,820) in ESRD dialysis patients. (A high-quality color representation of this figure is available in the online issue.)
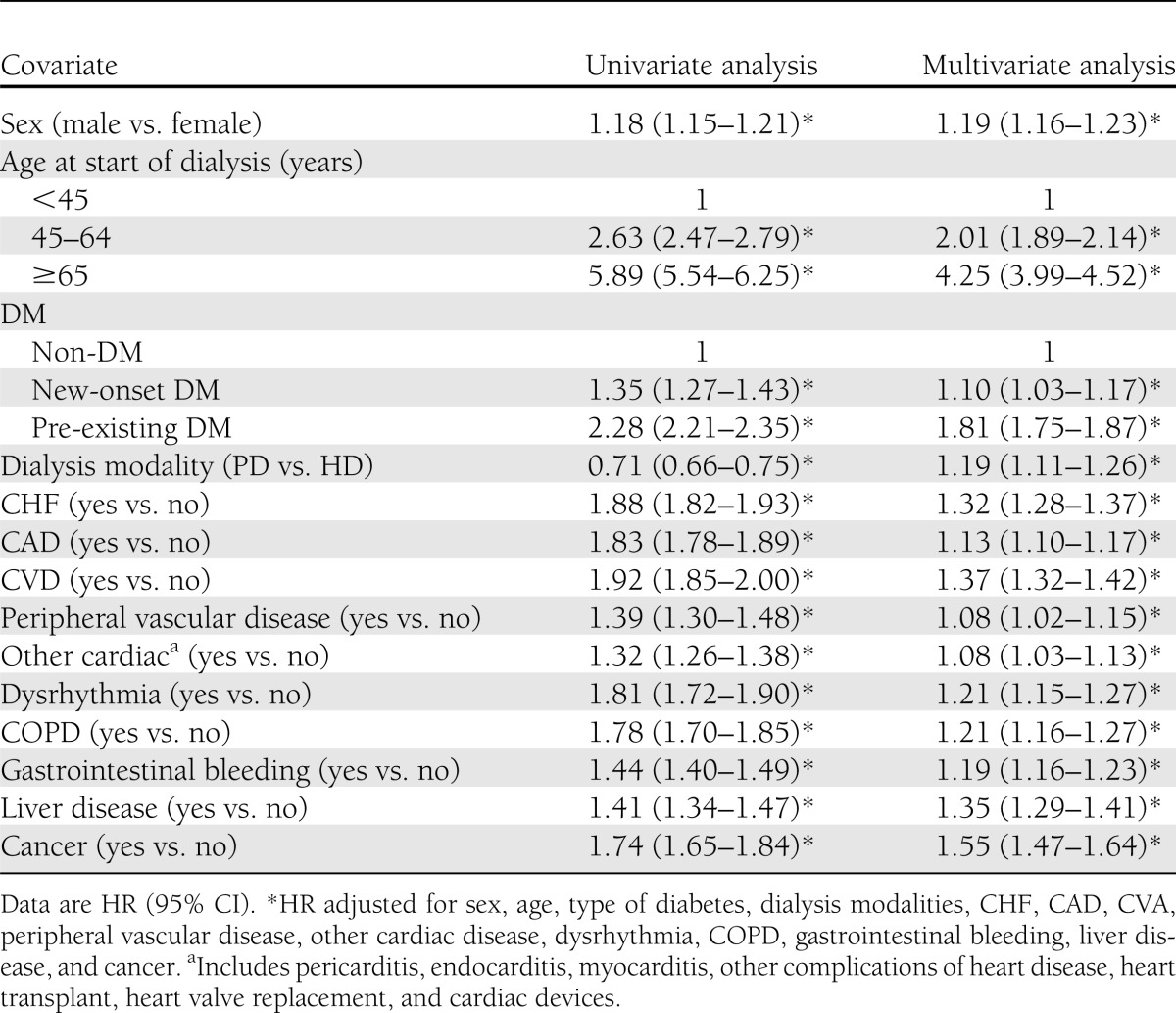
Pre-existing DM was associated with 80% higher death risk (HR 1.81 [95% CI 1.75–1.87]), whereas the new-onset DM was associated with 10% increased death risk (HR 1.10 [95% CI 1.03–1.17]) (Table 3). As can be seen in Supplementary Table 2, further analysis revealed individuals with pre-existing DM <45 years old were 2.99 (95% CI 2.65–3.39) times more likely to die than individuals of a similar age without DM, with the HR decreasing in elderly individuals with pre-existing DM. The trend was similar to those with new-onset DM compared with those without DM. Risk estimates for mortality tended to be higher in women than in men in both pre-existing DM and new-onset DM groups.
Table 3.
Risk factor for all-cause mortality in ESRD dialysis patients (n = 51,487)
CONCLUSIONS
In this nationwide study of 51,487 incident dialysis patients, we found no significant association between dialysis modality and new-onset DM. However, new-onset DM was significantly associated with sex, age, and baseline comorbidity. It was a risk factor for long-term mortality in patients on maintenance dialysis.
The incidence of new-onset DM after dialysis varies. One study (5) reported that the incidence after HD was 20 per 1,000 patient-years, and the prevalence was 7.6% during only 3 years of follow-up. Our nearly 10-year follow-up study found a higher incidence (29 per 1,000 patient-years) and prevalence (12.8%) rate after HD. Another 6-year follow-up study (4) reported that 8.5% of dialysis patients, including HD and PD, who initiated dialysis developed new-onset DM within 6 years. In our study, 12.7% of dialysis patients, including HD and PD, developed new-onset DM within 10 years. This higher rate of new-onset DM may reflect the longer follow-up period in our study. Woodward et al. (6) also found that immunosuppressant agents had great impact on the new-onset DM and reported that new-onset DM over the first 2 years posttransplant had a very high incidence of almost 18–30% among patients receiving cyclosporine and tacrolimus.
We found no significant difference in percentage of new-onset DM after the initiation of dialysis between patients undergoing HD (12.80%) and patients undergoing PD (12.20%), even after adjustment. This finding differs from that for the wait-listed transplanted renal allograft recipients in Woodward et al. (6). This discrepancy may be because the results in Woodward et al. (6) were not adjusted.
Being female and being older were significant risk factors for the development of new-onset DM in our patients. Age not only affected the prevalence of new-onset DM, but also the mortality. The prevalence of DM increases with age (1), which is considered one parameter of diabetes risk scores (19). The pathogenesis of age-related DM is related to insulin resistance and decreased β-cell function (20). We also found that cardiovascular disease (CVD) to be a significant risk factor for the development of new-onset DM. This relationship might be explained by the fact that atherosclerosis contributes to most of the macrovascular disease. Dyslipidemia and vascular inflammation result in endothelial dysfunction and atherosclerosis (21). Elevated values of circulatory makers such as interleukin-6 and high-sensitivity C-reactive protein (CRP) commonly accompany CVD. Vascular inflammation and endothelial dysfunction may also be associated with an increased risk of developing type 2 DM. Hu et al. (22) conducted a prospective, case-control study of inflammatory markers as predictors of type 2 DM among 32,826 subjects. These data support the role of inflammation in the pathogenesis in type 2 DM. A 4-year follow-up study in a nationwide cohort of 27,628 subjects shows elevated levels of CRP and interleukin-6 predict the development of type 2 DM (23). A 7.2-year follow-up study also showed that subjects with elevated CRP had a higher risk of developing diabetes and concluded that inflammation could be one of the risk factors for developing DM (24). Meigs et al. (25) performed a prospective study that showed that endothelial dysfunction could predict type 2 DM independent of other known risk factors. One study enrolled 8,291 Italian patients with a myocardial infarction within the previous 3 months who were free of diabetes at baseline. During 26,785 person-years follow-up, the study showed that patients with a recent myocardial infarction had a higher annual incidence rate of impaired fasting glucose and DM (26). In addition, some common prescribed drugs for CVD, such as statin, might also result in dysglycemia. Many meta-analyses reported an association between statin use and a 10% increase in risk for incident diabetes (27–29).
HTN might be a significant factor associated with new-onset DM independent of other cardiovascular comorbidities, such as CHF, CAD, or other CVD. In our study, we found HTN to be a risk factor for new-onset DM in patients with ESRD under maintenance dialysis. One prospective study by Conen et al. (30) examined the relationship for blood pressure and blood pressure progression with the incident diabetes in the general population. They found patients with baseline HTN had a higher risk of developing DM independent of BMI and other components of metabolic syndrome. The reasons for this relationship are uncertain. Endothelial dysfunction might be one of the common pathophysiological pathways between HTN and incident type 2 DM.
Other studies have reported similar findings. One reported the death rate at the end of a 3-year follow-up to be significantly higher in patients with new-onset DM undergoing HD and second highest in patients with pre-existing DM than in those without DM (49.2 vs. 50.6 vs. 41%, respectively) (5). Another study reported that ESRD patients on PD, with increased fasting plasma glucose levels, had a greater mortality rate (7). In our study, patients undergoing HD and PD were enrolled and followed-up for ∼10 years. We found the cumulative survival rate to be highest in those without DM, moderate in those with new-onset DM, and lowest in those with pre-existing DM. From the Kaplan-Meier survival plot, we found survival curves of patients with new-onset DM and without DM began to diverge 3 years after initiation of dialysis therapy. This may reflect cumulative or delayed damage caused by the increased glucose level. We have further analyzed the survival rate after new-onset DM was diagnosed (Supplementary Fig. 3) and found the mean duration between new-onset DM diagnosed and death was 6.10 ± 1.01 years.
There are several limitations to our study. First, the comorbidity diagnoses relied on the claims data and ICD-9-CM diagnosis codes and may have some disease misclassifications. Second, we were unable to take into account the severity of the diseases. Third, it would be interesting to consider what would happen to patients with pre-existing DM who stopped taking insulin and/or oral agents due to recurrent hypoglycemia and lowered A1C <6% while off all medications. However, our study lacked specific data on medical prescriptions and laboratory data for this analysis. Finally, it would be better to describe the association with cardiovascular mortality; however, the Taiwan Bureau of NHI does not afford the cross-link information between this and the database of causes of death.
In conclusion, we found that female sex, old age, and some comorbidities were associated with new-onset DM. Dialysis modality was not a significant predictor of new-onset DM. In addition, new-onset DM increased long-term mortality more than non-DM. Physicians might want to pay attention to the plasma glucose level of high-risk patients undergoing dialysis.
Acknowledgments
The study was supported by Grant CMFHR10124 from Chi-Mei Medical Center and Grant NHRI-NHIRD-99182 from the National Health Research Institutes in Taiwan.
No potential conflicts of interest relevant to this article were reported.
K.-J.T. contributed to the discussion and reviewed and edited the manuscript. Z.-Z.L. contributed to the discussion. C.-C.Chio drafted the manuscript. J.-J.W. designed the study, researched data, and drafted the manuscript. C.-C.Chu drafted the manuscript. Y.-M.S. drafted the manuscript. W.-C.K. and C.-C.Chien designed the study and researched data. C.-C.Chien is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2148/-/DC1.
References
- 1.Wild SH, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann F, Haastert B, Koch M, Giani G, Glaeske G, Icks A. The effect of diabetes on incidence and mortality in end-stage renal disease in Germany. Nephrol Dial Transplant 2011;26:1634–1640 [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2011 [Google Scholar]
- 4.Lok CE, Oliver MJ, Rothwell DM, Hux JE. The growing volume of diabetes-related dialysis: a population based study. Nephrol Dial Transplant 2004;19:3098–3103 [DOI] [PubMed] [Google Scholar]
- 5.Salifu MO, Abbott KC, Aytug S, et al. New-onset diabetes after hemodialysis initiation: impact on survival. Am J Nephrol 2010;31:239–246 [DOI] [PubMed] [Google Scholar]
- 6.Woodward RS, Schnitzler MA, Baty J, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 2003;3:590–598 [DOI] [PubMed] [Google Scholar]
- 7.Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. New-onset hyperglycemia in nondiabetic chinese patients started on peritoneal dialysis. Am J Kidney Dis 2007;49:524–532 [DOI] [PubMed] [Google Scholar]
- 8.Raimann JG, Kruse A, Thijssen S, et al. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrol Dial Transplant 2012;27:1559–1568 [DOI] [PubMed] [Google Scholar]
- 9.Wideröe TE, Smeby LC, Myking OL. Plasma concentrations and transperitoneal transport of native insulin and C-peptide in patients on continuous ambulatory peritoneal dialysis. Kidney Int 1984;25:82–87 [DOI] [PubMed] [Google Scholar]
- 10.Lindholm B, Karlander SG. Glucose tolerance in patients undergoing continuous ambulatory peritoneal dialysis. Acta Med Scand 1986;220:477–483 [DOI] [PubMed] [Google Scholar]
- 11.Villegas R, Liu S, Gao YT, et al. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 2007;167:2310–2316 [DOI] [PubMed] [Google Scholar]
- 12.Mosdøl A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr 2007;86:988–994 [DOI] [PubMed] [Google Scholar]
- 13.Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr 2011;106:1649–1654 [DOI] [PubMed] [Google Scholar]
- 14.Yang WC, Hwang SJ, Taiwan Society of Nephrology Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant 2008;23:3977–3982 [DOI] [PubMed] [Google Scholar]
- 15.Chen HF, Ho CA, Li CY. Age and sex may significantly interact with diabetes on the risks of lower-extremity amputation and peripheral revascularization procedures: evidence from a cohort of a half-million diabetic patients. Diabetes Care 2006;29:2409–2414 [DOI] [PubMed] [Google Scholar]
- 16.Chen HF, Chen P, Li CY. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology 2010;52:155–163 [DOI] [PubMed] [Google Scholar]
- 17.Chen PC, Chan YT, Chen HF, Ko MC, Li CY. Population-Based Cohort Analyses of the Bidirectional Relationship Between Type 2 Diabetes and Depression. Diabetes Care 2012;36:376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 2012;35:1047–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–731 [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab 1985;60:13–20 [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–1143 [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004;53:693–700 [DOI] [PubMed] [Google Scholar]
- 23.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 24.Thorand B, Löwel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984-1998. Arch Intern Med 2003;163:93–99 [DOI] [PubMed] [Google Scholar]
- 25.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004;291:1978–1986 [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Marfisi R, Levantesi G, et al. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet 2007;370:667–675 [DOI] [PubMed] [Google Scholar]
- 27.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med 2012;172:144–152 [DOI] [PubMed] [Google Scholar]
- 28.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564 [DOI] [PubMed] [Google Scholar]
- 29.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742 [DOI] [PubMed] [Google Scholar]
- 30.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women’s Health Study. Eur Heart J 2007;28:2937–2943 [DOI] [PubMed] [Google Scholar]