Abstract
OBJECTIVE
To assess the success and baseline predictors of maintaining glycemic control for up to 5 years of therapy using basal insulin glargine or standard glycemic care in people with dysglycemia treated with zero or one oral glucose-lowering agents.
RESEARCH DESIGN AND METHODS
Data from 12,537 participants in the Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial were examined by baseline glycemic status (with or without type 2 diabetes) and by therapeutic approach (titrated insulin glargine or standard therapy) using an intention-to-treat analysis. Median values for fasting plasma glucose (FPG) and A1C and percentages with A1C <6.5% (48 mmol/mol) during randomized treatment were calculated. Factors independently associated with maintaining updated mean A1C <6.5% were analyzed with linear regression models.
RESULTS
Median A1C in the whole population was 6.4% at baseline; at 5 years, it was 6.2% with glargine treatment and 6.5% with standard care. Of those with diabetes at baseline, 60% using glargine and 45% using standard care had A1C <6.5% at 5 years. Lack of diabetes and lower baseline A1C were independently associated with 5-year mean A1C <6.5%. Maintaining mean A1C <6.5% was more likely with glargine (odds ratio [OR] 2.98 [95% CI 2.67–3.32], P < 0.001) than standard care after adjustment for other independent predictors.
CONCLUSIONS
Systematic intervention with basal insulin glargine or standard care early in the natural history of dysglycemia can maintain glycemic control near baseline levels for at least 5 years, whether diabetes is present at baseline or not. Keeping mean A1C <6.5% is more likely in people with lower baseline A1C and with the glargine-based regimen.
There is a strong epidemiologic relationship between hyperglycemia and long-term complications of type 2 diabetes (1–4), and intensive treatment studies have verified that improving glycemic control can delay or prevent some of these complications (5–8). However, interventional studies have shown that diabetes is a progressive disorder, and the treatments used often do not prevent a gradual increase of hyperglycemia over time (9–11). The Outcome Reduction with Initial Glargine Intervention (ORIGIN; NCT00069784) trial compared the medical outcomes of two different glycemic treatment approaches for people with dysglycemia either with or without a diagnosis of diabetes who were taking no more than one oral glucose-lowering drug (12). The 12,537 participants were randomized to treatment with either basal insulin glargine, which was systematically titrated to maintain fasting plasma glucose (FPG) ≤5.3 mmol/L (≤95 mg/dL), or to standard care based on oral therapy. Cardiovascular and other medical outcomes of ORIGIN and the glycemic control results for the whole population have been reported previously (13,14). Here we report the glycemic control results separately for the subgroups with and without diabetes at enrollment and assess the independent associations of glycemic status and other baseline characteristics, and the treatment regimen used, with maintenance of updated mean A1C below <6.5% for up to 5 years of follow-up.
RESEARCH DESIGN AND METHODS
Participants
The rationale and design of the ORIGIN trial were reported previously (9). In brief, it was a multinational randomized trial with a 2 × 2 factorial design that tested two pairs of interventions. Titrated basal insulin glargine was compared with standard stepwise oral therapy, and an omega-3 fatty acid supplement with placebo. Participants were required to have a prior cardiovascular (CV) event or other evidence of high CV risk together with documented dysglycemia, defined as either impaired fasting glucose, impaired glucose tolerance (or the two together), or newly detected or previously diagnosed type 2 diabetes. Participants with diabetes could be treated with lifestyle alone or accompanied by no more than a single oral glucose-lowering agent. The present analysis concerns the glycemic intervention, with use of omega-3 fatty acids included only as a covariate. Data from all 12,537 individuals in 40 countries were assessed.
Interventions
Participants assigned to standard care continued their prior glucose-lowering treatment and were managed according to the investigators’ judgment and local guidelines for glycemic control and therapeutic approaches. Investigators were advised not to prescribe insulin for standard participants unless they were on full doses of two or more oral agents, and if insulin was added, glargine was not to be used. Participants assigned to basal insulin glargine who were taking a thiazolidinedione prior to randomization stopped this medication but continued to take other glucose-lowering agents. Insulin glargine (Lantus; Sanofi) was added to their regimen starting at 2–6 units daily, based on fasting glucose levels. Participants were advised to inject the glargine in the evening and to self-titrate the dosage using a simple algorithm supported by the site investigators. Self-measured, plasma-referenced fasting capillary blood glucose tests were done at least twice-weekly to guide titration, with the goal of achieving and maintaining fasting glucose at ≤5.3 mmol/L (≤95 mg/dL). Other oral agents could be continued, reduced, or discontinued as judged appropriate during treatment with insulin glargine. The only oral agent that could be added (if not previously used) was metformin, which the site investigator initiated for individual participants if judged necessary to limit the risk of hypoglycemia. The importance of lifestyle management was periodically reinforced in both treatment groups.
Measurements
In addition to self-measured glucose tests, venous blood for measurement of FPG and A1C at local laboratories was collected at intervals during treatment. Measurements of A1C were done at baseline, yearly thereafter, and at the end of treatment for all participants. All participants in the glargine treatment group and the nondiabetic participants in the standard care group had FPG levels measured at baseline, annually, and at the end of treatment. Participants with diabetes in the standard care group had FPG measurements at baseline, after 2 years, and at the end of treatment. Historical information about participants (such as smoking and alcohol habits, prior CV events, depression, and other medical problems) was systematically collected at baseline by participant self-report.
Statistical analysis
Summary statistics were computed for baseline characteristics of the whole population and for subgroups by glycemic treatment allocation and by glycemic status at enrollment (diabetes absent or present). Median FPG and A1C with interquartile ranges were computed for each subgroup for all time points. Percentages of participants in each subgroup having A1C <6.5 and <7.0% (two levels commonly identified as targets for glycemic control) (15,16) were calculated for each time point from the beginning to the end of the trial.
To determine the relationships between baseline characteristics and glycemic outcomes, findings for all randomized participants during the first 5 years of treatment were analyzed. Maintenance of an A1C <6.5% during treatment was defined as having the mean of all available annual measurements, including the 1-year value, up to and including the 5-year value (i.e., the updated mean A1C) below those levels. Univariable analyses of the relationships between clinical characteristics and a maintained A1C <6.5% were performed using linear regression models. Characteristics with a P < 0.1 in univariate analyses were entered into multivariable models. Model 1 included all baseline characteristics meeting this criterion, including baseline glycemic status (diabetes vs. no diabetes), but not glycemic treatment allocation. The independent effect of allocation to basal insulin glargine versus standard care was assessed by adding this variable to model 2, which included all variables statistically significant at P < 0.05 in model 1. The unadjusted effect of allocation to insulin glargine within subgroups was estimated using logistic regression and statistical tests for interactions between allocation and these subgroups. The results of these analyses were displayed as a forest plot.
RESULTS
Baseline characteristics
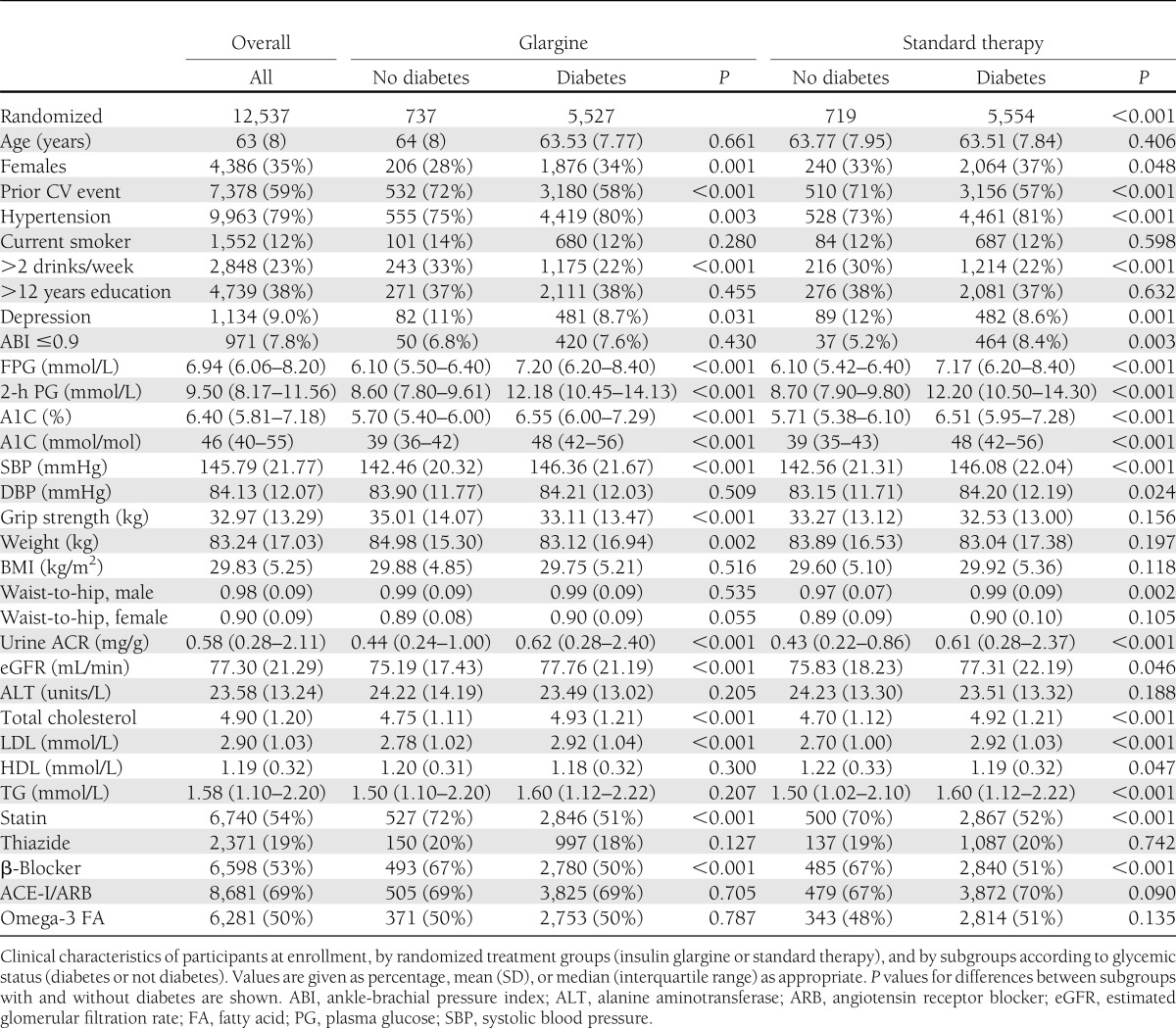
The characteristics of the ORIGIN population at enrollment, divided by treatment assignment and glycemic status, are shown in Table 1. Of 12,537 subjects randomized, 6,264 were assigned to treatment with insulin glargine and 6,273 to standard care. For the whole population, the mean age was 63.5 years, median FPG 6.9 mmol/L (125 mg/dL), and median A1C 6.4% (46 mmol/mol). The two randomized treatment groups were alike in baseline characteristics. Eighty-eight percent of participants had either a prior diagnosis of diabetes (of mean duration of 5.4 years) or newly detected diabetes. The 12% without diabetes clearly differed from those with diabetes in FPG and A1C levels and also in other ways, including more frequent prior CV events, use of alcohol, depression, and use of statins and β-blockers.
Table 1.
Baseline characteristics of participants
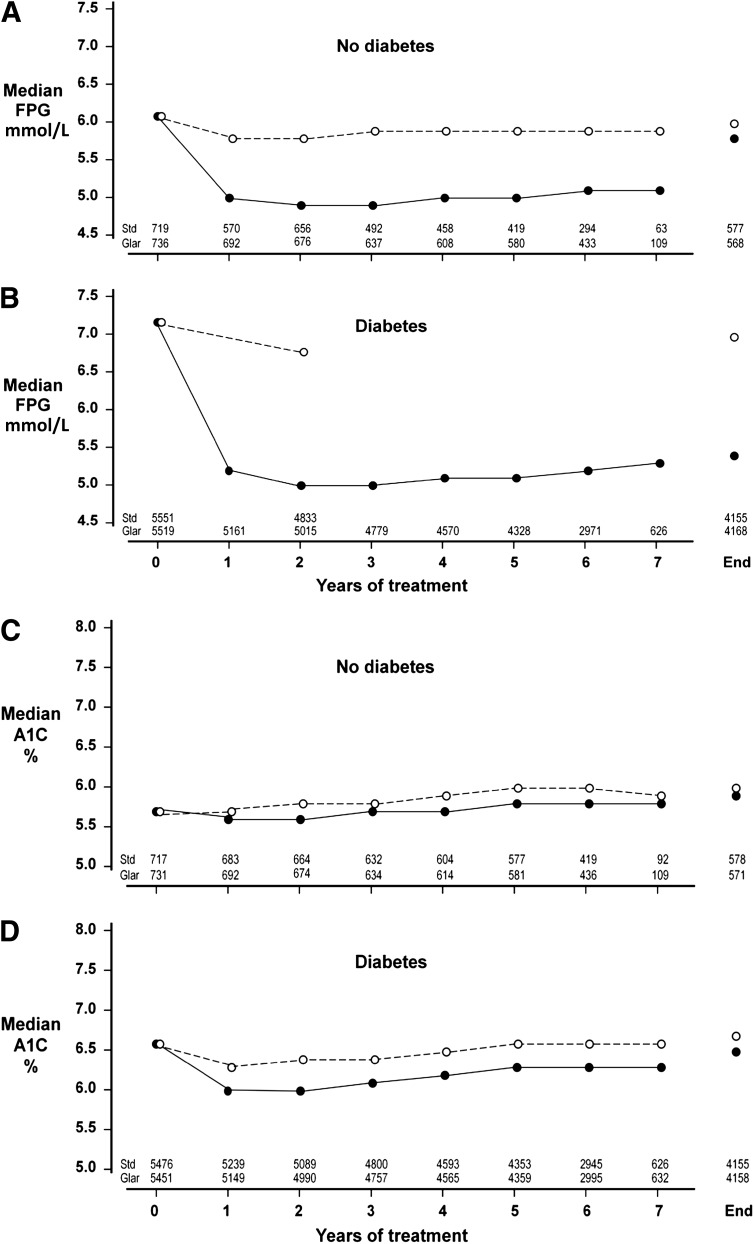
Glycemic responses of participants with and without diabetes
Median FPG.
The median period of follow-up on randomized treatment was 6.2 years. The effect of treatment allocation on the responses of FPG and A1C during treatment is shown in Fig. 1. For participants without diabetes, the median FPG (interquartile range) was 6.1 mmol/L (5.5–6.4) prior to randomized treatment (Fig. 1A). Standard care led to little change of FPG in this subgroup, but insulin glargine caused a sustained decrease to median values of 5.0 (4.5–5.5), 4.9 (4.4–5.5), 5.0 (4.5–5.7), and 5.1 mmol/L (4.5–5.8) at 1, 2, 5, and 7 years. For participants with diabetes, the median baseline FPG was 7.2 mmol/L (6.2–8.4). With standard care, the values at 2 years and the end of treatment were 6.8 (5.9–8.1) and 7.0 mmol/L (5.9–8.4) (Fig. 1B). Treatment with glargine reduced median FPG to 5.2 (4.6–5.9), 5.0 (4.4–5.8), 5.1 (4.5–6.1), and 5.3 mmol/L (4.5–6.4) after 1, 2, 5, and 7 years.
Figure 1.
Median FPG values and median A1C values at baseline, yearly during randomized treatment, and at the end of treatment are shown separately for participants without diabetes (A for FPG, C for A1C) and with diabetes (B for FPG, D for A1C) at baseline. The group assigned to use basal insulin glargine is shown by solid lines and solid circles, and the group assigned to standard care by broken lines and open circles. The numbers at the bottom of each panel show the number of observations included at each point in time.
Median A1C.
For participants without diabetes, A1C changed little from baseline with either regimen (Fig. 1C and Supplementary Table 1). With standard therapy, the median A1C was 5.7% (5.4–6.1) at baseline, 5.7% (5.4–6.1) at 1 year, and 6.0% (5.6–6.4) after 5 years. For glargine-treated participants, median A1C was 5.7% (5.4–6.0) at baseline, 5.6% (5.3–5.9) at 1 year, and 5.8% (5.5–6.1) at 5 years. For participants with diabetes, the median A1C at baseline was 6.6% (6.0–7.3) (Fig. 1D). During standard care, the median A1C values at 1, 2, 5, and 7 years were 6.3 (5.8–7.0), 6.4 (5.9–7.0), 6.6 (6.1–7.3), and 6.6% (6.1–7.3). Corresponding values during treatment with glargine declined to 6.0 (5.5–6.5), 6.0 (5.6–6.6), 6.3 (5.8–6.9), and 6.3% (5.8–6.9). For comparison with these median values, mean A1C during treatment is also provided in Supplementary Table 1.
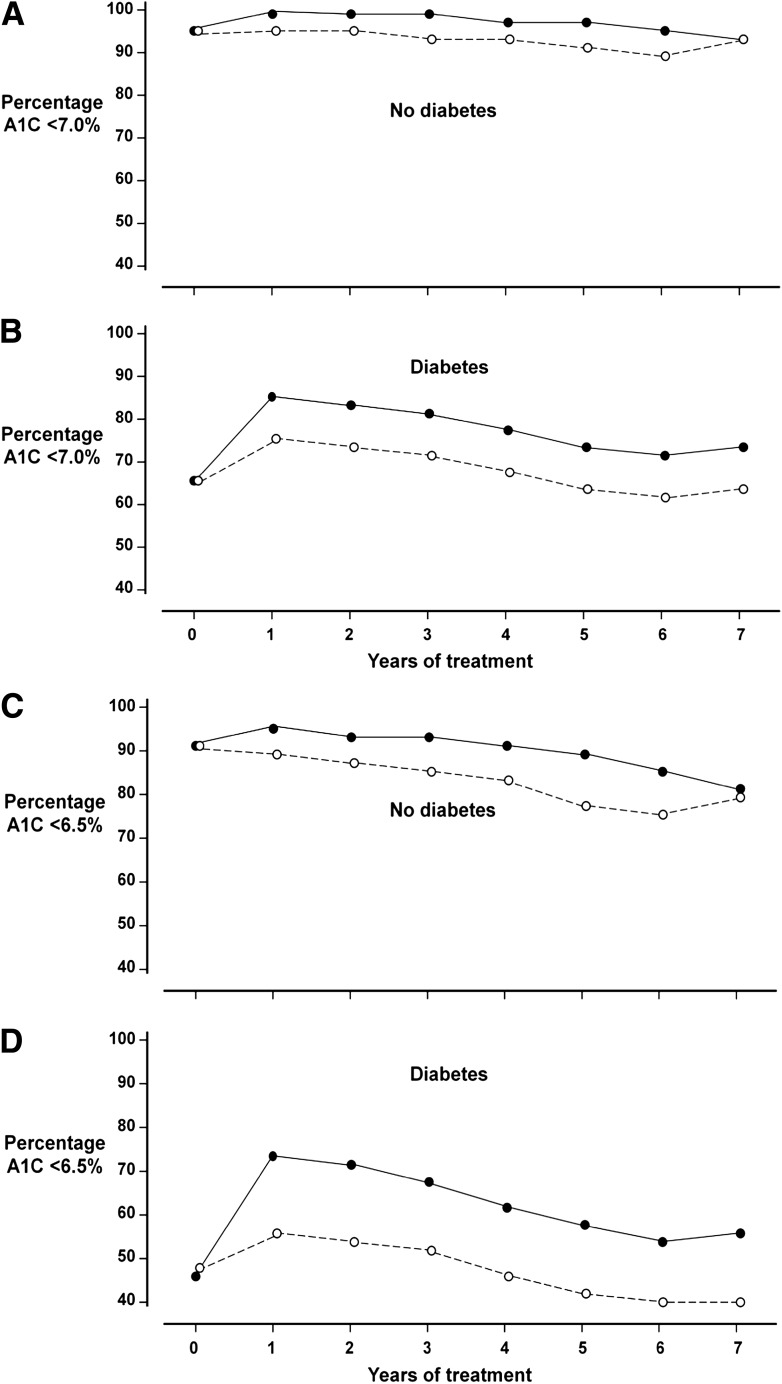
Percentages <7.0 and 6.5% A1C.
Of participants without diabetes at entry, >90% sustained A1C levels <7.0% (53 mmol/mol), and >75% achieved an A1C <6.5% (48 mmol/mol) throughout randomized treatment with both regimens (Fig. 2A and C). Of participants with diabetes, 66% had an A1C <7.0% and 47% had A1C <6.5% before starting treatment (Fig. 2B and D). During glargine treatment, the percentage in the diabetic subgroup achieving an A1C <7.0% increased to 88% at 1 year and 77% at 5 years, and the percentage achieving an A1C <6.5% was 74% at 1 year and 60% after 5 years. With standard care, the percentages in the diabetic subgroup were 76% with A1C <7.0% at 1 year and 66% at 5 years, and 58% with A1C <6.5% at 1 year and 45% at 5 years.
Figure 2.
Percentages of participants with A1C values <7.0 and <6.5% at baseline and yearly during randomized treatment are shown separately for those without diabetes (A for <7.0%, C for <6.5%) and with diabetes (B for <7.0%, D for <6.5%) at baseline. The group assigned to use basal insulin glargine is shown by solid lines and solid circles, and the group assigned to standard care by broken lines and open circles.
Predictors of success in maintaining mean A1C <6.5%
Associations of various clinical factors with maintaining a mean level of <6.5% for up to 5 years are shown in Table 2. Univariate associations with this outcome are shown for all baseline characteristics. Multivariable model 1, which included baseline characteristics with a univariate P < 0.1 for this association, displays several factors with independent associations with maintaining A1C <6.5%. These were greater age, reported use of alcohol, diagnosis of depression, lower A1C, lower waist-to-hip ratio, greater grip strength, lower albumin-to-creatinine ratio (ACR), lack of diabetes at baseline, and perhaps lack of statin use (P = 0.053). Model 2 included the baseline characteristics independently associated with mean A1C <6.5% in model 1 with a P value <0.05 as well as the effect of allocation to insulin glargine versus standard care. In model 2, greater age, use of alcohol, depression, lower A1C, lower waist-to-hip ratio, greater grip strength, lower ACR, and lack of diabetes were significant independent predictors. The adjusted odds ratio (OR) for success in maintaining A1C <6.5% when using glargine compared with standard care was 2.98 (P < 0.001). In a further multivariable analysis not reported in detail here, independent predictors of maintaining mean updated A1C <7.0% during 5 years of treatment were similar. Greater age, use of alcohol, lower A1C at baseline, and lack of diabetes at baseline were independent predictors, and the OR for glargine versus standard care was 2.41 (P < 0.001).
Table 2.
OR (95% CI) of maintaining a cumulative 5-year mean A1C <6.5%
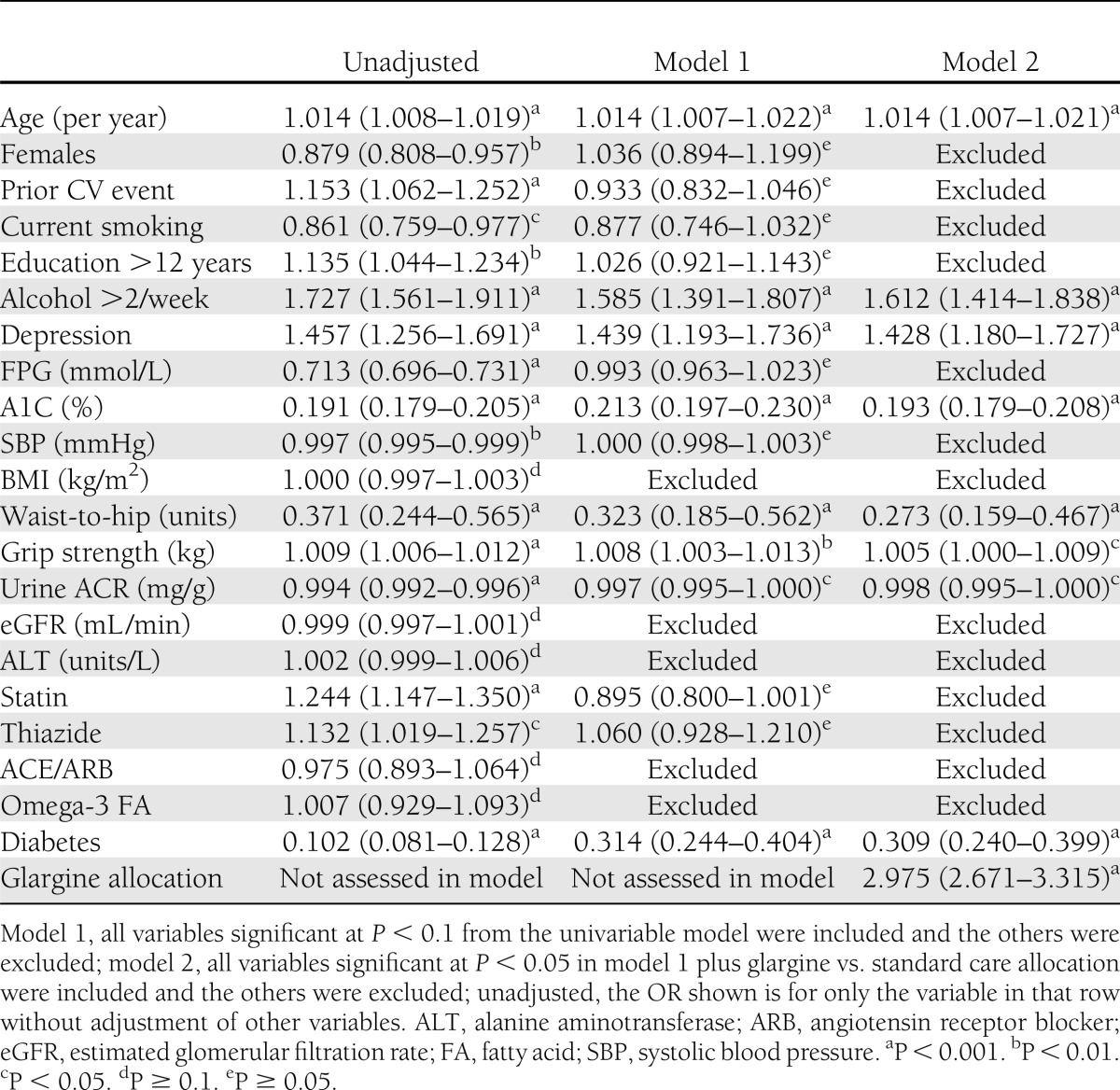
In an unadjusted subgroup analysis, the glargine-based regimen was more effective than standard care in all subgroups, including those with and without diabetes at baseline, with ORs close to 2 and no overlap of 95% CIs with unity (Supplementary Fig. 1). Three subgroup comparisons showed a nominally significant interaction with treatment assignment; glargine may have been modestly more effective in participants with higher waist-to-hip ratios (P = 0.011), greater grip strength (P < 0.001), and moderate use of alcohol (P = 0.029).
Glucose-lowering therapies and confirmed hypoglycemia
The usage of oral glucose-lowering agents prior to randomization is listed in Supplementary Table 2. Less than 2% of participants with dysglycemia not meeting the criteria of diabetes had used such agents prior to entry, and none at the time of oral glucose tolerance testing during screening. Of the participants with diabetes at enrollment, 33% were taking no oral therapy, 31% were taking metformin, and 33% were taking a sulfonylurea. Supplementary Table 3 displays usage of oral agents and insulin at the end of treatment. Of the participants without diabetes at entry, 69% of those assigned to glargine and 0.3% of those assigned to standard care were taking insulin at the end of the study. At the end of follow-up, 21% of those randomized to glargine and 29% of those randomized to standard care were taking one or more oral agents, most often metformin (18 and 24%; P < 0.003). Of the participants with diabetes at entry, insulin was used at the end by 82% of those who were assigned to glargine treatment and by 12% of those assigned to standard care (P < 0.001). Oral therapies were used by 71% of participants with diabetes assigned to glargine and 88% of those assigned to standard care (P < 0.001). Metformin was taken by 51 and 65% of subjects in the glargine and standard groups, respectively, and sulfonylureas were used by 28 and 52% (each <0.001). Two or more oral agents were taken by 14% of the glargine-treated group and 42% of the standard care group. The percentages of people with diabetes at enrollment having one or more hypoglycemic episodes confirmed by a glucose test of <3 mmol/L (<54 mg/dL) was 10.5 per 100 person-years with glargine and 3.0 per 100 person-years with standard treatment. The corresponding frequencies for those without diabetes at enrollment were 5.7 and 0.3 per 100 person-years.
CONCLUSIONS
As previously reported, the methods of therapy used in ORIGIN maintained excellent control of both FPG and A1C for at least 5 years of follow-up in the whole study population with dysglycemia. The present analysis expands this demonstration by examining the subpopulations with and without diabetes at baseline separately. In the subgroup without diabetes at baseline, there was a small increase of median A1C from 5.7% (39 mmol/mol) initially to 6.0% (42 mmol/mol) with standard care and to 5.8% (40 mmol/mol) with glargine after 5 years of follow-up. In those with diabetes at entry, median A1C decreased slightly from the 6.5% (48 mmol/mol) baseline after initiation of either glargine or standard care, and then it slowly increased with both treatments. After 5 years, the median A1C was 6.6% (49 mmol/mol) with standard care and 6.3% (45 mmol/mol) with glargine. Relative stability of A1C levels over time was not unexpected for the subpopulation without diabetes, but the finding that both treatment regimens had sustained success in people with overt diabetes at entry is reassuring. In contrast, glycemic control steadily worsened over the course of 5–10 years in some other long-term studies of type 2 diabetes (9–11). Sustained glycemic control in ORIGIN presumably was related to the use in each treatment arm of “treat-to-target” schemes, which called for intensification of treatment in response to evidence of rising levels of glucose. The dosage of glargine was systematically adjusted, seeking FPG levels ≤5.3 mmol/L, and metformin could be added to mitigate the risk of hypoglycemia. Similarly, during standard therapy, oral medications were added and their dosage increased, with the aim of keeping A1C below either 6.5 or 7.0%, depending on locally accepted guidelines. At the end of treatment, 42% of those using the standard regimen were taking two or more oral agents, and 14% of participants assigned to glargine therapy were doing so. Treatment in the Belfast (9), UK Prospective Diabetes Study (UKPDS) (10), and A Diabetes Outcome Progression Trial (ADOPT) (11) studies, in contrast, was based on assignment to monotherapy regimens, including diet alone, metformin, sulfonylurea, thiazolidinedione, or basal insulin, with escalation of therapy only under certain conditions.
The glycemic control attained in ORIGIN resembled that achieved in Action in Vascular Disease: Preterax and Diamocron Modified Release Controlled Evaluation (ADVANCE) (17), Action to Control Cardiovascular Risk in Diabetes (ACCORD) (18), and Veterans Affairs Diabetes Trial (VADT) (19), other trials in which glucose-lowering therapies in the intensive treatment groups were systematically adjusted seeking near-normal glycemic control. However, compared with the population in ORIGIN, participants in these studies had a longer duration of diabetes and in many cases established therapy with multiple glucose-lowering agents prior to enrollment. The A1C levels attained in ADVANCE (6.5% [48 mmol/mol]) and ACCORD (6.4% [46 mmol/mol]) were close to those at baseline in ORIGIN (6.5%), whereas those in VADT were slightly higher (6.9% [52 mmol/mol]). In all three studies, these values were achieved by strenuous efforts to improve control from higher A1C levels at baseline. Hence, maintenance of A1C at or below near-normal entry levels in ORIGIN contrasts with the other trials’ efforts to restore previously inadequate glycemic control. Keeping glycemic control below a level associated with increasing risk of diabetes complications by advancing therapy as needed may be a more desirable approach than the historically common practice of allowing marked hyperglycemia to occur and then attempting to reduce levels to a lower target (20–22). This concept is in keeping with the recent adoption of A1C 6.5% as one option for timely diagnosis of diabetes to allow intervention to minimize the risk of complications (23,24).
This analysis also identified baseline characteristics of ORIGIN participants that were predictive of maintaining an updated mean A1C from the first year of treatment to the end of treatment or 5 years of follow-up at <6.5%. This measure of treatment success was selected because it was close to the median value at entry to the trial and also reflects a level of control now endorsed as a threshold for diagnosing diabetes. Data after 5 years of treatment were not included because of the smaller numbers of participants who had been in the study long enough to complete 6 or 7 years. Individuals with diabetes at baseline were understandably less likely than those without diabetes to have this level of success in controlling A1C, having an adjusted OR of 0.309 (P < 0.0001). An association of long-term treatment success with lower baseline A1C, independent of the presence of overt diabetes, was also demonstrated, consistent with other studies. Other predictive factors included greater age, use of alcohol more than two times weekly, depression, lower A1C, and lower waist-to-hip ratio (all P < 0.001). Less clearly associated factors were greater grip strength (P = 0.038) and lower urinary albumin excretion (P = 0.035). Speculative explanations may be offered for these associations. Better success for individuals who were older at enrollment might reflect slower progression of the underlying defects of diabetes in those with later onset. Lower waist-to-hip ratio, greater grip strength (25), and lower albumin excretion might be markers for better physical fitness and a lower burden of microvascular complications of diabetes. Moderate use of alcohol may be associated with favorable physiological patterns and reduced risk of type 2 diabetes and mortality (26), and participants who reported depression might have been more willing to make changes to reduce depression or otherwise improve health-related behavior. Several of these associations support the assumption that various behavioral characteristics of participants influenced the success of these treatment regimens in maintaining glycemic control.
Finally, allocation to basal insulin glargine with titration of dosage seeking normal FPG levels led to a nearly threefold increase in the likelihood of maintaining mean A1C <6.5% for 5 years. Moreover, this effect was observed in subgroups representing characteristics that were associated with success in maintaining A1C <6.5% for 5 years. This observation is not surprising given that an ambitious glycemic target for glargine titration, fasting glucose <5.3 mmol/L, was systematically sought. Experience in ORIGIN showed that early use of this basal insulin was able to both attain and maintain this level of control of FPG, and thereby keep A1C from rising above baseline levels (median 6.5% [48 mmol/mol]) for a majority of people with overt diabetes at baseline for up to 5 years (5-year median 6.3% [45 mmol/mol], 60% of participants <6.5%). However, the short- and long-term risks versus benefits of this method of treatment relative to standard care in ORIGIN are not yet well-defined. As previously reported (13,27), the use of systematically titrated glargine in ORIGIN was associated with 1.6-kg gain of weight and 0.7% increased incidence of severe hypoglycemia. These unwanted effects of seeking nearly normal glycemic control were less prominent in ORIGIN than in the trials in which participants had a longer duration of diabetes and more elevated A1C levels at baseline (18,19). For example, the mean gain of weight with the intensive treatment regimen in the VADT was 8.2 kg (19). Also, the annual incidence of severe hypoglycemia with intensive treatment in ACCORD was 3.1% (27), whereas it was 1.0% with basal insulin and 0.3% with standard therapy in ORIGIN (13). Although maintenance of nearly normal glycemic control for 5 years may be predicted to delay the development of complications of diabetes, the present analysis lacks information about the predictors and consequences of hypoglycemia and other unwanted effects of insulin glargine relative to standard care. Therefore, further examination of data from ORIGIN is needed to clarify the balance of risks to potential benefits from early intervention with insulin glargine.
In conclusion, early intervention with basal insulin glargine or with standard care kept median A1C near the starting level for at least 5 years in both the subgroup without diabetes and the subgroup with diabetes at entry. Maintaining the mean of yearly A1C measurements at <6.5% (48 mmol/mol) was more often accomplished when the initial A1C was lower and with titrated basal insulin glargine than with standard care. However, the risks versus benefits of this approach remain to be determined by further analyses and additional follow-up of ORIGIN participants.
Acknowledgments
The ORIGIN study was sponsored by Sanofi, but the study was managed by and the analyses presented here were performed at the Population Health Research Institute (Hamilton, Ontario, Canada), with guidance by the ORIGIN Trial Investigators.
M.C.R. has received honoraria for consulting and research support from Sanofi, Eli Lilly, and Amylin, and honoraria for consulting from Elcelyx and Valeritas; these potential conflicts of interest have been reviewed and managed by Oregon Health & Science University. H.C.G. has received consulting fees from Sanofi, Novo Nordisk, Eli Lilly, Bristol-Myers Squibb, Roche, AstraZeneca, Novartis, GlaxoSmithKline, and Bayer; lecture fees from Sanofi and Bayer; and support for research or continuing education through his institution from Sanofi, Eli Lilly, Merck, Novo Nordisk, Boehringer Ingelheim, Bristol-Myers Squibb, and AstraZeneca. M.H. has received speaker honoraria from Takeda, GlaxoSmithKline, Roche, Bayer, Eli Lilly, and Sanofi and advisory board honoraria from Sanofi, Takeda, Bristol-Myers Squibb, and GlaxoSmithKline. P.J. is a full-time employee of Sanofi. J.L.P. has received honoraria for consulting and research grant and contract support from Sanofi. J.R. has received honoraria for consulting and research grant support from all insulin manufacturers: Sanofi, Eli Lilly, and Novo Nordisk. L.E.R. has received honoraria for advisory boards, steering committees, and lectures from Sanofi and other commercial companies and Swedish organizations and research grant support from Sanofi, F. Hoffmann-La Roche, Swedish Heart Lung Foundation, the Swedish Medical Research Council, AFA Insurance, Karolinska Institute, and County Council of Stockholm. G.A.S. has received honoraria for consulting from AstraZeneca, Bristol-Myers Squibb, Medtronic, Eli Lilly, Merck, Novo Nordisk, Novartis, Daiichi Sankyo, and Sanofi and research grant support from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
All members of the writing group were involved in the conduct of the trial and/or management of the data and all participated in the preparation of the manuscript. M.C.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of these findings were presented in preliminary form at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
APPENDIX
The members of the writing group were Matthew C. Riddle (Oregon Health & Science University, Portland, OR), Hertzel C. Gerstein (Population Health Research Institute, Hamilton, Ontario, Canada), Leanne Dyal (McMaster University and Population Health Research Institute, Hamilton, Ontario, Canada), Markolf Hanefeld (Dresden Technical University, Dresden, Germany), Peter Johnston (Sanofi, Bridgewater, NJ), Jeffrey L. Probstfield (University of Washington, Seattle, WA), Ambady Ramachandran (India Diabetes Research Foundation, Chennai, India), Julio Rosenstock (Dallas Diabetes and Endocrine Center at Medical City, Dallas, Texas), Lars E. Ryden (Karolinska Institute, Stockholm, Sweden), and Giatgen A. Spinas (University Hospital Zurich, Zurich, Switzerland).
Footnotes
Clinical trial reg. no. NCT00069784, www.clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2238/-/DC1.
The members of the writing group are listed in the APPENDIX.
References
- 1.Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saydah S, Tao M, Imperatore G, Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care 2009;32:1440–1446 [DOI] [PMC free article] [PubMed]
- 3.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstein HC, Islam S, Anand S, et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia 2010;53:2509–2517 [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 7.Chew EY, Ambrosius WT, Davis MD, et al. ACCORD Study Group. ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismail-Beigi F, Craven T, Banerji MA, et al. ; ACCORD Trial Group. Effect of intensive treatment of hyperglycemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadden DR, Blair ALT, Wilson EA, et al. Natural history of diabetes presenting age 40-69 years: a prospective study of the influence of intensive dietary therapy. Q J Med 1986;59:579–598 [PubMed] [Google Scholar]
- 10.U.K. Prospective Diabetes Study Group. U.K. prospective diabetes study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed]
- 11.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 12.The ORIGIN Trial Investigators. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am Heart J 2008;155:26–32, 32.e1–e6 [DOI] [PubMed]
- 13.Gerstein HC, Bosch J, Dagenais GR, et al. ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 14.ORIGIN Trial Investigators , Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–318 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 17.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 20.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004;27:1535–1540 [DOI] [PubMed] [Google Scholar]
- 21.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010;33:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddle MC, Yuen KCJ. Reevaluating goals of insulin therapy: perspectives from large clinical trials. Endocrinol Metab Clin North Am 2012;41:41–56 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. Available at http://www.who.int/diabetes/publications/diagnosis_diabetes2011/en/index.html Accessed 1 September 2011 [PubMed]
- 25.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA 1999;281:558–560 [DOI] [PubMed] [Google Scholar]
- 26.Bonnet F, Disse E, Laville M, et al. RISC Study Group Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 2012;55:3228–3237 [DOI] [PubMed] [Google Scholar]
- 27.Miller ME, Bonds DE, Gerstein HC, et al. ACCORD Investigators The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]