Abstract
OBJECTIVE
To investigate whether the risk of bladder cancer in individuals with newly diagnosed type 2 diabetes is influenced by the frequency of physician visits before diagnosis as a measure of detection bias.
RESEARCH DESIGN AND METHODS
With the use of linked administrative databases from 1996 to 2006, we established a cohort of 185,100 adults from British Columbia, Canada, with incident type 2 diabetes matched one to one with nondiabetic individuals on age, sex, and index date. Incidence rates and adjusted hazard ratios (aHRs) for bladder cancer were calculated during annual time windows following the index date. Analyses were stratified by number of physician visits in the 2 years before diabetes diagnosis and adjusted for age, sex, year of cohort entry, and socioeconomic status.
RESULTS
The study population was 54% men and had an average age of 60.7 ± 13.5 years; 1,171 new bladder cancers were diagnosed over a median follow-up of 4 years. In the first year after diabetes diagnosis, bladder cancer incidence in the diabetic cohort was 85.3 (95% CI 72.0–100.4) per 100,000 person-years and 66.1 (54.5–79.4) in the control cohort (aHR 1.30 [1.02–1.67], P = 0.03). This first-year increased bladder cancer risk was limited to those with the fewest physician visits 2 years before the index date (≤12 visits, aHR 2.14 [1.29–3.55], P = 0.003). After the first year, type 2 diabetes was not associated with bladder cancer.
CONCLUSIONS
The results suggest that early detection bias may account for an overestimation in previously reported increased risks of bladder cancer associated with type 2 diabetes.
Studies have suggested that people with type 2 diabetes are at an increased risk of several types of cancer, including breast, colorectal, pancreatic, endometrial, and liver (1–5). Similarly, a meta-analysis of observational studies suggested a statistically significant 37–43% increased risk of bladder cancer in those with diabetes (6).
Epidemiologic evidence also suggests an initial period of elevated risk for most solid cancers (colorectal, endometrial, lung, breast, cervical, ovarian, and prostate) in the months immediately following a diabetes diagnosis, which is followed by a decline and leveling off of risk after the first year (7,8). This pattern suggests a potential detection bias around the time of a new diabetes diagnosis, but the influence of this phenomenon on the seemingly increased risk of bladder cancer has not been assessed (1–5). Conceptually, individuals with fewer physician visits may have a lower likelihood of detection of a presymptomatic bladder cancer before diabetes diagnosis, whereas those with more visits may be more likely to have an incidental bladder cancer detected, such as during routine urinalysis. Thus, the risk of bladder cancer diagnosis after diabetes diagnosis may be higher among individuals with fewer physician visits (and lower among those with more frequent physician visits). If this is the case, the reported increase in bladder cancer risk may be attributable to detection bias.
With the recent attention to bladder cancer and diabetes, especially with reports of an increased risk associated with pioglitazone (9–12), we must understand temporal trends and the potential influence of detection bias on bladder cancer in individuals with diabetes (7). Therefore, we examined the time-varying risk of bladder cancer in a large population-based cohort of individuals with a new diagnosis of type 2 diabetes relative to nondiabetic controls to assess a potential detection bias.
RESEARCH DESIGN AND METHODS
The study population for this analysis has been previously described (7). Briefly, we used the British Columbia Linked Health Databases (BCLHD), which includes administrative health claims, demographic data, and information from the BC Cancer Agency from 1 April 1996 through 31 March 2006, to identify a retrospective cohort of individuals >30 years of age with incident type 2 diabetes (N = 185,100). A cohort of the same size was selected as control subjects from individuals who had not been identified as having diabetes as of 31 March 2006, with one-to-one matching on birth year and sex. The date of type 2 diabetes diagnosis was assigned as the index date for each matched pair. To protect patient confidentiality, the dataset was devoid of all traceable personal identifiers. Ethics approval from the University of British Columbia Behavioral Research Ethics Board and the University of Alberta Health Research Ethics Board was obtained.
Type 2 diabetes was identified with the previously validated case definition (13) used by the Canadian National Diabetes Surveillance System (14) and defined as the earlier of 1) a hospital admission for diabetes (ICD-9 code 250) or 2) the second of two medical fee-for-service claims coded with ICD-9 code 250 within a 2-year period. Individuals who met this definition before 1 April 1996 and women with gestational diabetes (ICD-9 code 648.8) were excluded.
After exclusion of individuals with any cancer diagnosis in the 2 years before the index date, we identified incident cases of bladder cancer (ICD-O-3 code C67.X) diagnosed after the index date. Individuals were censored at the earlier of the end of the study (31 March 2006) or departure from BCLHD (i.e., from British Columbia), and the follow-up was terminated at death.
Statistical analyses
We first calculated unadjusted bladder cancer incidence rates during the following time windows after the index date: <1.0, 1.0–2.0, 2.0–3.0, and 3.0–10.0 years. We then used Cox regression to estimate adjusted time-varying hazard ratios (HRs) for developing bladder cancer during the time windows, with time since the index date as the time scale and the nondiabetes cohort as the reference group. All models were adjusted for age, sex, index year, and socioeconomic status. To represent socioeconomic status, median neighborhood income quintiles were derived from 2006 Canadian census data and assigned according to postal code of residence, with a sixth missing information category also included.
To explore potential detection bias, we hypothesized that the risk of bladder cancer would differ by the frequency of visits to physicians in the 2 years before the index date. Rate of physician visits has recently been reported as a strong indicator for potential observational bias and is associated with illness adjustment (15). Physician visits in the 2 years before the index date were categorized in tertiles as ≤12 visits (low), 13–24 visits (medium), and ≥25 visits (high). We tested an interaction term between diabetes status and number of physician visits. Finding a statistically significant interaction, we then stratified the incidence rate calculations and regression models by the physician visit categories. To graphically display changes in bladder cancer risk over time, adjusted HRs were calculated and plotted at regular intervals throughout follow-up. We used a lowess curve to smooth the plotted representation of the time-varying bladder cancer risk in each category. Results with P < 0.05 were interpreted as statistically significant. All analyses were conducted with Stata/SE 11 (StataCorp, College Station, TX) statistical software; graphs were created in R (16).
RESULTS
The cohort comprised 185,100 individuals with incident type 2 diabetes and 775,398 person-years (PY) of follow-up and 185,100 matched individuals without diabetes and 795,167 PY of follow-up (Table 1). Fifty-four percent were male, and the mean (SD) age at the time of diabetes diagnosis (or index date for the control cohort) was 60.7 (13.5) years. Individuals with incident type 2 diabetes were more likely to have a low socioeconomic status, with 23% in the lowest socioeconomic quintile vs. 16% in the highest quintile compared with 19 and 21%, respectively, in the nondiabetes cohort. The incident type 2 diabetes and nondiabetes cohorts had an approximately equal median (interquartile range [IQR]) duration of cancer-free years in the database before the index date (4.0 [1.8–6.7] vs. 4.1 [1.9–6.8], respectively). Follow-up length after the index date was similar in both cohorts (3.8 [1.7–6.5] vs. 3.9 [1.8–6.6] years in the diabetes and nondiabetes cohorts, respectively) (Table 1).
Table 1.
Population characteristics at index date
Incidence of bladder cancer
During the entire follow-up period, 603 (0.33%) individuals with incident type 2 diabetes and 568 (0.31%) nondiabetic individuals were given a diagnosis of bladder cancer. Individuals who were eventually given a diagnosis of bladder cancer were older at the index date (mean 69.5 (SD 10.0) vs. 60.7 (13.5) years), were more often male (81 vs. 54%), and had more physician visits (median 22 [IQR 12–39] vs. 19 [9–34]) in the 2 years before the index date than those who were not given a diagnosis of bladder cancer (Table 1). The overall incidence of diagnosed bladder cancer over the duration of follow-up was 77.8 (95% CI 71.7–84.2) per 100,000 PY for those with diabetes vs. 71.4 (65.7–77.6) per 100,000 PY for those without. In adjusted analyses, diabetes was significantly associated with an increased risk of bladder cancer (adjusted HR 1.13 [95% CI 1.01–1.26], P = 0.04).
Time-varying risks of bladder cancer
In the first year following the index date, the incidence rates of bladder cancer in the diabetes and nondiabetes cohorts were 85.3 (95% CI 72.0–100.4) and 66.1 (54.5–79.4) per 100,000 PY, respectively (adjusted HR 1.30 [95% CI 1.02–1.67], P = 0.03). Diabetes was not associated with an increased risk of bladder cancer in any subsequent time window (Table 2), and the overall risk of bladder cancer, when excluding the first year of follow-up, was 1.08 (0.95–1.23) (P = 0.24) (Fig. 1 and Table 2).
Table 2.
Unadjusted bladder cancer IRs in newly diagnosed T2DM and no DM cohorts and aHRs by time since T2DM diagnosis and number of physician visits in the 2 years before the index date (T2DM diagnosis)
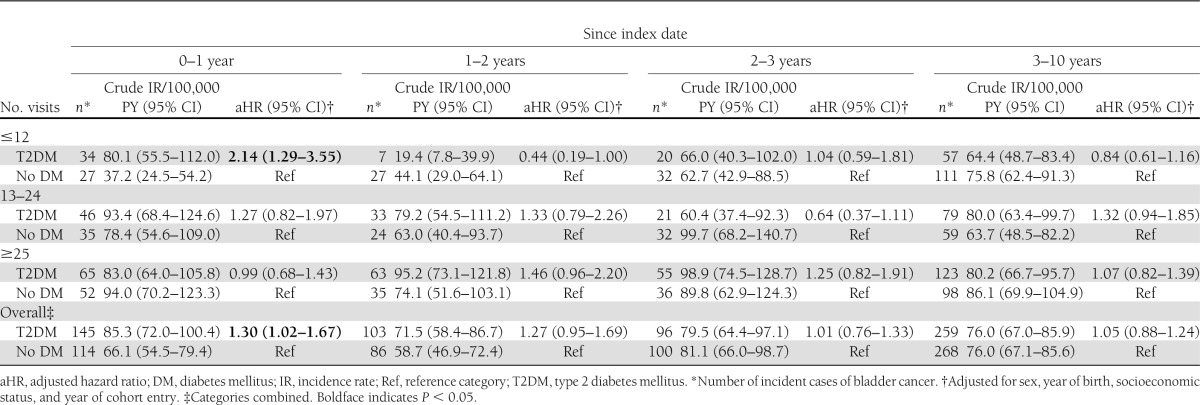
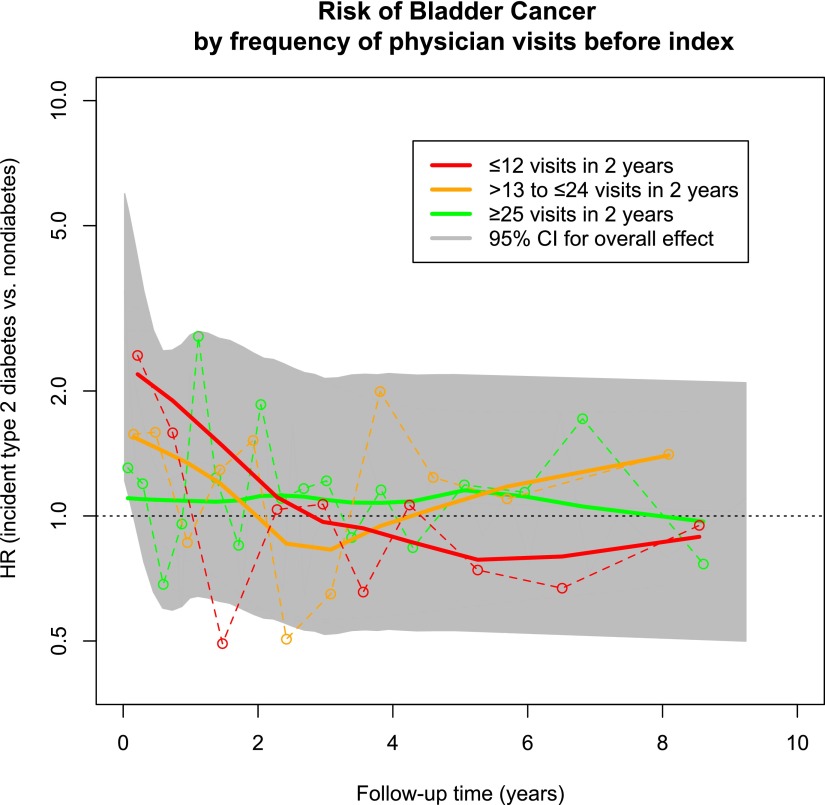
Figure 1.
Risk of bladder cancer by frequency of physician visits 2 years before the index date. Adjusted HRs for each physician visit category (≤12, 13–24, and ≥25 visits) were calculated at multiple points throughout follow-up and plotted (○ connected by dashed line). The time-varying risk of bladder cancer is estimated with the solid trend lines. The 95% CI for the overall (i.e., nonstratified) effect is shaded in gray (trend line for overall risk not shown).
Potential detection bias related to medical visits
The incident type 2 diabetes cohort had a greater number of physician visits in the 2 years before the index date (i.e., diabetes diagnosis) than the nondiabetes cohort (median 23 [IQR 13–39] vs. 16 [7–30]). We observed a statistically significant interaction between diabetes status and the frequency of physician visits in the 2 years before the index date in the time windows of 0–1 year (P = 0.017) and 1–2 years (P = 0.012). Therefore, we stratified regression models according to the number of physician visits before the index date (Table 2). In the first year of follow-up, the significantly elevated risk of bladder cancer was confined to those who had the fewest previous physician visits (adjusted HR 2.14 [95% CI 1.29–3.55] vs. 1.27 [0.82–1.97] vs. 0.99 [0.68–1.43] for ≤12, 13–24, and ≥25 visits, respectively, P = 0.018 for trend) (Table 2). In subsequent time periods, estimates in all physician visit frequency categories approached the null and were not statistically significant (Fig. 1 and Table 2).
CONCLUSIONS
Overall, we observed a statistically significant 13% relative increase in the risk of developing bladder cancer over a period of up to 10 years after the diagnosis of type 2 diabetes. However, our more detailed analyses suggest that the increased risk of bladder cancer occurred in the first year after type 2 diabetes diagnosis and predominantly among individuals who previously accessed physician services the least. Indeed, we found no significant increased risk of bladder cancer among those with type 2 diabetes in time periods of ≥2 years, regardless of controlling for detection bias (i.e., the frequency of previous physician visits).
Evidence for detection bias
There is a growing body of observational studies on the risk of various cancer in people with type 2 diabetes, including an estimated 43% increased risk of bladder cancer among those with diabetes of any duration (6,7,17,18). Consistent with evidence from several other cancers in people with diabetes (7,8), the present results show a significantly elevated overall risk of bladder cancer that when split into follow-up time windows shows a highly elevated risk of bladder cancer in the months immediately following type 2 diabetes diagnosis. This risk declines to approximately the level of the nondiabetes population over time.
Bladder cancer is not routinely screened for during a regular physician’s visit and often is discovered incidentally during routine urinalyses (19). Approximately 1 in 10 cases of hematuria are caused by an underlying bladder cancer (20). Individuals who previously visited the physician infrequently, and thus had less opportunity for investigation of potential symptoms, may be more likely to have an undiagnosed bladder cancer at the time of diabetes diagnosis than those who had more frequent physician contact, suggesting a mechanism for potential detection bias. Similarly, among individuals with fewer (≤12) physician visits in the 2 years before diabetes diagnosis, workup at the time of diagnosis may allow bladder cancer to be detected sooner. The below-the-null rebound of bladder cancer risk observed in this group during the second year after diabetes diagnosis (index date) suggests that cases that would have been detected in year 2 were shifted to the first year, thereby depleting these susceptible individuals from the subsequent time point. We observed no difference in bladder cancer risk between individuals with and without diabetes in the highest physician visit category. In this category, frequent physician visits may be driven by serious and/or multiple health problems; in this group, diabetes status may no longer differentially affect the likelihood of discovering bladder cancer. Alternatively, frequent physician visits may reflect health-seeking behavior (i.e., regular exercise, eating a healthy diet, not smoking); such behaviors may have prevented the otherwise potentially elevated bladder cancer risk in the diabetes group.
Study limitations
Despite some strengths, this work has several important limitations. First, we lacked potentially important clinical information, such as smoking (a known risk factor for bladder cancer and, thus, a potential confounder) or frequency of urinalyses (to further explore the detection bias hypothesis). We did, however, adjust for socioeconomic status, which is correlated with smoking status. Confounding by smoking is unlikely to be time dependent and, thus, the time-specific findings may not be subject to this limitation. Second, the follow-up period of up to 10 years (median 4 years) may not have been long enough to capture latent bladder cancer risk when the estimated latency period may extend up to 30 years (21). Third, diagnoses of diabetes were based entirely on claims data, and given the number of individuals with undiagnosed diabetes in the community, it is almost certain that there were individuals with diabetes in the control group. Given that the diagnostic workup for a diagnosis of diabetes is associated with a (short-term) increased risk of a bladder cancer diagnosis, undiagnosed diabetes in the nondiabetes cohort would not influence the findings in an important way because individuals in this group would not have received this workup. Finally, we did not examine another tracer condition. If our hypotheses are correct, other new diagnoses, such as of hypothyroidism or chronic obstructive pulmonary disease, could also lead to spuriously increased diagnoses of new cancers because of detection bias.
Implications
We observed a significantly increased risk of bladder cancer in individuals with newly diagnosed type 2 diabetes compared with individuals without diabetes. However, this increased risk in the type 2 diabetes population was limited to the first year following diabetes diagnosis and only among individuals with the fewest (≤12) physician visits in the previous 2 years. Subsequent to the first year following diabetes diagnosis, the risk of bladder cancer was equal to that of individuals without diabetes. This pattern suggests a potential detection bias of bladder cancer in those with type 2 diabetes. Studies that fail to account for time since diabetes diagnosis and frequency of physician visits may overestimate the long-term risk of bladder cancer in these individuals. Moreover, the present findings suggest that associations between diabetes (and possibly other newly diagnosed conditions) and risk of other cancers might, at least in part, be a result of detection biases. With the recent interest in the potential association between pioglitazone use and bladder cancer (9,11,12) and given the potential bias visiting the physician has on bladder cancer detection, this study begs the question of whether seeing the physician more frequently (e.g., to intensify or switch glucose-lowering agents) may bias the discovery of clinically present, but undiagnosed bladder cancer.
Acknowledgments
Support for this research was provided in part by an operating grant from the Canadian Institutes of Health Research (CIHR) (reference # MOP-82737) and a CIHR Team Grant to the Alliance for Canadian Health Outcomes Research in Diabetes (ACHORD) (reference # OTG-88588) sponsored by the CIHR Institute of Nutrition, Metabolism and Diabetes. I.N.C. holds a Studentship for Diabetes Research from the Alberta Diabetes Foundation and is supported by the ACHORD Research Trainee Program. S.R.M. holds the Endowed Chair in Patient Health Management (Faculties of Medicine and Dentistry and Pharmacy and Pharmaceutical Sciences, University of Alberta) and receives salary support from an Alberta Innovates - Health Solutions (AIHS) Health Scholar Award. Y.Y. holds a Canada Research Chair in Biostatistics and is a Health Senior Scholar with AIHS. C.A.M. holds a Canada Research Chair in Pharmaceutical Outcomes. J.A.J. is a Health Senior Scholar with AIHS and a Centennial Professor at the University of Alberta.
No potential conflicts of interest relevant to this article were reported.
The authors are independent of the study sponsors, which had no role in the study design; collection, analysis, and interpretation of the data; writing of the manuscript; or decision to submit for publication.
I.N.C. conducted the data analysis and drafted the manuscript. I.N.C., S.R.M., Y.Y., and J.A.J. designed and implemented the study and planned the data analysis. I.N.C., S.R.M., Y.Y., S.L.B., and J.A.J. interpreted the data. I.N.C., S.R.M., Y.Y., S.L.B., C.A.M., and J.A.J. reviewed the manuscript and approved the final version to be published. J.A.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as a poster at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
References
- 1.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–862 [DOI] [PubMed] [Google Scholar]
- 2.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–1687 [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007;50:1365–1374 [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 2012;130:1639–1648 [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia 2006;49:2819–2823 [DOI] [PubMed] [Google Scholar]
- 7.Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 2011;54:2263–2271 [DOI] [PubMed] [Google Scholar]
- 8.Carstensen B, Witte DR, Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia 2012;55:948–958 [DOI] [PubMed] [Google Scholar]
- 9.Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ 2012;184:E675–E683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia 2012;55:1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 2011;34:916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azoulay L, Yin H, Filion KB, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ 2012;344:e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–516 [DOI] [PubMed] [Google Scholar]
- 14.Responding to the challenge of diabetes in Canada: first report of the national diabetes surveillance system (NDSS) [Internet]. Ottawa, ON, Public Health Association of Canada, Government of Canada, 2003 [cited 2012 Aug 17]. Available from http://www.phac-aspc.gc.ca/ccdpc-cpcmc/ndss-snsd/english/pubs_reports/pdf/WEB_NDSS_English_Report-nocover.pdf Accessed 17 August 2012
- 15.Wennberg JE, Staiger DO, Sharp SM, et al. Observational intensity bias associated with illness adjustment: cross sectional analysis of insurance claims. BMJ 2013;346:f549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing [Internet]. Vienna, Austria, R Foundation for Statistical Computing, 2008. Available from http://www.R-project.org Accessed 17 August 2012
- 17.Renehan AG, Yeh H-C, Johnson JA, Wild SH, Gale EA, Møller H, Diabetes and Cancer Research Consortium Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia 2012;55:1619–1632 [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logsetty S. Screening for bladder cancer. In The Canadian Guide to Clinical Preventive Health Care [Internet]. Ottawa, ON, Public Health Agency of Canada, 1993. Available from http://www.phac-aspc.gc.ca/publicat/clinic-clinique/pdf/s10c68e.pdf Accessed 17 August 2012
- 20.Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–527 [PubMed] [Google Scholar]
- 21.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66(Suppl. 1):4–34 [DOI] [PubMed] [Google Scholar]