Abstract
OBJECTIVE
To describe the prevalence of physical function limitations among a nationally representative sample of adults with prediabetes.
RESEARCH DESIGN AND METHODS
We performed a cross-sectional analysis of 5,991 respondents ≥53 years of age from the 2006 wave of the Health and Retirement Study. All respondents self-reported physical function limitations and comorbidities (chronic diseases and geriatric conditions). Respondents with prediabetes reported no diabetes and had a measured glycosylated hemoglobin (HbA1c) of 5.7–6.4%. Descriptive analyses and logistic regressions were used to compare respondents with prediabetes versus diabetes (diabetes history or HbA1c ≥6.5%) or normoglycemia (no diabetes history and HbA1c <5.7%).
RESULTS
Twenty-eight percent of respondents ≥53 years of age had prediabetes; 32% had mobility limitations (walking several blocks and/or climbing a flight of stairs); 56% had lower-extremity limitations (getting up from a chair and/or stooping, kneeling, or crouching); and 33% had upper-extremity limitations (pushing or pulling heavy objects and/or lifting >10 lb). Respondents with diabetes had the highest prevalence of comorbidities and physical function limitations, followed by those with prediabetes, and then normoglycemia (P < 0.05). Compared with respondents with normoglycemia, respondents with prediabetes had a higher odds of having functional limitations that affected mobility (odds ratio [OR] 1.48), the lower extremities (OR 1.35), and the upper extremities (OR 1.37) (all P < 0.01). The higher odds of having lower-extremity limitations remained after adjusting for age, sex, and body mass index (OR 1.21, P < 0.05).
CONCLUSIONS
Comorbidities and physical function limitations are prevalent among middle-aged and older adults with prediabetes. Effective lifestyle interventions to prevent diabetes must accommodate physical function limitations.
Type 2 diabetes affects 13% of U.S. adults 45–64 years of age and 26% of adults ≥65 years of age (1). To better identify people at risk for diabetes, the American Diabetes Association (ADA) now recommends the use of glycosylated hemoglobin (HbA1c) 5.7–6.4% (39–46 mmol/mol) as an alternative definition for prediabetes (2). However, few population-based studies based on HbA1c in adults with prediabetes have characterized this population comprehensively (3,4) or described its prevalence of physical function limitations.
To prevent or delay diabetes, the ADA recommends that adults with prediabetes be identified so that lifestyle (diet and physical activity) and pharmacologic interventions are applied. The Diabetes Prevention Program (DPP) showed that an intensive lifestyle intervention reduced the development of type 2 diabetes by 58% over 3 years (5). On the basis of these findings, the ADA recommends that health care providers encourage patients with prediabetes to lose 7% of their initial body weight and to increase their physical activity to at least 150 min/week of moderate activity (e.g., brisk walking) (6,7). Despite the findings from the DPP and the recommendations of the ADA, U.S. adults who are at risk for diabetes have not increased their physical activity levels (4,8,9). Older adults who participated in the lifestyle intervention in the DPP had a greater reduction in diabetes incidence than younger adults (5), yet older adults are more likely to be sedentary. Only 12% of adults ≥75 years of age engage in 30 min of moderate physical activity ≥5 days/week, and 65% report no leisure time physical activity (10).
Studies have described barriers to physical activity in adults with diabetes. Adults with diabetes have a high prevalence of physical function limitations (11–13) that are associated with decreased physical activity (8). Diabetes is also associated with comorbidities, including geriatric conditions such as falls, cognitive impairment, and chronic pain (14,15). Geriatric conditions themselves are associated with an increased risk for physical function limitations (16). In contrast, little is known about the prevalence of physical function limitations and comorbidities among older adults with prediabetes. One study suggested that lack of physician advice may be a barrier to physical activity for older adults with prediabetes (9). Other barriers, including comorbidities and physical function limitations, have not been described for this population.
The objective of the present study was to characterize the prevalence of comorbidities and physical function limitations in a nationally representative sample of adults with prediabetes as defined by HbA1c. As adults with glucose intolerance and recent-onset diabetes already have microvascular and neuropathic complications characteristic of diabetes (17,18), we hypothesized that physical function limitations may be more common among middle-aged and older adults with prediabetes than among those with normoglycemia. From a public health perspective, a better understanding of this high-risk group is important to inform implementation of lifestyle interventions in real-world settings.
RESEARCH DESIGN AND METHODS
We performed a secondary data analysis of the 2006 wave of the Health and Retirement Study (HRS). The HRS is a biennial longitudinal health interview survey designed to study health transitions in U.S. adults ≥51 years of age. It is sponsored by the National Institute on Aging and performed by the Institute for Social Research at the University of Michigan.
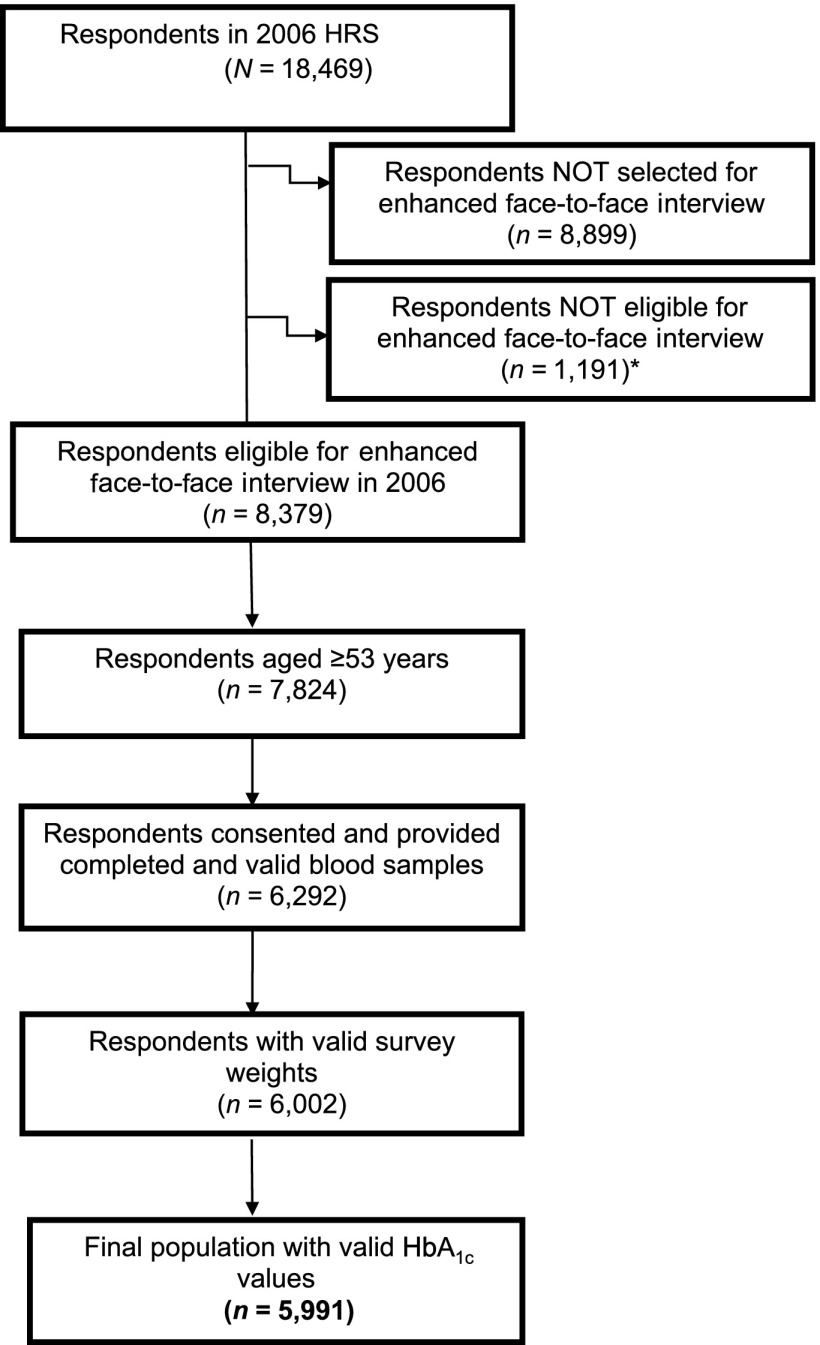
Of the 18,469 respondents eligible for the 2006 wave of the HRS interview, a random one-half sample (n = 8,379) of noninstitutionalized respondents was selected for an enhanced face-to-face interview (Fig. 1). All respondents completed the interviews without the assistance of a proxy. Interviewers obtained physical measures and blood samples from the respondents. We limited the study to the 5,991 respondents who were ≥53 years of age (the age group with nationally representative sample weights) and who had valid HbA1c measurements (76.5% of eligible respondents ≥53 years of age). This final study population represented 74.4 million Americans ≥53 years of age in 2006.
Figure 1.
Flowchart of the respondents selected from the 2006 wave of the HRS. *Respondents not eligible for the enhanced face-to-face interview included 224 nursing home residents, 459 people who required proxy respondents, and 508 people interviewed by telephone only.
The HRS was approved by the Behavioral Sciences Committee Institutional Review Board at the University of Michigan. HRS data are publicly available and contain no unique identifiers, thus ensuring respondent anonymity.
Physical measures and biomarkers
As part of the enhanced face-to-face interview, HbA1c was measured with random blood samples collected by trained interviewers (19). Instructions and kits from Biosafe Laboratories (Chicago, IL), a Clinical Laboratory Improvement Amendments–certified laboratory (19), were used for blood acquisition and HbA1c determination. The Biosafe HbA1c assay was not NGSP certified and had an expected normal range of 3.8–5.9% compared with 4.0–6.0% for NGSP-certified HbA1c assays (20). We considered respondents to have diabetes if they reported having received a physician diagnosis of diabetes, regardless of their HbA1c results (n = 1,270). We divided respondents who did not self-report diabetes into three groups: 1) normoglycemia (HbA1c <5.7% [39 mmol/mol], n = 2,752), 2) prediabetes (HbA1c 5.7–6.4% [39–46 mmol/mol], n = 1,803), and 3) undiagnosed diabetes (HbA1c ≥6.5% [48 mmol/mol], n = 166).
HRS interviewers also measured each respondent’s height and weight. We calculated the body mass index (BMI) for the 5,962 respondents having both measured height and measured weight. There were 29 respondents who either weighed ≥300 lb or were unable to stand; we used self-reported heights or weights to calculate their BMIs.
Covariates
Respondents self-reported their sociodemographic characteristics, including age, sex, race, marital status, educational achievement, and net worth (i.e., total household assets – current debt). Respondents also self-reported their comorbidities, which included both chronic diseases and geriatric conditions. We assessed the following seven chronic diseases: hypertension, arthritis, coronary artery disease, cancer (excluding minor skin cancers), psychiatric disorders, chronic lung disease, and stroke. We considered a chronic disease to be present if 1) a respondent reported having received a physician diagnosis and 2) the disease was active or severe (e.g., requiring medications for treatment) on the basis of follow-up questions about the disease. We assessed six geriatric conditions based on the following criteria:
Cognitive impairment: With the use of a performance-based measure adapted from the Telephone Interview for Cognitive Status (21), we defined respondents as cognitively impaired if they had dementia (a score of 0–6 on the 27-point cognitive scale) or mild cognitive impairment (a score of 7–11).
Falls: A fall resulting in injury or falling two or more times in the past 2 years. (Only respondents ≥65 years of age were asked about falls.)
Urinary incontinence: Incontinence requiring the use of pads or other absorbent undergarments.
Vision impairment: Decreased acuity despite the use of corrective lenses.
Hearing impairment: Decreased hearing despite the use of hearing aids.
Chronic pain: A report of being often troubled with pain.
Physical function
Each respondent was asked to report his or her difficulty with performing physical tasks because of a health condition. A physical function limitation was present if a respondent reported yes or can’t do to any one of the following tasks: 1) 5 activities of daily living (ADLs) (bathing, dressing, eating, transferring, and toileting); 2) 5 instrumental ADLs (IADLs) (meal preparation, shopping for groceries, managing medications, making telephone calls, and managing finances); and 3) 12 higher-level physical function items based on Rosow and Breslau (22) and Nagi (23) (RBN) tasks (walking ≥1 blocks; climbing ≥1 flights of stairs without resting; running [or jogging] ∼1 mile; sitting for 2 h; getting up from a chair after sitting for long periods; stooping, kneeling, or crouching; reaching or extending arms above shoulder level; pulling or pushing large objects such as a living room chair; lifting a weight >10 lb; and picking up a dime from the table).
Statistical analysis
To adjust for the complex sample design of the HRS, the differential probability of participation in the enhanced face-to-face interview, and lack of valid response from eligible respondents, all analyses were weighted with the use of Stata 9.0 (Stata Corp, College Station, TX) software to produce national population estimates.
We used standard descriptive methods (frequencies, means, and SEs) to estimate the prevalence of sociodemographic characteristics, physical function limitations, and comorbidities by glycemic status (i.e., normoglycemia, prediabetes, diabetes). We first performed weighted χ2 tests to make comparisons among the three glycemic groups. Then we compared two glycemic groups separately to focus on pairwise differences (prediabetes vs. normoglycemia and prediabetes vs. diabetes).
We used latent class factor analysis to identify discrete latent variables that best grouped the 12 higher-level physical function (RBN) tasks together (24). We selected models based on the smallest Bayesian information criterion and Akaike information criterion with best model fit (P > 0.05) and highest R2. Using the best model, we categorized respondents as belonging to one of three RBN groups: 1) mobility limitation (respondents reporting difficulty in walking several blocks and/or climbing a flight of stairs), 2) lower-extremity limitation (respondents reporting difficulty with getting up from a sitting position and/or stooping, kneeling, or crouching), and 3) upper-extremity limitation (respondents reporting difficulty with pulling or pushing large objects and/or lifting >10 lb). If a respondent had a physical function limitation in one of the three RBN groups, then he or she was considered part of that group. If a respondent had multiple physical function limitations, he or she was considered to be part of more than one RBN group.
We used logistic regression models to determine the odds of respondents with prediabetes having physical function limitations as defined by the three RBN groups, with the normoglycemia group serving as the reference. For comparison, we also determined the odds of respondents with diabetes having physical function limitations compared with those with normoglycemia. The models were adjusted for age, sex, BMI, and chronic diseases to examine whether prediabetes would independently predict limitations in physical functioning.
We performed sensitivity analyses by including the 166 respondents with undiagnosed diabetes in the diabetes group or by excluding them; the results did not change. Therefore, we included these 166 respondents with the respondents who reported having diabetes.
We also performed sensitivity analyses to define the three glycemic groups accounting for the small difference in the expected normal HbA1c range for the Biosafe HbA1c assay relative to the NGSP criteria. We divided respondents who did not self-report diabetes into three groups: 1) normoglycemia (HbA1c <5.6% [38 mmol/mol] n = 2,283), 2) prediabetes (HbA1c 5.6–6.3% [38–45 mmol/mol], n = 1,497), and 3) undiagnosed diabetes (HbA1c ≥6.4% [46 mmol/mol], n = 227). The results did not change; therefore, we present the results with the original HbA1c classification for the three glycemic groups.
RESULTS
The prevalence of prediabetes as defined by HbA1c in respondents ≥53 years of age was 29%, representing 21 million U.S. adults. In comparison, the prevalence of diabetes was 21%, representing 16 million adults. The prevalence of prediabetes increased with age. Of adults ≥65 years of age, 33% were estimated to have prediabetes, representing 12 million adults.
Table 1 presents the characteristics of respondents with prediabetes, normoglycemia, and diabetes. The mean age of the adults with prediabetes was 67.6 years, which was higher than those with normoglycemia (P < 0.001), although nearly one-half (46%) of respondents with prediabetes were 53–64 years of age. Respondents with prediabetes were more likely to be female than in the other two groups (P < 0.001). They were more likely than respondents with normoglycemia but less likely than respondents with diabetes to be black or Hispanic and to have less education, a lower net worth, a higher BMI, more chronic diseases, and more geriatric conditions (P < 0.001).
Table 1.
Characteristics of U.S. adults ≥53 years of age and older by glycemic status
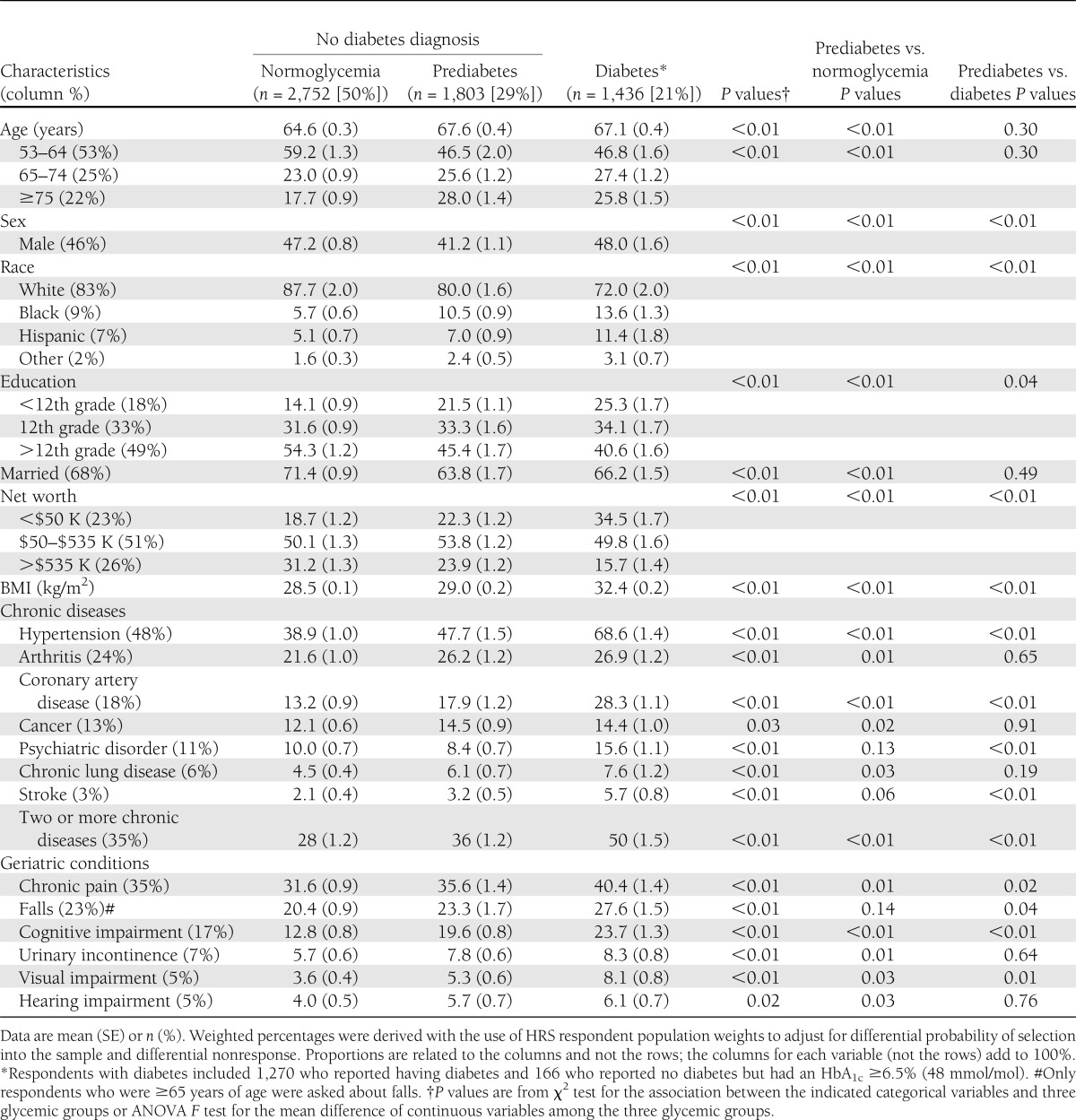
Like respondents with normoglycemia or diabetes, respondents with prediabetes were likely to have the same leading chronic diseases and geriatric conditions, including hypertension (48%), arthritis (26%), chronic pain (36%), and falls (23%). Thirty-six percent of respondents with prediabetes reported two or more chronic diseases compared with 28% of those with normoglycemia and 50% of those with diabetes (all P < 0.001). Additionally, 20% of respondents with prediabetes had at least mild cognitive impairment compared with 13% of those with normoglycemia and 24% of those with diabetes (all P < 0.001).
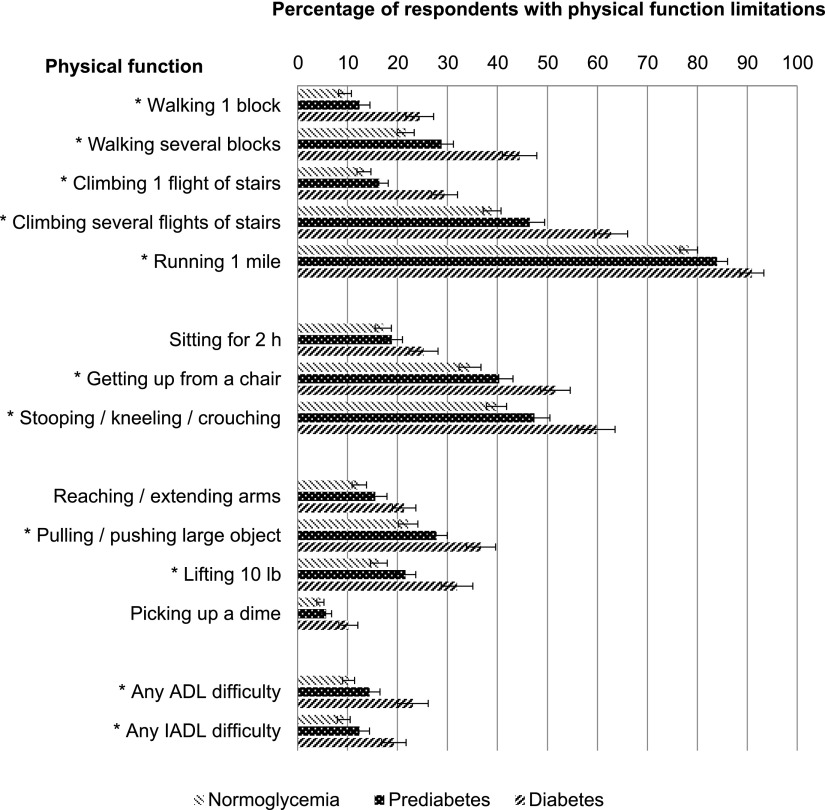
Figure 2 shows the prevalence of physical function limitations as measured by the 12 RBN tasks, any ADLs, and any IADLs by glycemic status. The pattern of physical function limitations among the three glycemic groups was similar; all respondents were most likely to report difficulty running 1 mile; stooping, kneeling, or crouching; and climbing several flights of stairs. Among respondents with prediabetes, 84% reported difficulty with running 1 mile; 47% with stooping, kneeling, or crouching; and 46% with climbing several flights of stairs. Fourteen percent of respondents with prediabetes reported difficulty with at least one ADL, which was significantly fewer than those with diabetes but more than those with normoglycemia (all P < 0.001).
Figure 2.
Prevalence of physical function limitations among U.S. adults ≥53 years of age with normoglycemia, prediabetes, and diabetes. Weighted percentages were derived with the use of HRS respondent population weights to adjust for differential probability of selection into the sample and differential nonresponse. P values are from χ2 test for the association between the indicated variables and the three glycemic groups. SE bars are presented for each physical function. For all physical function items, P < 0.01 when comparing the three glycemic groups and prediabetes vs. diabetes. *P < 0.02 comparing respondents with prediabetes vs. normoglycemia.
Respondents with diabetes had the highest prevalence of physical function limitations as measured by the 12 RBN tasks, any ADLs, and any IADLs; this was followed by respondents with prediabetes and then those with normoglycemia. This pattern was statistically significant for each physical function task (all P < 0.01). Subgroup analyses showed that respondents with prediabetes had a significantly higher prevalence of limitations for 10 of the 12 RBN tasks (except for sitting for 2 h and picking up a dime), any ADLs, and any IADLs than those with normoglycemia (P ≤ 0.02) (data not shown). Fewer respondents with prediabetes than those with diabetes reported any physical function limitations (P < 0.002).
Supplementary Fig. 1 shows the prevalence of physical function limitations for men and women separately. The pattern of physical function limitations for men and women is similar to that shown in Fig. 2. The prevalence was highest among diabetic respondents, lower in prediabetic respondents, and lowest in those with normoglycemia regardless of sex (P < 0.01 for each physical function task).
We grouped the 12 RBN tasks into three RBN groups (see statistical analysis) to simplify the analysis and presentation. Among respondents with prediabetes, 32% reported difficulty with walking several blocks and/or climbing a flight of stairs (mobility limitation group), 56% reported difficulty with getting up from a chair and/or stooping, kneeling, or crouching (lower-extremity limitation group), and 33% reported difficulty with pulling or pushing large objects and/or lifting >10 lb (upper-extremity limitation group).
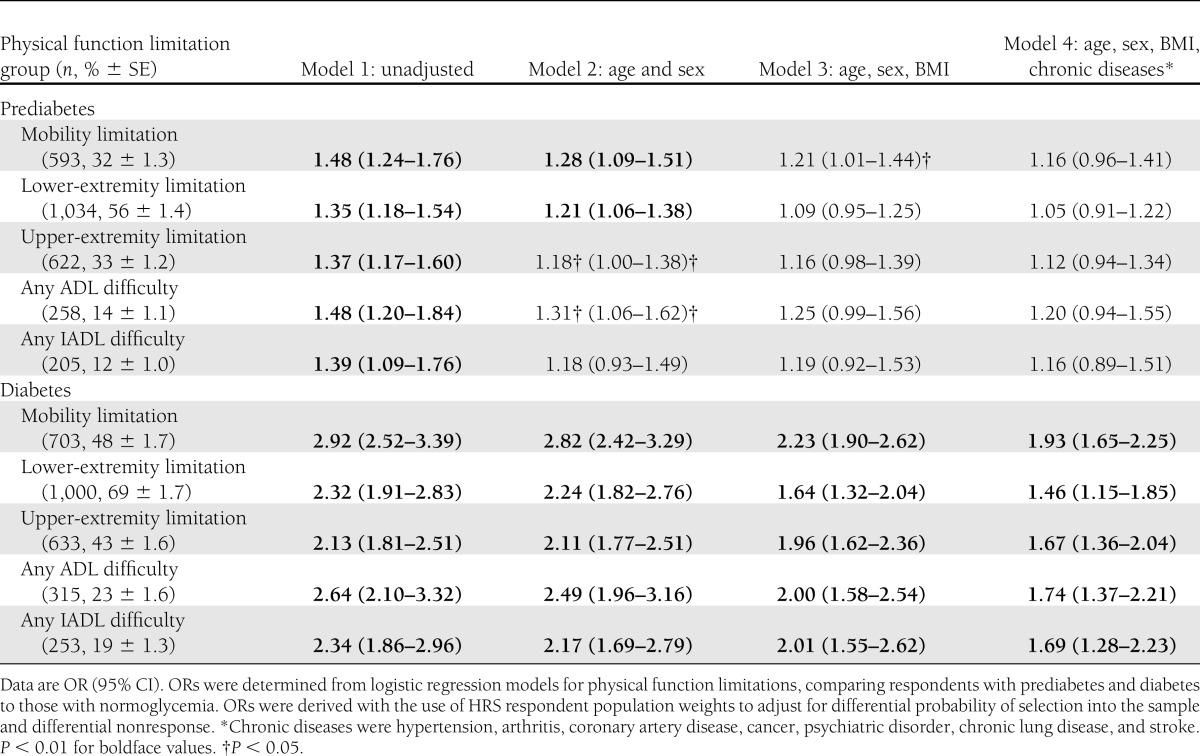
Table 2 shows the odds of respondents with prediabetes and diabetes reporting physical function limitations according to the three RBN groups compared with those with normoglycemia. In the unadjusted model (model 1), respondents with prediabetes compared with normoglycemia had a higher odds of having limitations in all three RBN groups, any ADLs, and any IADLs (odds ratio [OR] 1.35–1.48, P < 0.01). After adjusting for age and sex (model 2), there was a slight reduction in the odds of limitations among all physical function groups. The odds of having a limitation in mobility remained statistically significant after adjusting for age, sex, and BMI (model 3). When chronic diseases were added (model 4), the odds of having limitations in mobility were no longer statistically significant. By comparison, respondents with diabetes had consistently higher odds of physical function limitations than those with normoglycemia in all three RBN groups, any ADLs, and any IADLs, even after adjusting for age, sex, BMI, and chronic diseases (1.46–1.93, P < 0.01). The C statistic (area under the curve) to assess model fit for all physical function limitations (model 4 for each) was >0.72, confirming good model fit.
Table 2.
Association of prediabetes and diabetes with physical function limitations among U.S. adults ≥53 years of age
CONCLUSIONS
With the use of the ADA-recommended definition based on HbA1c measurement, we estimated the prevalence of prediabetes in a nationally representative sample of U.S. adults. We found that 29% of U.S. adults ≥53 years of age have prediabetes. Confirming our hypothesis, we found that individuals with prediabetes had more physical function limitations and comorbidities than those with normoglycemia but fewer than those with diabetes.
The findings add to the existing literature on prediabetes prevalence. In general, our findings are consistent with prior U.S. estimates of prediabetes prevalence based on assessment of impaired fasting glucose, impaired glucose tolerance, and HbA1c, which range from 15 to 43% for adults ≥45 years of age (3). Our estimate that 33% of adults ≥65 years of age have prediabetes is higher than the 26% estimate from the 2005 to2008 National Health and Nutrition Examination Survey (NHANES) (3). Although both the HRS and the NHANES are population-based health surveys, the HRS includes a larger sample of middle-aged and older adults, which may partially explain the different estimates in these two samples. We were able to analyze HRS data for >1,800 adults ≥53 years of age with prediabetes, whereas NHANES included only 696 adults ≥18 years of age with prediabetes. Other potential reasons for the different estimates are different study methods and respondent characteristics.
Like a prior study that identified individuals with prediabetes based on impaired fasting glucose or impaired glucose tolerance criteria (9), we found that adults with prediabetes were older, had lower educational attainment, and a higher BMI than those with normoglycemia. However, we found that women were more likely to have prediabetes than men likely because this population was older than that in the prior study and because there were more women in the older age group. The findings further highlight the high prevalence of prediabetes among middle-aged and older adults.
We have furthered the understanding of physical function limitations and comorbidities associated with glycemic abnormalities by including the full spectrum of individuals with normoglycemia, prediabetes, and diabetes in our analysis. Previous studies described the high rates of physical function limitations (12,13) and comorbidities (14,15,25,26) among adults with diabetes but not among those with prediabetes. The present finding of a high level of physical function limitation among adults with prediabetes is consistent with a previous report on older Mexican Americans with the metabolic syndrome (27). Furthermore, the present results support the hypothesis that diabetes diagnosis may lag the development of its associated complications and physical function limitations, suggesting that interventions are needed early, before diabetes diagnosis, to delay or prevent the development of complications and physical function limitations.
Our findings have important implications for the design of lifestyle interventions for middle-aged and older adults at risk for diabetes. Clinical trials such as the DPP (5) and the Finnish Diabetes Prevention Study (28) demonstrated that intensive lifestyle interventions can effectively delay or prevent diabetes. However, adults with multiple comorbidities and physical function limitations were excluded from these trials because their comorbidities and functional limitations might interfere with their participation in the interventions (29). As demonstrated in the present study, comorbidities that might preclude participation in intensive lifestyle interventions were prevalent among adults with prediabetes. More than one-third of the respondents reported chronic pain, one-fourth reported arthritis requiring medical treatment, nearly 20% were found to have at least mild cognitive impairment, and nearly one-fourth reported having had an injurious fall or two or more falls in the past 2 years. Furthermore, because nearly one-third of respondents with prediabetes reported difficulty walking several blocks or climbing a flight of stairs, they would have been excluded from the DPP because it excluded subjects who were unable to walk 0.25 mile in 10 min (30).
Because most lifestyle interventions involve brisk walking, if not jogging or cycling (29), we would expect that most, if not all the respondents with prediabetes might have difficulty in performing this activity. Physical function limitations were prevalent among respondents with prediabetes (32 and 56% had mobility limitations and lower-extremity limitations, respectively). More women than men reported physical function limitations (walking several blocks 32 vs. 23%, stooping, kneeling, or crouching 52 vs. 40%). Women may be especially limited in their ability to participate in lifestyle interventions. Although age, sex, obesity, and chronic diseases partially explained the difference in physical function limitation between respondents with prediabetes and respondents with normoglycemia, the fact remains that physical function limitations are prevalent among adults with prediabetes.
A major strength of this study is that it involved a large, nationally representative sample of middle-aged and older adults; therefore, the results can be generalized to the U.S. population in the corresponding age groups. Additionally, compared with other population surveys, the HRS collected detailed data on chronic diseases and geriatric conditions. Geriatric conditions, such as chronic pain, falls, and cognitive impairment, often are not considered to be chronic diseases and, thus, have not been well investigated (9). However, these conditions are associated with physical disability (16) and can certainly affect the ability of individuals to participate in lifestyle interventions.
This study has several limitations. First, prediabetes was determined by a single measurement of HbA1c. The HRS did not determine fasting plasma glucose (FPG) levels or perform oral glucose tolerance tests (OGTTs). Although the ADA and other expert panels recommend the use of HbA1c to identify adults with prediabetes and diabetes, a number of studies identified discordance among FPG, OGTT, and HbA1c (31,32). On the other hand, the use of HbA1c for epidemiologic studies has advantages over FPG or OGTT, including convenience, less day-to-day variability, and international standardization (31). Second, the HRS used self-reported data to define diabetes, other chronic diseases, geriatric conditions, and physical function limitations. The data chosen for this study were limited by the questions included in the HRS and were not verified by medical record review. We were unable to determine the severity of the conditions on the basis of the responses. Third, only 76.5% of the eligible respondents were included in the study because others did not consent to the study or provide valid HbA1c measurements. Sakshaug et al. (33) found that younger HRS respondents and respondents with more physical function limitations were less likely to consent to the enhanced face-to-face interview and that respondents with diabetes were more likely to consent. Therefore, our estimates of physical function limitations among respondents with prediabetes or normoglycemia are likely conservative estimates.
This study highlights the high prevalence of physical function limitations and comorbidities among middle-aged and older adults with prediabetes. Particularly striking is the high prevalence of difficulty with physical function essential for lifestyle interventions. The findings may help to explain the low rates of physical activity participation among adults despite their knowledge of having prediabetes (8). On the other hand, physician advice can potentially improve physical activity participation (34). Our findings suggest that when providing advice on physical activity, health providers should inquire about the patient’s physical function limitations and make specific recommendations to accommodate those limitations. For example, it would be impractical to simply recommend walking 30 min/day to a patient who has chronic knee pain from arthritis. Additional guidance about how to be physically active with a chronic pain condition may be more helpful. Knowledge about physical function limitations among patients with prediabetes will help providers to make tailored physical activity recommendations, to consider referring patients to rehabilitation programs to increase physical activity, or to perhaps recommend pharmacologic therapy for diabetes prevention.
In summary, we found a high prevalence of comorbidities and physical function limitations in a nationally representative sample of middle-aged and older adults with prediabetes. Effective lifestyle interventions to prevent diabetes should be tailored to accommodate these individuals.
Acknowledgments
P.G.L. was supported by the Claude D. Pepper Older Americans Independence Centers at the University of Michigan (National Institute on Aging [NIA] Grant AG024824 2010–2012), the John A. Hartford Foundation, the Veterans Affairs Ann Arbor Healthcare System (VA Ann Arbor) Geriatric Research, Education and Clinical Center (GRECC), and National Institute of Diabetes and Digestive and Kidney Diseases Grant P60-DK-020572 (to the Michigan Diabetes Research and Training Center). C.T.C. was supported by an NIA Mentored Clinical Scientist Research Career Development Award (K08-AG-031837) and the VA Ann Arbor GRECC. L.M. was supported by the VA Ann Arbor GRECC and the John A. Hartford Foundation Center of Excellence in Geriatrics at the University of Michigan. C.S.B. was supported by NIA Grant R01-AG-021493A and the VA Ann Arbor GRECC. W.H.H. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-092926 (to the Michigan Center for Diabetes Translational Research). The NIA provided funding for the HRS (U01-AG-09740), data from which were used in this study.
No potential conflicts of interest relevant to this article were reported.
P.G.L. designed the study; researched data; and wrote, reviewed, and edited the manuscript. C.T.G. and L.M. contributed to the study methodology and discussion and reviewed and edited the manuscript. J.H. contributed to the statistical analysis. S.L.M., C.S.B., and W.H.H. contributed to the discussion and reviewed and edited the manuscript. P.G.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 63rd Annual Scientific Meeting of the Gerontological Society of America, New Orleans, Louisiana, 19–23 November 2010.
The authors acknowledge Dr. Jeffrey Halter for his critical review of the manuscript and Ms. Hechuan Hou for her assistance in statistical analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0412/-/DC1.
References
- 1.U.S. Department of Health and Human Services Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011 Atlanta, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2009;32(Suppl. 1):S62–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James C, Bullard KM, Rolka DB, et al. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care 2011;34:387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Self-reported prediabetes and risk-reduction activities—United States, 2006. MMWR Morb Mortal Wkly Rep 2008;57:1203–1205 [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl. 1):S11–S66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colberg SR, Sigal RJ, Fernhall B, et al. American College of Sports Medicine. American Diabetes Association Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010;33:e147–e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care 2007;30:203–209 [DOI] [PubMed] [Google Scholar]
- 9.Geiss LS, James C, Gregg EW, Albright A, Williamson DF, Cowie CC. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am J Prev Med 2010;38:403–409 [DOI] [PubMed] [Google Scholar]
- 10.Conn VS, Minor MA, Burks KJ, Rantz MJ, Pomeroy SH. Integrative review of physical activity intervention research with aging adults. J Am Geriatr Soc 2003;51:1159–1168 [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Mangione CM, Cauley JA, et al. Study of Osteoporotic Fractures Research Group Diabetes and incidence of functional disability in older women. Diabetes Care 2002;25:61–67 [DOI] [PubMed] [Google Scholar]
- 12.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care 2000;23:1272–1277 [DOI] [PubMed] [Google Scholar]
- 13.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care 2010;33:1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PG, Cigolle C, Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc 2009;57:511–516 [DOI] [PubMed] [Google Scholar]
- 15.Cigolle CT, Lee PG, Langa KM, Lee YY, Tian Z, Blaum CS. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med 2011;26:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med 2007;147:156–164 [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–144 [DOI] [PMC free article] [PubMed]
- 18.Meigs JB, D’Agostino RB, Sr, Nathan DM, Rifai N, Wilson PW, Framingham Offspring Study Longitudinal association of glycemia and microalbuminuria: the Framingham Offspring Study. Diabetes Care 2002;25:977–983 [DOI] [PubMed] [Google Scholar]
- 19.Crimmins E. Documentation of Physical Measures, Anthropometrics and Blood Pressure in the Health and Retirement Study [Internet]. Ann Arbor, MI, Survey Research Center, University of Michigan, 2008. Available from http://hrsonline.isr.umich.edu/sitedocs/userg/dr-011.pdf Accessed 23 August 2012
- 20.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care 2008;31:408–414 [DOI] [PubMed] [Google Scholar]
- 21.Cigolle CT, Kabeto MU, Lee PG, Blaum CS. Clinical complexity and mortality in middle-aged and older adults with diabetes. J Gerontol A Biol Sci Med Sci 2012;67:1313–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol 1966;21:556–559 [DOI] [PubMed] [Google Scholar]
- 23.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc 1976;54:439–467 [PubMed] [Google Scholar]
- 24.Magidson J, Vermunt JK. Latent class factor and cluster models, bi-plots, and related graphical displays. Sociol Methodol 2001;31:223–264
- 25.Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes. An epidemiological evaluation. Diabetes Care 1993;16:1167–1178 [DOI] [PubMed] [Google Scholar]
- 26.Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med 2000;160:174–180 [DOI] [PubMed] [Google Scholar]
- 27.Blaum CS, West NA, Haan MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci 2007;62:766–773 [DOI] [PubMed] [Google Scholar]
- 28.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 29.Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roqué I Figuls M, Richter B, Mauricio D. Exercise or exercise and diet for preventing type 2 diabetes mellitus. Cochrane Database Syst Rev 2008;(3):CD003054. [DOI] [PubMed] [Google Scholar]
- 30.Diabetes Prevention Program Research Group. Protocol for the Diabetes Prevention Program [Internet]. Rockville, MD, Biostatistics Center, George Washington University, 2001. Available from http://www.bsc.gwu.edu/dpp/PROTOCOL.PDF Accessed 13 February 2013
- 31.Cohen RM, Haggerty S, Herman WH. HbA1c for the diagnosis of diabetes and prediabetes: is it time for a mid-course correction? J Clin Endocrinol Metab 2010;95:5203–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipska KJ, De Rekeneire N, Van Ness PH, et al. Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. J Clin Endocrinol Metab 2010;95:5289–5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakshaug JW, Couper MP, Ofstedal MB. Characteristics of physical measurement consent in a population-based survey of older adults. Med Care 2010;48:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costello E, Kafchinski M, Vrazel JE, Sullivan P. Motivators, barriers, and beliefs regarding physical activity in an older adult population. J Geriatr Phys Ther 2011;34:138–147 [DOI] [PubMed] [Google Scholar]