SUMMARY
Mature lymphocyte immigration into the thymus has been documented in mouse, rat and pig models and highly increases when cells acquire an activated phenotype. Entrance of peripheral B and T cells into the thymus has been described in normal and pathological situations. However it has not been proposed that leukocyte recirculation to the thymus could be a common feature occurring during the early phase of a Th1 inflammatory/infectious process when a large number of peripheral cells acquire an activated phenotype and the cellularity of the thymus is seriously compromised. The data we present here demonstrate that in well-established Th1 models triggered by different types of immunogens, e.g. LPS treatment (a bacterial product), C. albicans infection (a fungus) and after T. cruzi infection (a parasite), a large number of mature peripheral B and T cells enter the thymus. This effect is dependent on, but not exclusive of, the available space in the thymus. Our data also demonstrate that MCP-1/CCR2 interaction is responsible for the infiltration of peripheral cells to the thymus in these Th1-inflammatory/infectious situations. Finally, systemic expression of IL-12 and IL-18 produced during the inflammatory process is ultimately responsible for these migratory events.
Keywords: thymus re-entry, infection, IL-12 and IL-18, MCP-1, peripheral B and T cells
INTRODUCTION
The thymus is the primary source of T cells for peripheral lymphoid organs. T cells produced in the thymus migrate to the spleen and lymph nodes (LNs), especially early in life. The reverse pathway, i.e. mature T cells migrating from the periphery back into the thymus is less often considered although some studies have shown that this is a common pathway in healthy animals [1–5]. Moreover, it has been suggested that this pathway might preferentially be used by activated T cells [4;6–8]. For example, it was shown that activated T cells homed to the thymus, and represented approximately 0.4% of mature T thymocytes [6]. Others have shown that, as compared with naive CD4+ T cells, there is a preferential accumulation of antigen-experienced T cells in the rat thymus [9]. Interestingly, the rate of homing was greatly increased when thymocyte depletion occurred after host irradiation [6]. In any case, accumulation of peripheral T cells within the thymus is largely restricted to the medulla [6;10].
Although a small number of mature B cells can be found in a healthy thymus, the migration of peripheral B cells to the thymic medulla could increase several fold in certain pathological situations such as thymic lymphoma [11] and certain autoimmune diseases murine models [12].
The functional consequences of cellular migration of both T and B cells back to the thymus have been addressed by several investigators. For example, it has been proposed that B cells enter the thymus in order to achieve T-cell tolerance to immunoglobulins and to other B-cell-specific antigens [13]. Moreover, it has also been proposed that B cells found in the thymus could participate in negative selection by acting as Ag-presenting cells [14]. As for T cells, it has been proposed that the thymus can function as a repository of memory T cells [15], while others have demonstrated an important role of peripheral mature T cells in central tolerance during the processes of positive and negative selection in the thymus [10;16]. It has also been proposed that migrating lymphocytes can participate in transplantation tolerance [17] and that mature T cells in the thymus are important in maintaining medullary epithelial cells [18].
Whereas naïve syngeneic T cells preferentially home to the peripheral lymphoid organs, they rarely reenter the thymus. In contrast, in vivo peptide-activated peripheral T cells migrate to and accumulate in the thymus, thus confirming that reentry of T cells to the thymus is mostly restricted to activated T cells [4;6–8]. Based on this data, it is surprising that the possibility that the entrance of mature cells into the thymus could be a common occurrence during the acute phase of an infectious/inflammatory process has not been generally addressed, since a large proportion of T and B cells acquire an activated phenotype in these situations. Moreover, thymocyte depletion observed in several infectious disease models could even increase the possibility of peripheral cell migration into the thymus considering reports describing that when the cellularity of this organ is compromised (neonatal, irradiation, SCID mice, atrophic aged thymi, etc), peripheral cell infiltration into the thymus considerably increases [4;6;18;19]. In this context, the aim of this work is to demonstrate that migration of peripheral T and B cells to the thymus occurs during the early phase of Th1 inflammatory/infectious processes triggered by different type of pathogens. In support of this hypothesis, we examine the entrance of B and T cells into the thymus in well-established Th1 infectious/inflammatory murine models. Furthermore, we demonstrate that peripheral T cells and B cells but not NK cells, macrophages or DCs largely migrate to the thymus under inflammatory/infectious conditions but only when the cellularity of the organ is compromised. Moreover, the entrance of peripheral lymphocytes to the thymus necessarily requires MCP-1 production in this organ and CCR2 expression on migrating lymphocytes. Importantly, we demonstrate as a general mechanism that this phenomenon is triggered by IL-12 and IL-18 produced during the acute phase of Th1/inflammatory/infectious processes. Moreover, our data with OVA-specific TCR transgenic mice suggest that rather than being a TCR-dependent mechanism, any T cell has the potential to migrate to the thymus in response to inflammatory conditions.
RESULTS
Peripheral T and B cell largely migrate to the thymus during Th1 inflammatory/infectious processes
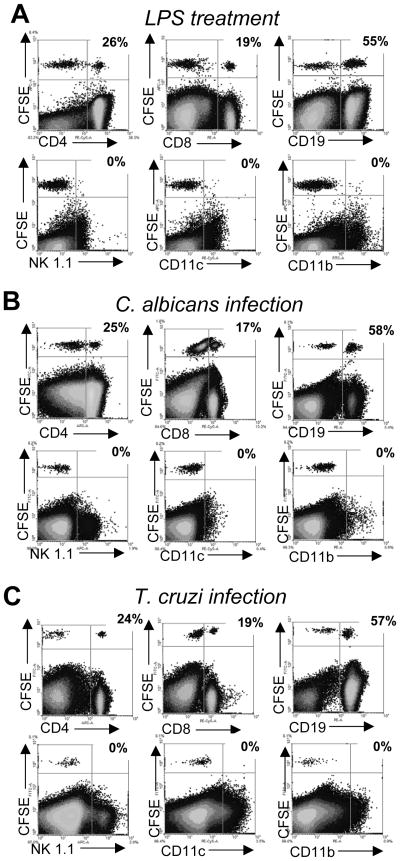
To address if migration into the thymus of mature peripheral lymphocytes is a common feature of Th1-driven inflammatory/infectious processes, we adoptively transferred CFSE-labeled splenocytes from mice either treated in vivo with LPS (a bacterial product), or infected with a fungus (C. albicans) or a parasite (T. cruzi) to recipient hosts that have received the same treatments. All these pathological conditions are characterized by a potent Th1 immune response especially during the acute phase of the process[20–23]. Data presented in figure 1 demonstrates that after LPS treatment (fig. 1A), C. albicans (fig. 1B) or T. cruzi (fig. 1C) infections, CD4+ and CD8+ T cells together with B cells entered the thymus in different proportions. Interestingly, other cell populations present in the spleen such as NK cells, macrophages or DCs were excluded from the thymus in these conditions, although they normally appeared as CFSE+ cells in the spleen or LNs of recipient mice (data not shown).
Figure 1. Entrance of peripheral T and B cells in the thymus after systemic LPS treatment and C. albicans or T. cruzi infection.
B6 mice (WT) were injected i.p. daily with 20μg of LPS or infected with 5 × 107 C. albicans or 5 × 105 trypomastigotes of T. cruzi. On (A) day 3 for LPS-treated mice, (B) day 7 for C. albicans infection or (C) day 12–14 for T. cruzi infection, half of the mice of each group were sacrificed, spleen cells were obtained and after red cell depletion, 2–3 × 107 cells were stained with CFSE and adoptively transferred (i.v. injection) into the other half of mice of the same group. Twenty four hours later, the thymi from recipient mice were obtained and surface stained with anti-CD4, -CD8, -CD19, -NK1.1, -CD11b and –CD11c and analyzed in a flow cytometer. The values indicate the percentage of CFSE+ cells that are lineage+. Data shown are from a single mouse per treatment and are representative of 3 such mice per treatment. Experiment was repeated 3 times. Data was obtained by gating on the viable cells (Supporting Information fig. 3A).
Space availability is important but is not the only requirement to permit the ingress of peripheral cells into the thymus
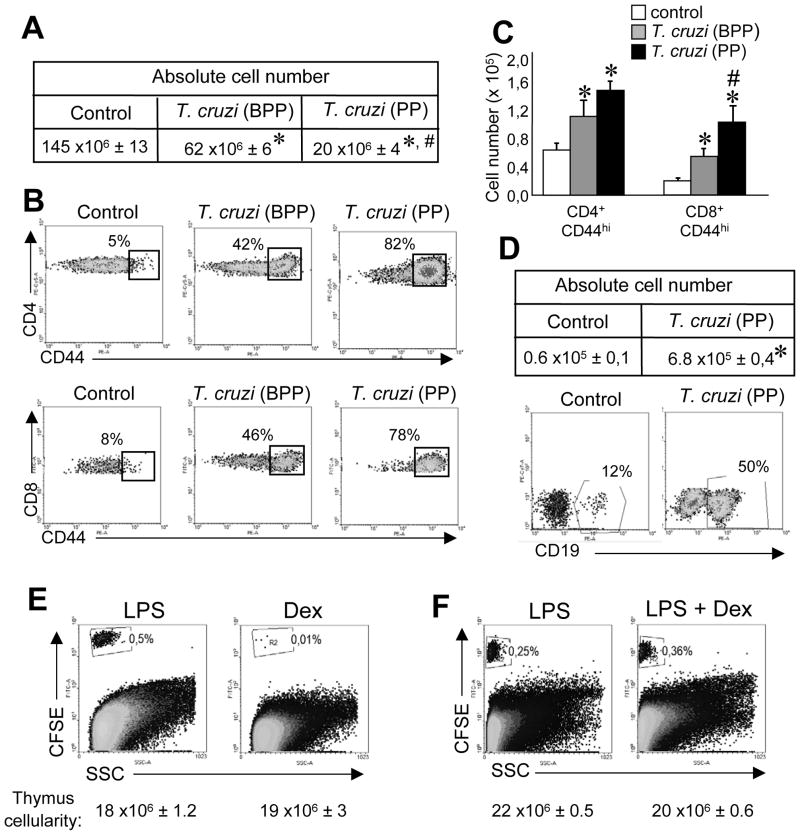
Loss of thymus cellularity is a common feature among inflammatory/infectious processes [24]. Moreover, it has been reported that when the cellularity of this organ is compromised, the number of peripheral cells infiltrating into the thymus considerably increases [4;6;18;19]. Then, we speculated that available space could represent a crucial situation for cell migration to the thymus in inflammatory conditions. To test this hypothesis we examined T. cruzi-infected mice at 2 different times: before the parasitemia peak (BPP, between 9–11 days post-infection) where part of resident thymocytes (especially DP cells) are depleted and during the parasitemia peak (PP, between 12–14 days post-infection) when a larger number of thymocytes are depleted (fig. 2A). As CD4+ and CD8+ cells are found in the thymus as single positive (SP) thymocytes, it is difficult to discriminate between resident and peripheral mature T cells; however, we and others have demonstrated that expression of CD44, an activation marker for T cells is preferentially expressed by mature T cells that enter the thymus [7;17;25] (and fig. 3A). Thus we evaluated the percentage and the absolute number of CD44hi T cells present in the thymi of T. cruzi infected mice. As shown in figure 2, the percentage (fig. 2B) as well as the absolute number (fig 2C) of CD44hi cells in the CD4+ or CD8+ SP compartment significantly increase when the total cellularity of the thymus decreases (fig. 2A) (compare BPP and PP). Based on the high percentages of CFSE+ CD19+ cells that enter the thymus in the 3 inflammatory conditions evaluated (fig. 1), we also analyzed the absolute number of B cells in the thymi of control or T. cruzi-infected mice. Both the percentage and the absolute number of B cells increased (fig. 2D) with the reduction in the cellularity of the organ (fig. 2A). Interestingly, the kinetics of cell entry to the thymus varies depending upon the inflammatory/infection process being evaluated (after 3 days of LPS treatment, around days 12–14 in T. cruzi infected mice and around days 6–7 in C. albicans infected mice). However, what they all have in common is the fact that cells enter the thymus when cellularity of this organ starts to diminish. Based on the later data, we speculated that any situation where the total thymocyte number is reduced would favor the entrance of peripheral cells to the thymus. To prove this hypothesis, we treated mice with dexamethasone (Dex) since it has been demonstrated that this hormone considerably decreases the cellularity of the thymus [26;27]. We adoptively transferred CFSE-splenocytes from LPS-treated mice into LPS- or Dex-treated recipient mice[26]. Even though the total cell number of thymocytes is highly diminished in both LPS- and Dex-treated mice, peripheral cells could enter the thymus only in LPS-treated mice (fig. 2E). Moreover, the data indicates that both transferred cells and recipient mice need to be in an inflammatory environment to permit the entrance of mature cells to the thymus. To examine this possibility, CFSE-splenocytes from LPS-treated mice were transferred into recipient mice treated with Dex after the inflammatory process was triggered by LPS (fig. 2F). Interestingly, in this experimental condition the entrance of peripheral cells into the thymus occurred. Similar data was observed when T.cruzi–infected mice were used instead of LPS–treated mice (not shown). Overall, this data indicates that space is necessary but not sufficient for the entrance of cells into the thymus and we hypothesize that specific signals that recruit peripheral cells into the organ are also required.
Figure 2. Thymic cell loss is necessary but it is not the only requirement to permit the ingress of peripheral cells into the thymus.
Mice infected with 5 × 105 trypomastigotes (i.p) were killed the day of parasitemia peak (PP) or before the day of parasitemia peak (BPP). (A) Total cell number or (B) the percentages and (C) absolute cell numbers of CD4+ CD44+ or CD8+ CD44+ cells at both times. (D) The absolute cell number and the percentage of B cells (CD19+) in the thymic DN compartment were determined in control mice and in T. cruzi-infected mice sacrificed during PP. (E) Percentage of CFSE+ cells in the thymi of LPS treated mice (see the Materials and methods) versus mice treated with a single dose of 0.3 mg of dexamethasone (Dex). (F) Percentage of CFSE+ cells in the thymi of LPS treated mice versus mice treated with LPS and a single dose of 0.3 mg of Dex. Data from B and D was obtained by gating on the viable cells from thymi of control or T. cruzi-infected mice and later on the CD4+, CD8+ or DN cells as shown in Supporting Information fig. 3B. Data from A, C and D are shown as the mean + SEM of 18 mice per group pooled from 6 independent experiments. (B, D, E and F) Representative dot plots from individual recipient mice are shown. Data are representative of at least 3 independent experiments with 2–3 mice per group. *Control vs T. cruzi-infected, p<0.05, #T. cruzi-BPP vs T. cruzi-PP, p<0.05. Student unpaired t test.
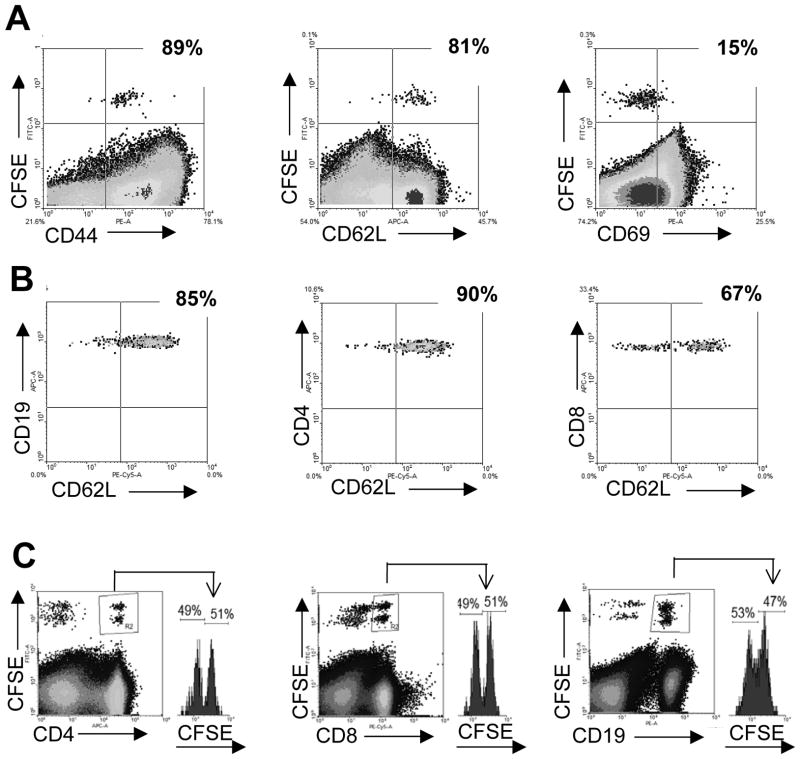
Figure 3. CD62L expression during the entrance of peripheral T cells to the thymus.
WT mice were infected i.p. with 5 × 105 trypomastigotes on day 0. On the day of parasitemia peak, splenocytes were obtained and after red cell depletion, 2–3 × 107 cells were stained with CFSE and adoptively transferred into T.cruzi-infected mice. Twenty four hours later, the thymi from recipient mice were obtained and (A) the percentage of CD44+, CD62L+ and CD69+ cells in the CFSE+ population was evaluated by flow cytometry. (B) The percentage of CD62L+ cells was analyzed by flow cytometry in the CSFE+ CD19+, CD4+ or CD8+ populations. Plots represent data from an individual recipient mouse, and the data are representative of 2 independent experiments with 3 mice each (C) Splenocytes from T.cruzi-infected mice were obtained and 2 × 107 cells were stained with either 1 μM (low) CFSE (untreated) or with 4 μM (high) CFSE and treated for 30 min with 100 μg/ml of a MEL-14 Ab (anti-CD62L). After washing the cells, equal amounts of low and high CFSE+ cells were mixed together and 3 × 107 cells of the mix were adoptively transferred to T.cruzi-infected mice. Eighteen hours later, the thymi from recipient mice were obtained and the percentage of CD19+, CD4+ or CD8+ cells was analyzed by flow cytometry in both low and high CSFE+ stained cells. Data shown are representative of 2 independent experiments with 3 mice per group. Data from B were obtained gating first on the viable cells from T. cruzi-infected mice and later on the CD4+ or CD8+ or CD19+ CFSE+ cells as shown in Supporting Information fig. 3C.
CD62L is highly expressed in mature T and B cells entering the thymus but does not participate in the migration to the organ
To characterize the phenotype of cells that enter the thymus during Th1-inflammatory/infectious processes, we analyzed the expression of markers that discriminate between naïve, recently activated or memory T cells (CD44, CD62L and CD69). Data shown in figure 3A demonstrates that cells that enter the thymus exhibited high expression of CD44 and CD62L but low expression of CD69. Together, cells migrating to the thymus exhibited surface expression markers compatible with a central memory phenotype. It has been demonstrated that traffic of peripheral B and T cells to the thymus in AKR mouse is mediated by the expression of L-selectin on immigrating lymphocytes [11]. Thus, we analyzed CD62L expression in all the cell types recruited to the thymus in LPS-treated and T. cruzi-infected mice. As shown in fig. 3B, CD62L was expressed by most immigrating B and CD4+ T cells and about 70% of CD8+ lymphocytes, suggesting that the integrin could represent an important pathway for cells to extravasate into the thymus. However, data presented in figure 3C demonstrate that CD62L is not involved in cell migration to the thymus since splenocytes from LPS-treated mice incubated with an anti-CD62L neutralizing Ab before the adoptive transfer did not affect migration of either mature T or B cells to the thymus (fig. 4C) but highly diminished the entrance of transferred cells to popliteal LNs (data not shown) [28]. Similar results were found in the LPS model (data not shown).
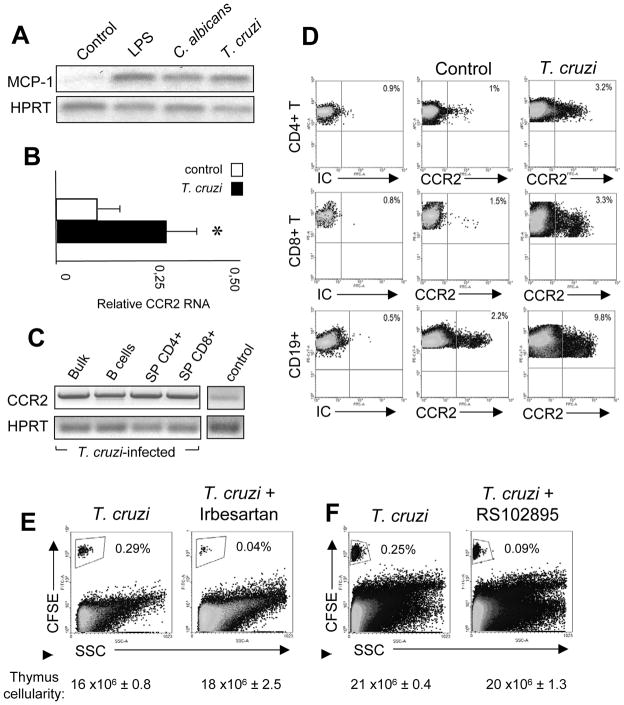
Figure 4. Thymic MCP-1 expression and entrance of CCR2+ peripheral cells to the thymus.
WT mice were infected i.p. with 5 × 105 trypomastigotes on day 0. (A) MCP-1 mRNA expression was determined in the thymi of control, LPS-treated or C. albicans or T. cruzi-infected mice by RT PCR. Data are representative of 2 independent experiments with 2–3 mice each. HPRT was used as a loading control. (B) CCR2 mRNA expression was determined by real time PCR in the thymi of control of T. cruzi-infected mice. Data are expressed as the mean+SEM of 2 separate experiments with 3 mice each. (C) CCR2 mRNA expression was determined by RT PCR in the sorted B cells, T CD4+, T CD8+ and DN thymic population from T. cruzi-infected mice and in a thymus from an uninfected control mice. Data are representative of 2 independent experiments where thymocytes were pooled from 3 mice before the cell sorting. (D) Splenocytes from control or T.cruzi-infected mice were obtained and CCR2 expression was evaluated in B cells and CD4+ or CD8+ T cells by flow cytometry. Dotplots are representative of 2 different experiments with 3 mice per group. (E–F) Splenocytes from T.cruzi-infected mice were obtained and 2 × 107 cells were stained with 4 μM CFSE and adoptively transferred to T. cruzi-infected mice treated with (E) irbesartan or (F) RS102895 for 2 days before the day of sacrifice. Data are representative of 2 independent experiments with 3 mice per group. Data from D were obtained gating first on the viable splenic cells from T. cruzi-infected mice and later on the CD4+ or CD8+ or CD19+ cells as shown in Supporting Information fig. 3D. *Control vs T. cruzi-infected, p<0.05. Student unpaired t test. IC: Isotype control.
Expression of MCP-1 and CCR2 is required for extravasation of peripheral cells into the thymus
Since CD62L did not participate in the entry of mature lymphocytes into the thymus, we focused our attention on other integrin/chemokines candidates. We found that the expression of the chemokine MCP-1 was highly up-regulated in the thymi of LPS-treated, C. albicans or T. cruzi-infected mice compared with that of controls (fig. 4A). Ex-vivo treatment of thymocytes from T. cruzi-infected mice with Brefeldin A for 4 h and then intracellular staining with an anti-MCP-1 Ab demonstrated a low but consistent detection of MCP-1+ cells (Supporting Information fig. 1). The expression of MCP-1 was mainly restricted to B and CD4+ and CD8+ CD44lo resident thymocytes, but not to CD44hi peripheral T cell counterparts or CD11b+ and CD11c+ subsets (Supporting Information fig. 1). As CCR2 is the most important receptor for MCP-1, we next analyzed the expression of this marker in uninfected (control) and T. cruzi-infected mice (fig 4B). We observed that CCR2 mRNA expression is increased in the thymi of T. cruzi-infected mice. Moreover, analysis of CCR2 expression revealed that after the infection, B and T cells in the thymus increase the expression of this receptor compared to uninfected mice (fig. 4C). These results led us to speculate that peripheral cells that infiltrate the organ would express this receptor. Interestingly, the data in figure 4D suggest that in non-pathological condition, a proportion of T and B cells express CCR2; however such cells are not attracted to the thymus since MCP-1 is not expressed in this organ. When an inflammatory/infectious process is triggered, not only is MCP-1 expressed in the thymus but also the number of CCR2+ peripheral T and B cells increases. Moreover, comparing naïve with infected mice, we can see that the percentage of CCR2+ B cells increases more than the percentage of CCR2+ T cells. This could explain why a larger number of peripheral B cells migrate to the thymus as compared with T cells in infectious/inflammatory processes.
Our data demonstrates that thymic MCP-1 expression is triggered in the thymus during Th1 inflammatory/infectious processes, thus facilitating the recruitment of certain peripheral CCR2+ T and B cells. To confirm this hypothesis, we treated T. cruzi-infected mice with two specific antagonists of the MCP-1 ligand [29;30]. As can be seen in figure 4E, administration of irbesartan to T. cruzi-infected recipient mice for 2 days prior to the adoptive transfer of splenocytes from T. cruzi-infected mice resulted in a strong diminution in the percentage of peripheral cells that enter the organ (about a 10-fold reduction). Furthermore, treatment of recipient mice and transferred cells with a CCR2 antagonist (RS102895) induced a ~60% reduction in the entrance of cells to the thymus (fig. 4F).
Systemic expression of IL-12 and IL-18 during an acute Th1 inflammatory/infectious process drives peripheral cell migration to the thymus
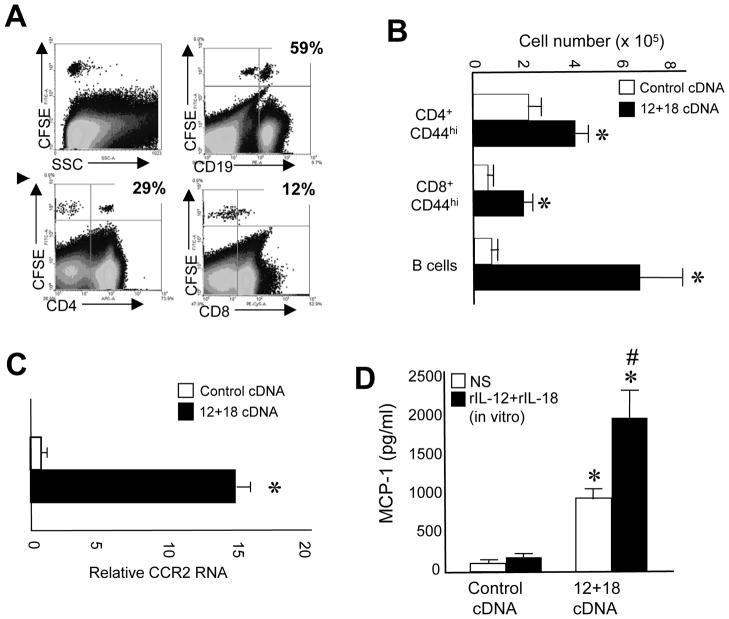
Thus far, using different experimental models with a strong Th1 bias, we have demonstrated that peripheral mature T and B cells are able to enter the thymus. Then, as a general mechanism, we speculated that cytokines such as IL-12 and IL-18 could be participating in this phenomenon since they are known to be important early mediators of the Th1 immune response that developed in these inflammatory models [20–23]. To evaluate this possibility, we treated mice with IL-12+IL-18 cDNAs by hydrodynamic injection in order to induce a systemic expression of both cytokines as we previously reported [31;32]. Seven days later, splenocytes from IL-12+IL-18 cDNA-treated mice were adoptively transferred into mice treated with IL-12+IL-18 cDNAs. As shown in fig. 5A, peripheral B and T cells enter the thymus of recipient mice in similar numbers as that observed in the infectious disease models. Accordingly, a significant increase occurred in B cells and CD4+ and CD8+ CD44hi T cell numbers in the thymi of IL-12+IL-18 cDNA-treated mice (fig. 5B). Next, we analyzed CCR2 and MCP-1 expression in the thymi of IL-12+IL-18 cDNA-treated mice. We observed a significant increment in CCR2 mRNA expression in the bulk thymocyte population of IL-12+IL-18 cDNA-treated mice (fig 5C). Moreover, thymocytes from IL-12+IL-18 cDNA-treated mice cultured ex-vivo, spontaneously produced much larger amounts of MCP-1 than thymocytes from control mice (fig. 5D). Interestingly, an important boost in MCP-1 expression is observed in thymocytes from IL-12+IL-18 cDNA-treated mice when rIL-12 and rIL-18 are added to the cultures but not in thymocytes from control mice, suggesting that rIL-12 and rIL-18 are able to drive MCP-1 expression only from thymocytes that have been exposed to IL-12 and IL-18 in vivo (fig. 5E).
Figure 5. Entrance of peripheral T and B cells in the thymus upon expression of IL-12 and IL-18.
B6 mice were hydrodynamically injected with control or IL-12+IL-18 cDNAs. (A) On day 7 post-injection, half of the mice were sacrificed, spleen cells were obtained and after red cell depletion, 2–3 × 107 cells were stained with CFSE and adoptively transferred into the other half of mice. Twenty four hours later, the thymi from recipient mice were obtained and surface stained with anti-CD4, -CD8, or -CD19 Abs and analyzed in a flow cytometer. Data are representative of 2 different experiments with 3–4 mice each. (B) Absolute cell numbers of CD4 CD44+ or CD8 CD44+ cells or B cells were determined in the thymi of control or IL-12+IL-18 cDNA-treated mice. Data are expressed as the mean + SEM of 3 separate experiments with 3 mice each. (C) CCR2 mRNA expression was determined by real time PCR in the thymi of control or IL-12+IL-18 cDNA-treated mice. (D) MCP-1 expression was evaluated by ELISA in thymi of control or IL-12+IL-18 cDNA-treated mice re-stimulated or not in vitro with rIL-12+rIL-18. Data from C and D are expressed as the mean+SEM of 2 separate experiments with 3 mice each. *Control vs IL-12+IL-18 cDNA-treated mice, p<0.05, #in vitro rIL-12+rIL-18-treated vs non-treated, p<0.05. Student unpaired t test.
Migration of peripheral immune cells to the thymus is independent of TCR specificity
Based on this data, we next speculated if T cells entering the thymus expressed a particular TCR or if it is a general polyclonal process. To evaluate whether T cell recruitment depends on the TCR, we administered T. cruzi infection in OT-I mice that express a transgenic TCR specific for OVA peptide in CD8+ T cells, an antigen not expressed by the parasite Trypanosoma cruzi. Similarly to what we observed in WT mice, when CFSE-splenocytes from OT-I T. cruzi-infected mice are adoptively transferred to T. cruzi-infected WT mice, only B cells and CD4+ and CD8+ T cells are able to enter the organ (Supporting Information fig. 2). Importantly, we observed that all CFSE+CD8+ splenocytes from OT-I infected mice that enter the thymus of WT-infected mice express the TCR Vβ5 chain (OVA specific), demonstrating that those clones are probably activated during the infection in a bystander way and then acquire the capacity to re-enter the thymus (Supporting Information fig. 2).
DISCUSSION
The entrance of peripheral mature T cells has been described in mouse [6;8] rat [9;33] and pig [34] models, especially after T cell activation by an Ag [6;8;10;16]. In the case of B cells, recruitment of a low number of these cells to the thymus seems to be a normal process, however it could highly increase in certain pathological situations such thymic lymphoma [11] and certain autoimmune-prone mouse strains [12]. To examine this concept in greater detail, we report here that entrance of mature peripheral B cells as well as T cells is a common feature that occurs during an acute Th1 inflammatory/infectious process. There is one report that demonstrates the entrance of T cells to the thymus during a viral infection, but in this case it is the consequence of peripheral CD8+ T cells entrance in order to eliminate infected cells in the thymus [35]. In the present manuscript we demonstrate that entrance of peripheral cells to the thymus during inflammatory/infectious disease processes is more a consequence of a bystander activation of certain peripheral B and T cells that express CD62L, CD44 and CCR2, thus allowing them to ingress the thymus due to local production of MCP-1. Moreover, our data with LPS treatment, C. albicans and T. cruzi infections demonstrate that the entrance of peripheral B and T cells into the thymus, rather than being a pathogen-specific phenomenon is the consequence of an acute inflammatory process triggered by an early production of the Th1 cytokines IL-12 and IL-18. One concern we needed to address is whether or not activated (CD44hi) cells or also naïve T cells are able to reach the thymus in these inflammatory conditions. To examine this question, we adoptively transferred splenocytes from a normal uninfected mouse to a T. cruzi-infected mouse and evaluated phenotype of the cells that entered to the thymus. We observed that they are CD44int/hi and CD62Lhi despite the fact that the cells expressed lower levels of CD44 and CD62L before the injection (not shown). Thus, we concluded that because we inject naïve cells into recipient mice that are actively expressing high levels of inflammatory cytokines, naïve cells get activated themselves during the 18h they reside into the recipient mice. This data supports the fact that only cells with an activated phenotype and expressing CD62L are able to reach the thymus in the context of these inflammatory conditions.
Even though we do not describe here what subset of peripheral leukocytes could migrate to the thymus in situations when IL-12 and IL-18 are systemically expressed, it is interesting to note that other investigators have characterized a subset of splenic CD44hi CD8+ T cells that, in the presence of both IL-12 and IL-18, can rapidly secrete IFNγ in the absence of specific Ag [36;37]. In vivo, the activation of these cells is triggered by different pathogens such as L. monocytogenes [36] or during certain acute viral infections [22].
Based on these reports and our own data with OVA transgenic mice that demonstrate that T cells that enter the thymus are not exclusively clones activated by Ags expressed by the pathogen itself, we speculate that in a normal non-immunized mouse there exists a subset of B and T cells that are able to rapidly respond to IL-12 and IL-18 (or to cytokines induced thereafter), become activated and acquire the capacity to migrate back to the thymus. We still need to determine if these cells originate from the pre-existing CD44hi pool or if they derive from naïve CD44lo cells that somehow get activated and up-regulate CD44, CD62L and CCR2 in the presence of inflammatory cytokines and these studies will be the focus of future research.
Even though most of the reports that evaluate migration of cells to the thymus use the i.v. route [6–8;16;17], we also performed adoptive transfer experiments with splenocytes stained with CFSE and injected i.p. (not shown in this manuscript) and demonstrate that in this case, utilizing a different route other than the bloodstream, peripheral cells migrate in similar proportion to the thymus as when cells were injected intravenously. Moreover, the large number of CD44hi T cells as well as B cells found in the thymi of T. cruzi-infected and LPS-treated mice in the absence of any adoptive transfer procedure further confirm that this is a phenomenon that naturally occurs during acute Th1 inflammatory conditions and it does not represent an artifact induced after i.v. cell injections.
It has been described that lymphopenic thymi are more permeable to peripheral leukocyte infiltration. For example, it has been reported that thymus lobes from aged or neonatal mice are much more leaky to peripheral T cells than are those from adult mice [4;19]. Certain disease states have also been shown to promote thymic immigration by recirculating T cells; for instance, mature resting T cells readily enter the atrophic thymus of T cell deficient SCID mice and persist there for months [18]. Interestingly, our data show that after LPS treatment, Candida albicans or T. cruzi infection or simply after IL-12+IL-18 systemic expression, thymi experience a great loss of their cellularity, especially of DP cells [31]. However, data suggest that permeability to peripheral cells to the thymus is unlikely to be due solely to the sparse DP compartment found in the thymi, since dexamethasone treatment of a normal mice, known to deplete the DP compartment [26;27], failed to promote the thymic immigration of adoptively transferred peripheral B and T cells from T.cruzi-infected mice.
This data let us to believe that not only free space is necessary but also certain molecules involved in cell migration induced in these inflammatory models are needed in the migration of cells to the thymus. The first candidate that we analyzed was the selectin CD62L, since it has been previously reported that cells that enter the thymus are CD62Lhi [11]. Moreover, expression of CD62L on T cells has been demonstrated to mediate the interaction between peripheral node addressin on the thymic vasculature or stromal cells, thereby promoting T cell immigration [28]. However, our data demonstrate that CD62L does not participate in this migratory effect.
In a different experimental model, it has been reported that memory T cells that migrate to bone marrow express higher levels of CCR2 than memory T cells that reside in the spleen [38]. This fact led us to investigate if CCR2 is also involved in peripheral cell migration to the thymus. We found that when mice are treated with 12+18-cDNA or T. cruzi infection, CCR2 expression in the thymus is increased. Moreover, B and T cells in the thymus of T.cruzi–infected mice show positive expression of CCR2. Monocyte chemoattractant protein-1 (MCP-1) is one of the C-C chemokines that has been reported to induces chemotaxis of B and memory T cells through its receptor CCR2 [39]. Moreover, MCP-1 has been reported to be important in mediating migration of CD8+ TCM cells to inflammatory sites [40] which is compatible with the TCM phenotype of T cells that enter the thymus in these 3 inflammatory/infectious conditions. Furthermore, MCP-1 is highly expressed in the thymus of LPS-treated, C. albicans and T. cruzi-infected and IL-12+IL-18-treated mice. Data using specific inhibitors of MCP-1 and CCR2 further confirm this hypothesis. Interestingly, our data support the fact that IL-12 and IL-18 are the cytokines responsible for MCP-1 up-regulation in the thymus since we observed that in vitro, recombinant IL-12 and IL-18 are able to significantly increase MCP-1 only in thymocytes from IL-12+IL-18-cDNA treated mice, indicating that cells present in the thymi of mice exposed to systemic IL-12+IL-18 but not in normal mice contain cells with the ability to produce this chemokine. Accordingly, further analysis demonstrates that thymic B cells and T cells CD44lo are the main producers of this chemokine in the thymus under these inflammatory conditions.
Based on the data presented in this work, we propose a novel concept of peripheral lymphocyte recirculation during non-physiological conditions. We demonstrate that in any potential situation where large amounts of IL-12 and IL-18 are produced as a consequence of an infectious/inflammatory process, the thymus cell number is reduced favoring the creation of new niches in this organ that facilitate peripheral B and T cells entrance to the thymus. Interestingly, this phenomenon occurs in the absence of any antigenic stimulation and seems to be part of bystander activation of certain peripheral mature B and T cells. The fact that systemic IL-12 and IL-18 expression is observed in numerous situations opens the possibility that this migratory events described here are also possible in a numerous type of pathological processes. At the present moment we are evaluating if the entrance of B and T cells is due to a mere opportunism of cells during a moment of large expansion of leukocytes or if it is a coordinated process that plays a role in thymus physiology. Moreover, evaluation of peripheral cell localization in the thymus could provide important information not only about the source of required factors peripheral B and T cells use to survive in the thymus but also about the role they might have in different thymic processes such as negative and positive selection and differentiation of immature cells in this organ.
MATERIAL AND METHODS
Mice
Female or male C57BL/6 (B6) and OT-I mice (Jackson Laboratory) used in this study were 6–10 week old of age and were maintained under specific pathogen-free conditions. The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Our animal facility obtained NIH animal welfare assurance (assurance number A5802-01, OLAW, NIH, USA).
LPS treatment, Candida albicans and Trypanosoma cruzi infections
B6 mice were injected i.p. with LPS (055-B5, Sigma) in a sublethal concentration of 20μg per mouse in 200μl PBS once a day for 3 consecutive days. Trypanosoma cruzi trypomastigotes were maintained by serial passages in B6 mice. B6 mice were i.p. infected with 5 × 105 trypomastigotes from T. cruzi diluted in PBS. Mice were sacrificed the days before parasitemia peak (BPP, between days 9–11 post-infection) or during parasitemia peak (PP, between days 12–14 post-infection) [41]. Yeast cells of C. albicans were grown on Sabouraud glucose agar slopes at 28°C, maintained by weekly subculture. B6 mice were i.p. infected with 5 × 107 viable yeast diluted in PBS. Mice were sacrificed 5 days after the infection.
Hydrodynamic cDNA injections
The hydrodynamic gene transfer procedure was described previously [42]. The designated amount of each DNA was dissolved in 1.6 ml of sterile 0.9% sodium chloride solution. Animals were injected in the tail vein with the cDNAs in less than 8 seconds and separated in 2 groups, control: 15μg of ORF empty vector control cDNA and IL-12+IL-18: 5μg of IL-12 cDNA (pscIL-12, p40-p35 fusion gene) plus 10μg of IL-18 cDNA (pDEF pro-IL-18). All the expression plasmids utilize the human elongation 1-α promoter to drive transcription.
Adoptive transfer experiments
Spleens from LPS-treated, C. albicans-infected or T. cruzi-infected mice were obtained and 2–3 × 107 splenocytes were stained with 1 or 4 μM CFSE (Molecular Probes, Eugene, OR) in PBS-5% SBF at a concentration of 107 cells/ml for 15 minute at RT, in the dark. Cells were washed, resuspended in 0.2 ml of PBS and injected i.p. or i.v. into the recipient’s tail vein. Thymi from recipient mice were gently disaggregated and cell suspensions were obtained 24 h post-adoptive transfer.
Flow cytometry analysis
For multicolor staining, fluorocrome-conjugated Abs (BD-Pharmingen, La Jolla, CA) were used in various combinations. Briefly, cells were stained for surface markers for 30 min at 4°C and washed twice. To detect intracellular expression of MCP-1, cells were cultured with no stimulus for 4 h in the presence of 10μg/ml brefeldin A (Sigma). Cells were then stained for surface markers, washed, and fixed with Cytofix/Cytoperm buffer (BD-Pharmingen) for 15 min at 4°C. Cells were washed with Perm/Wash buffer (BD-Pharmingen) and incubated with the PE anti-mouse Abs or PE isotype-matched Ab (BD-Pharmingen) for 30 min at 4°C and then analyzed by flow cytometry in a BD FACS Canto™ II cytometer (BD Biosciences, San José, CA, USA).
Irbesartan, RS102895 and dexamethasone treatments
Irbesartan(Sigma-Aldrich, USA) is reported to act as an antagonist of the monocyte chemoattractant protein-1 (MCP-1) and was administered i.p. at 10 mg/kg/day for 2 days before the sacrifice of the mice [30]. To block CCR2 interaction with its ligand, RS 102895 (Sigma-Aldrich, USA), a CCR2 antagonist was injected i.p. at 3 mg/kg in recipient mice twice, 24 h and 1 h before the adoptive transfer of cells and also CCR2 was blocked in CFSE labeled cells by incubation with the antagonist (10μM) for 30 min before the adoptive transfer to recipient mice [29]. To induce thymocyte apoptosis in vivo, dexamethasone (0.3 mg) was injected i.p. to untreated mice or 4 h after LPS treatment as described above [26]. The mice were sacrificed after 72h of the treatments. All treated mice were adoptively transferred with 2–3 × 107 splenocytes from LPS-treated mice 24h before the sacrifice.
mRNA extraction and analysis
Total RNA was isolated using a single-step phenol/chloroform extraction procedure (TRIzol; Invitrogen Life Technologies). RT-PCR was performed with 100 ng of total RNA for each sample (Super Script III one step RT-PCR with platinum Taq, Invitrogen), consisting of a 15 min reverse transcription at 45°C, 40 cycles of denaturing at 94°C (15 s), annealing at 55°C (30s) and extension at 68°C (60 s), with a final extension for 5 min at 68°C. Primers used were: MCP-1, 5′-CCCACTCACCTGCTGCTACT-3′ (sense) and 5′-TCTGGACCCATTCCTTCTTG-3′(antisense); CCR2, 5′-GTACCCAAGAGCTTGATGAA-3′ (sense) and 5′-GTGTAATGGTGATCATCTTGT-3′(antisense). Gene expression for CCR2 was also assessed using semiquantitative real-time PCR. Briefly, RNAs were treated with DNase I prior to reverse transcription. Reverse transcription was performed on 1 μg of RNA using random hexamers as primers. Semiquantitative real time PCR was performed on cDNAs using TaqMan® expression assays (Life Technologies) specific for each target gene. All reactions were run on a 96 well, 7300 Real Time PCR System (Life Technologies). Expression of all target genes was normalized using HPRT as the control housekeeping gene.
Statistical analysis
Data was compared in all cases between each treated-mice group with its own control group. For statistical significance data was analyzed by means of a Student unpaired t test with p<0.05 considered as significant.
Supplementary Material
Supporting Information Figure 1: MCP-1 expression in the thymi of T. cruzi-infected mice is restricted to B cells and resident CD4+ and CD8+ thymocytes. WT mice were infected with 5 × 105 trypomastigotes (i.p.). On day 12–14 post infection, thymocytes were obtained and cultured for 4 h in the presence of Brefeldin A. MCP-1 expression was determined by intracellular staining in CD44hi and CD44lo CD4+ and CD8+ SP cells, B cells, DCs cells and macrophages. First dotplot correspond to a positive control obtained from LPS-stimulated splenic macrophages. Representative plots from an individual mouse; data are derived from 2 independent experiments with 3 mice each. Intracellular MCP-1 data was obtained by gating on the viable cells from thymi of control or T. cruzi-infected mice and later on the CD4+, CD8+ or CD19+ cells similarly as shown in Supporting Information fig. 3D but in the thymus.
Supporting Information Figure 2: Recirculation of peripheral T cells to the thymus is independent of TCR specificity. OT-I mice (OVA specific TCR transgenic mice) were infected with 5 × 105 trypomastigotes (i.p.) and were sacrificed the day of parasitemia peak. Splenocytes (2–3 × 107) from OT-I infected mice were obtained, CFSE labeled and adoptively transferred to WT- infected recipients. Twenty four hours later thymocytes from recipient mice were obtained and the percentage of CD4+ cells, CD8+ cells and B cells (CD19+) was determined in the CFSE+ population by flow cytometry. The expression of OVA specific Vβ5+ cells was determined in the CD8+CFSE+ cells. Plots are representative of an individual recipient mouse. Data are derived from 2 independent experiments with 2 mice each. Data were obtained by gating on the viable cells (Supporting Information fig. 3A).
Supporting Information Figure 3: Gating strategies used in the flow cytometry data in this work. (A) Viable cells from a thymus in a forward vs side scatter dotplot. (B) Viable cells from a thymus of a control or a T. cruzi-infected mice in a forward vs side scatter dotplot. Then CD4+ or CD8+ or double-negative cells were gated. (C) CD4, CD8 or CD19 expression in CFSE+ cells. (D) CD4+, CD8+ or CD19+ cells on viable splenocytes from control or T. cruzi-infected mice.
Acknowledgments
We thank Mike Sanford for performing ELISA and analysis, Joseph Sarhan and Catherine Razzook for real time PCR analysis and Fabricio and Luis Navarro, John Wine and Tim Back for their support in animal care and experimentation. We also thank Dr. Claudia Sotomayor for providing C. albicans cultures, Paula Icely for technical assistance and Lic. Luciano Pedrotti for hydrodynamic injections.
This project has been funded in part with federal funds from the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health and also by Agencia Nacional de Promoción Científica y Tecnológica (Argentina) and Secretaria de Ciencia y Técnica de la Universidad Nacional de Córdoba (SeCyT-UNC).
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. government.
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
References
- 1.Galton M, Reed PB. Entry of lymph node cells into the normal thymus. Transplantation. 1966;4:168–177. doi: 10.1097/00007890-196603000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Naparstek Y, Holoshitz J, Eisenstein S, Reshef T, Rappaport S, Chemke J, Ben-Nun A, et al. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982;300:262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- 3.Fink PJ, Bevan MJ, Weissman IL. Thymic cytotoxic T lymphocytes are primed in vivo to minor histocompatibility antigens. J Exp Med. 1984;159:436–451. doi: 10.1084/jem.159.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181:2265–2270. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau LA, Rohekar S, Wang JJ, Lian D, Chakrabarti S, Zhang L, Zhong R, et al. Thymic re-entry of mature activated T cells and increased negative selection in vascularized allograft recipients. Clin Exp Immunol. 2002;127:43–52. doi: 10.1046/j.1365-2249.2002.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy CL, Godfrey DI, Scollay R. The effect of antigen stimulation on the migration of mature T cells from the peripheral lymphoid tissues to the thymus. Dev Immunol. 2001;8:123–131. doi: 10.1155/2001/20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westermann J, Smith T, Peters U, Tschernig T, Pabst R, Steinhoff G, Sparshott SM, et al. Both activated and nonactivated leukocytes from the periphery continuously enter the thymic medulla of adult rats: phenotypes, sources and magnitude of traffic. Eur J Immunol. 1996;26:1866–1874. doi: 10.1002/eji.1830260830. [DOI] [PubMed] [Google Scholar]
- 10.Kirberg J, Bosco N, Deloulme JC, Ceredig R, Agenes F. Peripheral T lymphocytes recirculating back into the thymus can mediate thymocyte positive selection. J Immunol. 2008;181:1207–1214. doi: 10.4049/jimmunol.181.2.1207. [DOI] [PubMed] [Google Scholar]
- 11.Michie SA, Rouse RV. Traffic of peripheral B and T lymphocytes to hyperplastic, preneoplastic thymuses of AKR mice. Am J Pathol. 1991;138:1015–1025. [PMC free article] [PubMed] [Google Scholar]
- 12.Hart M, Zan-Bar I. Modulation of B cell maturation and migration to the thymus of SJL mice. B cell migration to the thymus. Thymus. 1991;18:209–223. [PubMed] [Google Scholar]
- 13.Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993;12:357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frommer F, Waisman A. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PLoS One. 2010;5:e15372. doi: 10.1371/journal.pone.0015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopinathan R, Depaz HA, Oluwole OO, Engelstad K, Ali AO, Garrovillo M, Fawwaz RA, et al. Mechanisms of acquired tolerance induced by adoptive transfer of MHC-specific alloreactive T cells: effector T cells migrate to the thymus. Transplant Proc. 2001;33:92. doi: 10.1016/s0041-1345(00)01919-9. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann SL, Marconi P, Brocker T. Peripheral T cells re-enter the thymus and interfere with central tolerance induction. J Immunol. 2011;186:5612–5619. doi: 10.4049/jimmunol.1004010. [DOI] [PubMed] [Google Scholar]
- 17.Ali A, Garrovillo M, Oluwole OO, Depaz HA, Gopinathan R, Engelstad K, Hardy MA, et al. Mechanisms of acquired thymic tolerance: induction of transplant tolerance by adoptive transfer of in vivo allomhc peptide activated syngeneic T cells. Transplantation. 2001;71:1442–1448. doi: 10.1097/00007890-200105270-00015. [DOI] [PubMed] [Google Scholar]
- 18.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. J Exp Med. 1992;176:611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surh CD, Sprent J, Webb SR. Exclusion of circulating T cells from the thymus does not apply in the neonatal period. J Exp Med. 1993;177:379–385. doi: 10.1084/jem.177.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardillo F, Postol E, Nihei J, Aroeira LS, Nomizo A, Mengel J. B cells modulate T cells so as to favour T helper type 1 and CD8+ T-cell responses in the acute phase of Trypanosoma cruzi infection. Immunology. 2007;122:584–595. doi: 10.1111/j.1365-2567.2007.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 22.Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- 23.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin Microbiol Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PLoS One. 2011;6:e17940. doi: 10.1371/journal.pone.0017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hale JS, Fink PJ. Back to the thymus: peripheral T cells come home. Immunol Cell Biol. 2009;87:58–64. doi: 10.1038/icb.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priceputu E, Bouallaga I, Zhang Y, Li X, Chrobak P, Hanna ZS, Poudrier J, et al. Structurally distinct ligand-binding or ligand-independent Notch1 mutants are leukemogenic but affect thymocyte development, apoptosis, and metastasis differently. J Immunol. 2006;177:2153–2166. doi: 10.4049/jimmunol.177.4.2153. [DOI] [PubMed] [Google Scholar]
- 27.Talaber G, Boldizsar F, Bartis D, Palinkas L, Szabo M, Berta G, Setalo G, et al. Mitochondrial translocation of the glucocorticoid receptor in double-positive thymocytes correlates with their sensitivity to glucocorticoid-induced apoptosis. Int Immunol. 2009;21:1269–1276. doi: 10.1093/intimm/dxp093. [DOI] [PubMed] [Google Scholar]
- 28.Michie SA, Streeter PR, Butcher EC, Rouse RV. L-selectin and alpha 4 beta 7 integrin homing receptor pathways mediate peripheral lymphocyte traffic to AKR mouse hyperplastic thymus. Am J Pathol. 1995;147:412–421. [PMC free article] [PubMed] [Google Scholar]
- 29.Rehni AK, Singh N. Ammonium pyrrolidine dithiocarbamate and RS 102895 attenuate opioid withdrawal in vivo and in vitro. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2489-8. [DOI] [PubMed] [Google Scholar]
- 30.Tsukuda K, Mogi M, Iwanami J, Min LJ, Jing F, Oshima K, Horiuchi M. Irbesartan attenuates ischemic brain damage by inhibition of MCP-1/CCR2 signaling pathway beyond AT receptor blockade. Biochem Biophys Res Commun. 2011;409:275–279. doi: 10.1016/j.bbrc.2011.04.142. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Galan MC, Bream JH, Farr A, Young HA. Synergistic effect of IL-2, IL-12, and IL-18 on thymocyte apoptosis and Th1/Th2 cytokine expression. J Immunol. 2005;174:2796–2804. doi: 10.4049/jimmunol.174.5.2796. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Galan MC, Reynolds D, Correa SG, Iribarren P, Watanabe M, Young HA. Coexpression of IL-18 strongly attenuates IL-12-induced systemic toxicity through a rapid induction of IL-10 without affecting its antitumor capacity. J Immunol. 2009;183:740–748. doi: 10.4049/jimmunol.0804166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopinathan R, Depaz HA, Oluwole OO, Ali AO, Garrovillo M, Engelstad K, Hardy MA, et al. Role of reentry of in vivo alloMHC peptide-activated T cells into the adult thymus in acquired systemic tolerance. Transplantation. 2001;72:1533–1541. doi: 10.1097/00007890-200111150-00011. [DOI] [PubMed] [Google Scholar]
- 34.Binns RM, Licence ST, Whyte A, Wilby M, Rothkotter HJ, Bacon M. Genetically determined CD45 variant of value in leucocyte tracing in vivo in the pig. Immunology. 1995;86:25–33. [PMC free article] [PubMed] [Google Scholar]
- 35.Gossmann J, Lohler J, Lehmann-Grube F. Entry of antivirally active T lymphocytes into the thymus of virus-infected mice. J Immunol. 1991;146:293–297. [PubMed] [Google Scholar]
- 36.Bou Ghanem EN, Nelson CC, D’Orazio SE. T cell-intrinsic factors contribute to the differential ability of CD8+ T cells to rapidly secrete IFN-gamma in the absence of antigen. J Immunol. 2011;186:1703–1712. doi: 10.4049/jimmunol.1001960. [DOI] [PubMed] [Google Scholar]
- 37.Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Qin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, et al. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640–647. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Dai H, Wan N, Moore Y, Dai Z. The role for monocyte chemoattractant protein-1 in the generation and function of memory CD8+ T cells. J Immunol. 2008;180:2886–2893. doi: 10.4049/jimmunol.180.5.2886. [DOI] [PubMed] [Google Scholar]
- 41.Stempin CC, Garrido VV, Dulgerian LR, Cerban FM. Cruzipain and SP600125 induce p38 activation, alter NO/arginase balance and favor the survival of Trypanosoma cruzi in macrophages. Acta Trop. 2008;106:119–127. doi: 10.1016/j.actatropica.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe M, McCormick KL, Volker K, Ortaldo JR, Wigginton JM, Brunda MJ, Wiltrout RH, et al. Regulation of local host-mediated anti-tumor mechanisms by cytokines: direct and indirect effects on leukocyte recruitment and angiogenesis. Am J Pathol. 1997;150:1869–1880. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1: MCP-1 expression in the thymi of T. cruzi-infected mice is restricted to B cells and resident CD4+ and CD8+ thymocytes. WT mice were infected with 5 × 105 trypomastigotes (i.p.). On day 12–14 post infection, thymocytes were obtained and cultured for 4 h in the presence of Brefeldin A. MCP-1 expression was determined by intracellular staining in CD44hi and CD44lo CD4+ and CD8+ SP cells, B cells, DCs cells and macrophages. First dotplot correspond to a positive control obtained from LPS-stimulated splenic macrophages. Representative plots from an individual mouse; data are derived from 2 independent experiments with 3 mice each. Intracellular MCP-1 data was obtained by gating on the viable cells from thymi of control or T. cruzi-infected mice and later on the CD4+, CD8+ or CD19+ cells similarly as shown in Supporting Information fig. 3D but in the thymus.
Supporting Information Figure 2: Recirculation of peripheral T cells to the thymus is independent of TCR specificity. OT-I mice (OVA specific TCR transgenic mice) were infected with 5 × 105 trypomastigotes (i.p.) and were sacrificed the day of parasitemia peak. Splenocytes (2–3 × 107) from OT-I infected mice were obtained, CFSE labeled and adoptively transferred to WT- infected recipients. Twenty four hours later thymocytes from recipient mice were obtained and the percentage of CD4+ cells, CD8+ cells and B cells (CD19+) was determined in the CFSE+ population by flow cytometry. The expression of OVA specific Vβ5+ cells was determined in the CD8+CFSE+ cells. Plots are representative of an individual recipient mouse. Data are derived from 2 independent experiments with 2 mice each. Data were obtained by gating on the viable cells (Supporting Information fig. 3A).
Supporting Information Figure 3: Gating strategies used in the flow cytometry data in this work. (A) Viable cells from a thymus in a forward vs side scatter dotplot. (B) Viable cells from a thymus of a control or a T. cruzi-infected mice in a forward vs side scatter dotplot. Then CD4+ or CD8+ or double-negative cells were gated. (C) CD4, CD8 or CD19 expression in CFSE+ cells. (D) CD4+, CD8+ or CD19+ cells on viable splenocytes from control or T. cruzi-infected mice.