Abstract
Objectives
Insulin is recognized to increase renal salt reabsorption in the distal nephron and hyperinsulinemic states have been shown to be associated with increased expression of the renal NaCl cotransporter, NCC. However, the effect of insulin on NCC functional activity has not been reported.
Methods
Using a heterologous expression system of Xenopus laevis oocytes, a mouse distal convoluted cell line, mDCT15 cells, endogenously expressing NCC, and an ex vivo kidney perfusion technique, we assessed the effect of insulin on the activity and phosphorylation of NCC. The signaling pathway involved was analyzed.
Results
In Xenopus oocytes insulin increases the activity of NCC together with its phosphorylation at threonine residue 58. Activation of NCC by insulin was also observed in mDCT15 cells. Additionally, insulin increased the NCC phosphorylation in kidney under the ex vivo perfusion technique. In oocytes and mDCT15 cells, insulin effect on NCC was prevented with inhibitors of PI3K, mTORC2, and AKT1 kinases, but not by inhibitors of MAP or mTORC1 kinases, suggesting that PI3K-mTORC2-AKT1 is the intracellular pathway required. Additionally, activation of NCC by insulin was not affected by wild type or mutant versions of WNK1, WNK4, or SGK1, but it was no longer observed in the presence of wild type or the dominant negative, catalytically inactive WNK3, implicating this kinase in the process.
Conclusion
Insulin induces activation and phosphorylation of NCC. This effect could play an important role in arterial hypertension associated with hyperinsulinemic states, such as obesity, metabolic syndrome, or type 2 diabetes mellitus.
Keywords: Thiazide, distal convoluted tubule, obesity, salt transport, hypertension, WNK3
Introduction
Metabolic syndrome, obesity, and type 2 diabetes mellitus (DM) are associated with arterial hypertension. The higher the body-mass index, the greater the hypertension prevalence, and several lines of evidence strongly suggest that obesity plays a causative role in hypertension and that connection could be explained, at least in part, by hyperinsulinemia [1–3]. Insulin resistance in the classical target organs for this hormone (liver, muscle, and adipose tissue) leads to increase circulating levels of insulin, which would then have amplified effects on other tissues, with the kidney being particularly sensitive, as it is by far the most perfused organ of the body.
Insulin is known to promote salt reabsorption in the kidney [3], particularly via its actions in the distal nephron [3–5]. Insulin receptors are expressed all along the nephron [3]. In obese Zucker rats or db/db mice, the increased salt reabsorption is associated with overexpression at the mRNA or protein levels of distal nephron NaCl transport proteins, such as the renal specific bumetanide-sensitive Na+:K+:2Cl− cotransporter, NKCC2, the thiazide-sensitive Na+:Cl− cotransporter, NCC [6–8], the α-subunit of the Na+:K+-ATPase, and the β-subunit of the amiloride-sensitive epithelial sodium channel, ENaC [9]. In addition, insulin increases the activity of ENaC by a phosphatidyl inositol kinase 3 (PI3K)-serum glucocorticoid kinase 1 (SGK1) dependent mechanism [10].
The NCC plays a key role in the regulation of arterial blood pressure. On the one hand, inactivating mutations of NCC lead to Gitelman’s disease which features arterial hypotension and hypokalemia [11]. Moreover, in the Framingham study, subjects with a missense mutation in one allele of NCC, which decreased its activity [12], were protected against the development of hypertension [13]. On the other hand, mutations in the with-no-lysine kinases 1 and 4, WNK1 and WNK4, respectively, cause pseudohypoaldosteronism type II (PHAII) in which arterial hypertension seems to be due to the disturbance of the physiological WNK-mediated regulation of NCC, thus increasing the cotransporter activity and arterial blood pressure. Additionally, inhibition of NCC by thiazide-type diuretics has been a cornerstone in the treatment of hypertension over the last 50 years [14].
Increasing the activity of NCC could be one of the mechanisms through which hyperinsulinism leads to arterial hypertension. This assumption is supported by (i) the observation that hyperinsulinism is associated with increased expression of NCC [9], (ii) chronic insulin infusion to normal rats increased the natriuretic response to hydrochlorothiazide, a specific inhibitor of NCC [8], and (iii) that rosiglitazone (antidiabetic drug) administration to obese Zucker rats reduces insulin blood levels and the sensibility to thiazides [6]. However, it is not known from these models if in addition to the expression, insulin up-regulates the functional activity of NCC. In the present study, we thus analyzed the effect of insulin on NCC activity and phosphorylation.
Methods
Clones and antibodies
The previously characterized clones and antibodies used were: rat Flag-NCC (rNCC) [15], human c-Myc-tagged WNK3 [16], mutant c-Myc-tagged WNK3-D294A [17], mouse wild type HA-tagged WNK4 and mutant HA-tagged WNK4-D318A [18], wild type SGK1 and mutant SGK1-K104M [19]. Monoclonal anti-Flag antibody and polyclonal anti-actin (Sigma). Polyclonal antibodies against total NCC or phospho-antibodies Thr 58-NCC (kindly provided by Dario Alessi, Dundee University) [20]. For the present study we generated a new mutant of human WNK3 by substituting the serine 308 for an alanine: WNK3-S308A. To this end, single point mutagenesis was performed using the Quick-change mutagenesis kit (Stratagene), as we have previously described [17, 21]. All mutations were corroborated by using a DNA automatic sequencing.
Preparation of Xenopus laevis oocytes
As previously described in detail [22, 23], under anesthesia induced by 0.17% tricaine immersion, oocytes were surgically collected from adult female Xenopus laevis frogs. One hour after incubation in a Ca2+-free ND96 medium supplemented with 2 mg/ml collagenase A (mM: 96 NaCl, 2 KCl, 1 MgCl2, and 5 HEPES/Tris, pH 7.4), oocytes were manually defolliculated and incubated overnight in ND96 at 16°C. The next day, mature oocytes were injected with cRNA transcribed in vitro from one or various constructs at a concentration of 0.2–0.4 µg/µl. Control oocytes were injected with water. For two days oocytes were incubated at 16°C in ND96 supplemented with sodium pyruvate (2.5 mM) and gentamicin (5 mg/100 ml). cRNA for microinjection was synthetized in vitro with T7 RNA polymerase from linearized cDNAs at the 3’ end. The cRNAs were analyzed for integrity, quantified and stored in aliquots at −80°C until use.
Functional Assays
After two days of incubation, the activity of NCC was assessed following our standard procedures [22, 23]. Briefly, a 30-min incubation in a Cl−-free ND96 medium containing 1 mM ouabain, 0.1 mM amiloride, and 0.1 mM bumetanide was followed by a 60-min uptake period in a K+-free, NaCl medium (40 mM NaCl, 56 mM Na-gluconate, 4.0 mM CaCl2, 1.0 mM MgCl2, and 5.0 mM Hepes/Tris, pH 7.4) containing ouabain, amiloride, bumetanide and 2 µCi of 22Na+ per ml. Insulin alone or in the presence of inhibitors of the insulin transduction pathways: Wortmannin (Sigma), Rapamycin, UO126 (Callbiochem) [24], SB203580 (Callbiochem), AKT IV Inhibitor (Callbiochem), AZD8055 (Selleck) [25] were dissolved at 10mM dimethylsulfoxido (DMSO) as stock solutions and preserved at −20°C. All compounds were added before the uptake period for 1 to 15 minutes. All uptake experiments were performed at least three times and included at least 10 oocytes in each experimental group; statistical significance was p < 0.05 and results were reported as means ± S.E. The uptake observed in control groups was taken as 100% (fold-1), and experimental groups were normalized accordingly. The significance of the differences between groups was tested by student-t-test or one-way ANOVA with multiple comparisons using Bonferroni corrections.
Western blotting
The expression and phosphorylation of NCC at T58 was assessed with proteins extracted from oocytes as previously described [17]. Briefly, groups of 10 oocytes exposed to each condition were homogenized in 4 µl/oocyte of Lysis buffer (50 mM Tris/HCl, pH 7.5, 1mM EGTA, 1mM EDTA, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1% (wt/vol) Nonidet P-40, 0.27 M sucrose, 0.1% (vol/vol) 2-mercaptoethanol, and protease inhibitors (Complete tablets, Roche, 1 tablet per 50 ml)) and centrifuged at 10,000 g to collect the supernatants.. 50 µg of total protein was resolved by 7.5% SDS-PAGE and electroblotted onto polyvinylidenedifluoride membranes (PVDF, Amersham Pharmacia Biotech, Piscataway, NJ, USA). Membranes were blocked for 2 h at room temperature in TBS buffer-0.02% Tween-20 plus 5% non-fat milk and exposed to either the mouse anti-Flag peroxidase-conjugated antibody (SIGMA), 1:5000, or the phospho-antibodies Thr 58-NCC.
Cell culture and treatments
mDCT15 cells, which endogenously express NCC [19, 26, 27], were plated on cell culture dishes and grown in growth medium containing a 50:50 mix of DMEM/F12, 5% heat-inactivated fetal bovine serum (FBS) and 1% Penicillin/Streptomycin/Neomycin (P/S/N), at 37°C. Experiments were conducted when the cells reached 90–95% confluence [19].
Assessment of NCC Function in mDCT15 Cells
mDCT15 cells were seeded in 12-well plates and prepared as described [27]. The cells were then incubated in a serum-free growth media (Opti-Mem) for 24 hours prior to being assayed. Cells were then treated with insulin for the indicated times. 30 minutes before uptake, 0.1 mM metolazone was added to the media. The medium was then changed to a 22Na+-containing medium (140 mM NaCl, 1 mM CaCl, 1 mM MgCl, 5 mM HEPES/Tris pH 7.4, 1 mM amiloride, 0.1 mM bumetanide, 0.1 mM benzamil, 1 mM ouabain, and 1 microCi/ml of 22Na+) with or without thiazide (0.1 mM metolazone) and incubated for 20 minutes. Tracer uptake was then stopped via washes with ice-cold wash buffer. Cells were subsequently lysed with 0.1% SDS (sodium dodecyl sulfate). Radioactivity was measured via liquid scintillation and protein concentrations of the lysates were determined (Bicinchoninic Acid (BCA) Protein Assay, Pierce). Uptakes were normalized to nmol/mg. Thiazide-sensitive uptake was determined by calculating the difference of the uptakes with and without thiazide.
NCC response to insulin in ex-vivo Perfused Rat Kidney
NCC phosphorylation was analyzed after insulin administration using the “ex vivo” perfusion system mounted in Langendorff preparation as previously described [28, 29]. In brief, we used male Wistar rats of 250 gr. Rats were anesthetized with 63 mg/kg sodium pentobarbital intraperitoneally. By laparotomy, the right kidney was exposed and the mesenteric and right renal arteries were cleared of surrounding tissue. The right renal artery was then cannulated. The right ureter was also cut and the animal was euthanized with sodium pentobarbital. The removed kidney was suspended in a water jacketed bath at 37°C and perfused at constant flow with Krebs’ solution at 37°C and gassed with O2–CO2 (95 : 5%). Flow was adjusted to obtain a basal perfusion pressure of 75–90 mmHg. The perfusion pressure was measured by a blood pressure transducer (Model PT300, Grass Instruments, West Warwick, RI, USA) and was recorded using a polygraph (Model 7D, Grass Instruments, Quincy, Mass, USA). Insulin was administered in the perfusate at a rate of 0.06 to 0.125 U/ml, which had no effect in the perfusion pressure. After 30 min of perfusion, the kidney was manually separated into cortex and medulla and corresponding fragments were frozen in liquid nitrogen for protein extraction to analyze NCC expression and phosphorylation by Western blot.
All procedures in the present study were approved by our institutional review committee.
Results
Insulin is a powerful activator of NCC
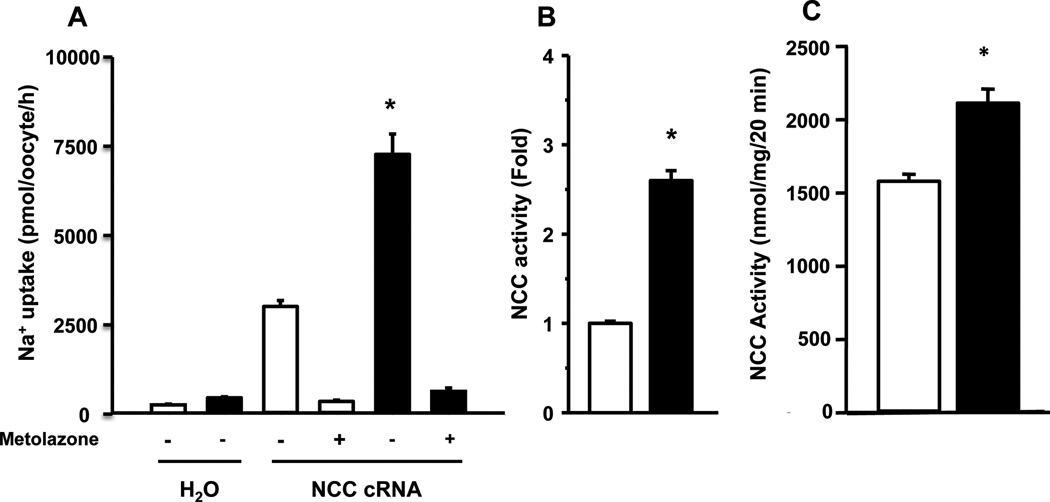
The heterologous expression system of Xenopus laevis oocytes has shown to be the one exhibiting the most robust and reproducible expression of transfected NCC. In addition, oocytes are able to respond to insulin owing to the expression of its receptor in the membrane [30]. As previously shown [23], oocytes microinjected with NCC cRNA induced the appearance of thiazide-sensitive Na+ uptake that was not present in water-injected oocytes (Fig. 1A), demonstrating the functional expression of the cotransporter. Exposing the oocytes to human recombinant insulin for 15 minutes in the pre-uptake solution at a concentration of 20 U/ml significantly increased the rNCC-induced Na+ uptake (Fig. 1A). On an average of 22 different experiments, insulin induced a 2.6 ± 0.1 fold increase in the rNCC activity (Fig 1B). The thiazide-sensitive Na+ uptake in mDCT15 also increased by insulin from 1,556 ± 32 nmol·mg−1·20 min−1 in its absence to 2,037 ± 68 mmol·mg−1·20 min−1 in its presence (p<0.05) (Fig. 1C). Thus, the positive effect of insulin on NCC was observed in both, Xenopus laevis oocytes and mDCT15 cells.
Figure 1. Insulin activates the renal thiazide-sensitive Na+:Cl− cotransporter, NCC, in X. laevis oocytes and in mDCT15 cells.
A. Na+ uptake was determined in the absence (white bars) or presence of 20 U/ml insulin (black bars) in microinjected oocytes with water or with rNCC cRNA and in the absence or presence of metolazone, as stated. (p<0.001 vs. all other groups). B. The thiazide-sensitive Na+ uptake observed in rNCC control in the absence of insulin was taken as 1, and the effect of insulin was normalized accordingly. (N=22; *p<0.001 vs control). (C) NCC activity was analyzed in mDCT15 cells endogenously expressing NCC in the absence (white bar) or presence (black bar) of insulin. (N=5; *p<0.001).
The effect of insulin on NCC was both dose and time dependent. We observed a significant effect of insulin on NCC-induced Na+ uptake with a little as one minute of exposure before the uptake period using 20 U/ml or with a concentration as low as 1 U/ml for 15 minutes before the uptake (data not shown). Stimulation of rNCC in oocytes was also observed using bovine insulin (Sigma). rNCC basal activity of 3,359 ± 56 pmol·oocyte−1·h−1 increased to 5,135 ± 58 pmol·oocyte−1·h−1 when exposed to bovine insulin (p<0.01). Activation of NCC was also observed using the human NCC cRNA. Microinjection of Xenopus oocytes with human NCC cRNA induced a Na+ uptake that was 6,416 ± 275 pmol·oocyte−1·h−1 in the absence and 9,641 ± 325 pmol·oocyte−1·h−1 in the presence of insulin (p<0.001).
Insulin increases phosphorylation of NCC
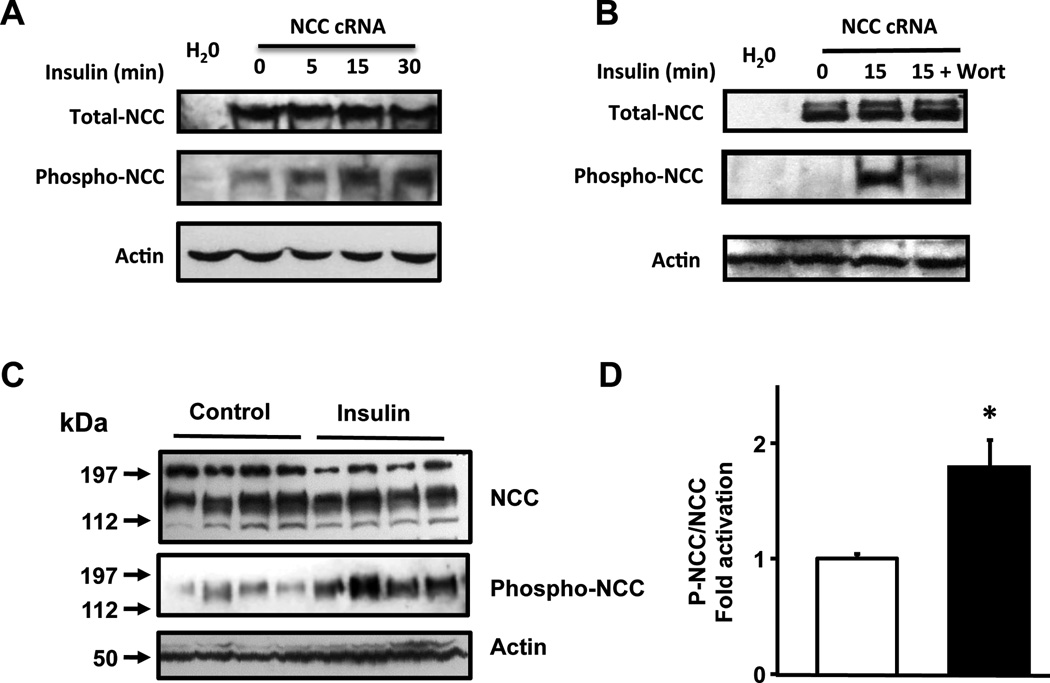
Activation of NCC is associated with increased phosphorylation of two threonine and one serine residues located in the amino terminal domain [15]: T53, T58, and S71, respectively in the rat NCC (T55, T60, and S73 in human NCC). Of these residues, T58 is the most important one, because preventing phosphorylation of T58, abrogates the phosphorylation in the other two sites [20] and the activity of the cotransporter [15]. As shown in Figure 2A, using a specific phosphoantibody against threonine 58, NCC phosphorylation in basal conditions was slightly detected. However, the signal increased after exposing oocytes to insulin. As a positive control, co-injection of NCC with WNK3 cRNA which is known to increase the activity and phosphorylation of NCC [17], was included. Note also that NCC phosphorylation induced by insulin was prevented by incubation of oocytes with the known PI3K inhibitor wortmannin (see below).
Figure 2. Insulin induces phosphorylation of rNCC at threonine 58.
(A and B) Representative Western blot assays performed using anti Flag monoclonal antibody, the pNCC-T58 phosphoantibody, and β-actin antibody, as stated. Total proteins were extracted from Xenopus laevis oocytes injected with water or rNCC cRNA and exposed to insulin for 0, 5, 15 and 30 minutes (A), or to 15 minutes in the absence or presence of wortmannin (B) (C) A representative Western blot analysis of protein extracts from ex-vivo perfused rat-kidneys. The kidneys were perfused with isotonic control solution or with isotonic solution containing 0.250 U/mL of insulin. (D) Densitometry analysis of phosphor-NCC over total rNCC from three different Western blot analyses compiling a total of 10 controls and 16 insulin perfused kidneys. (*p<0.01 vs control)
In order to define if the effect of insulin occurs in vivo, we used the ex vivo perfused rat kidney preparation, which allows analysis of hormonal effects on the kidney without the intervention of the central nervous system and/or extra renal hormonal networks. For this purpose, the right kidney from normal adult male Wistar rats was perfused with saline solution alone or containing insulin, at a concentration that did not change the perfusion pressure. As shown in Figure 2B and 2C, the level of T-58-NCC phosphorylation was significantly higher in the kidneys exposed to insulin than in controls. Thus, insulin induces NCC phosphorylation in the rat kidney.
Insulin activation of NCC occurs via PI3K-mTORC2-AKT
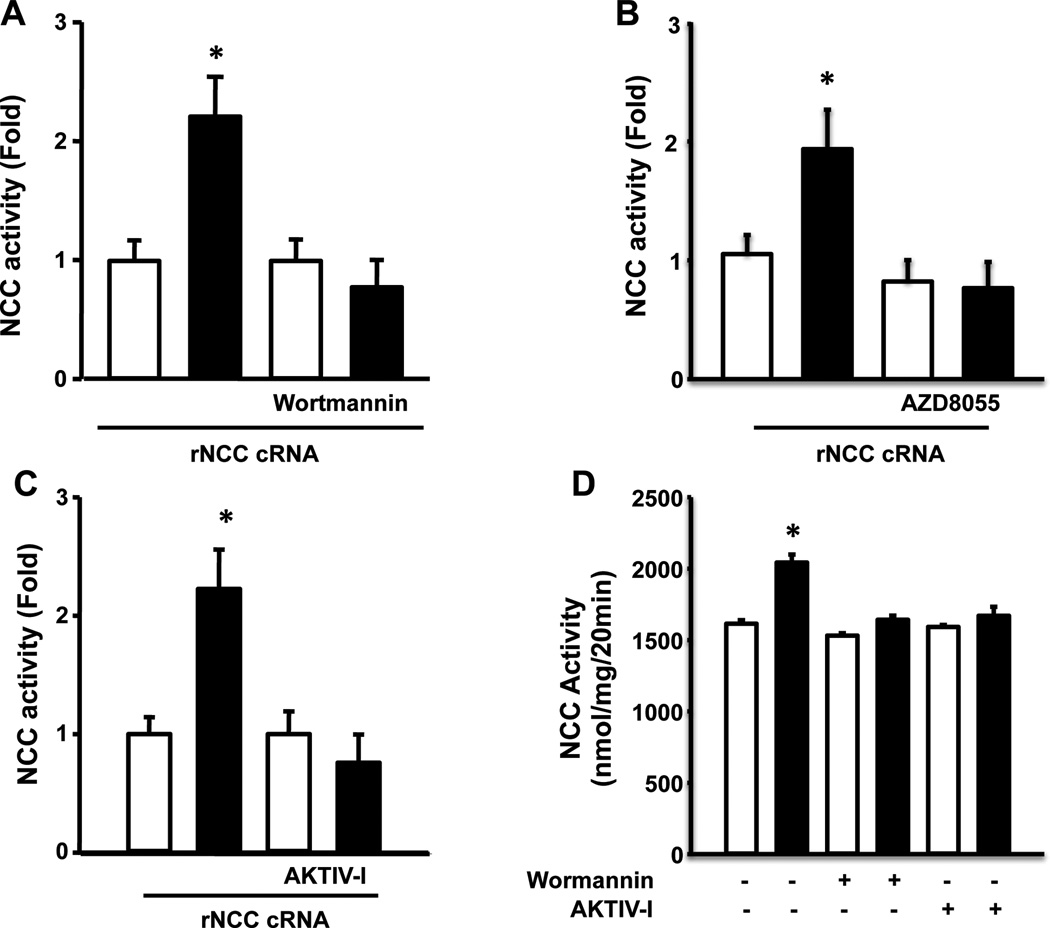
The known pathways through which insulin induces phosphorylation of threonine and serine residues were analyzed. Exposing oocytes to 50 nM wortmannin had no significant effect on the basal activity of NCC, but completely prevented the positive effect by insulin (Fig. 3A). This is consistent with results on Figure 2A in which the insulin-induced increase in NCC phosphorylation at T58 was completely prevented in the presence of wortmannin. In contrast, inhibitors of the MAP kinases did not affect basal activity of NCC or prevent the positive effect of insulin (Fig S1). Thus, the pathway implicated in insulin-induced NCC activation is via PI3K.
Figure 3. Insulin activates NCC cotransporter through a PI3K-mTORC2-AKT pathway.
Xenopus laevis oocytes were injected with rNCC cRNA and three days later Na+ uptake was assessed by incubated the oocytes in the absence (white bars) or presence (black bars) of insulin, in the absence or presence of the PI3K inhibitor wortmannin (A), the specific inhibitor of mTORc2, AZD8055 (B); or in the presence of the AKT Inhibitor, AKT IV-I (C). or the inhibitors of MAP kinases. The mean value of the cotransporter without insulin was taken as 1 and the effect of insulin was normalized to that value (*p<0.0001 vs own control in the absence of insulin). (D) NCC activity was analyzed in mDCT15 cells in the in the absence (white bars) or presence of 20 U/ml insulin (black bars) with or without wortmannin or the AKT inhibitor (*p<0.01 vs control).
We then assessed the effect of specific inhibitors of the mammalian target of rapamycin complex 1 and 2 (mTORC1 and mTORC2), and AKT enzymes. Rapamycin, an inhibitor of mTORC1, had no effect on the insulin-induced activation of NCC (Fig. S1). In contrast, the compound AZD8055, an inhibitor of mTORC2, was effective in preventing the NCC stimulating effect of insulin (Fig. 3B). Similarly, as was observed with wortmannin, the specific inhibitor of the AKT kinase (AKTIV inhibitor) had no effect on basal activity of NCC, but completely prevented the activation by insulin (Fig 3C). Thus, insulin promotes NCC activation via PI3K-mTORC2-AKT. This conclusion is supported by the observation in mDCT15 cells in which wortmannin and the AKTIV inhibitor also prevented the positive effect of insulin on NCC activity (Fig. 3D).
The kinases WNK1, WNK4, and SGK1 does not appear to be required for insulin effect on NCC
As previously reported [31] co-injection of oocytes with NCC and WNK4 cRNA resulted in a significant decrease of NCC activity. However, the level of NCC activation by insulin was similar in the NCC vs. the NCC + WNK4 group (p<0.01; Fig. S2). We next tested if the overexpression of the dominant negative version of WNK4 and/or SGK1 had any effect. Our data shows that even having both dominant negative WNK4-D318A and SGK1-K104M at the same time, the fold activation of NCC by insulin was similar to that seen in oocytes injected with NCC cRNA alone (Fig. S2). These observations are supported by results in mDCT15 cells in which WNK4 expression was reduced by knocking down WNK4 with specific RNAi. Although this treatment successfully reduced the WNK4 expression [32], exposure to insulin was still associated with an increased activity of NCC (Fig. S2). Thus, insulin positive effect on NCC in Xenopus laevis oocytes and mDCT15 cells does not appear to require the presence or activity of WNK4.
The WNK1 kinase has been shown to be required for the negative effect of PI3K on the potassium channel ROMK in a pathway in which phosphorylation of an Akt1-site located in the WNK1 threonine residue 58 is required [33]. To assess if this is also occurring for NCC, in a series of experiments (N=8), we tested the effect of insulin in oocytes injected with NCC cRNA alone or co-injected with NCC and a mutant WNK1-T58A, in which the Akt1 site was eliminated. Pooling 8 different experiments together we observed that the effect of insulin on NCC was similar in the absence or the presence of in the presence of WNK1-T58A (2.48 ± 0.35 vs 2.7 ± 0.4 fold, respectively (p=NS). Thus, it is unlikely that insulin signal pathway leading to NCC activation requires the WNK1 kinase.
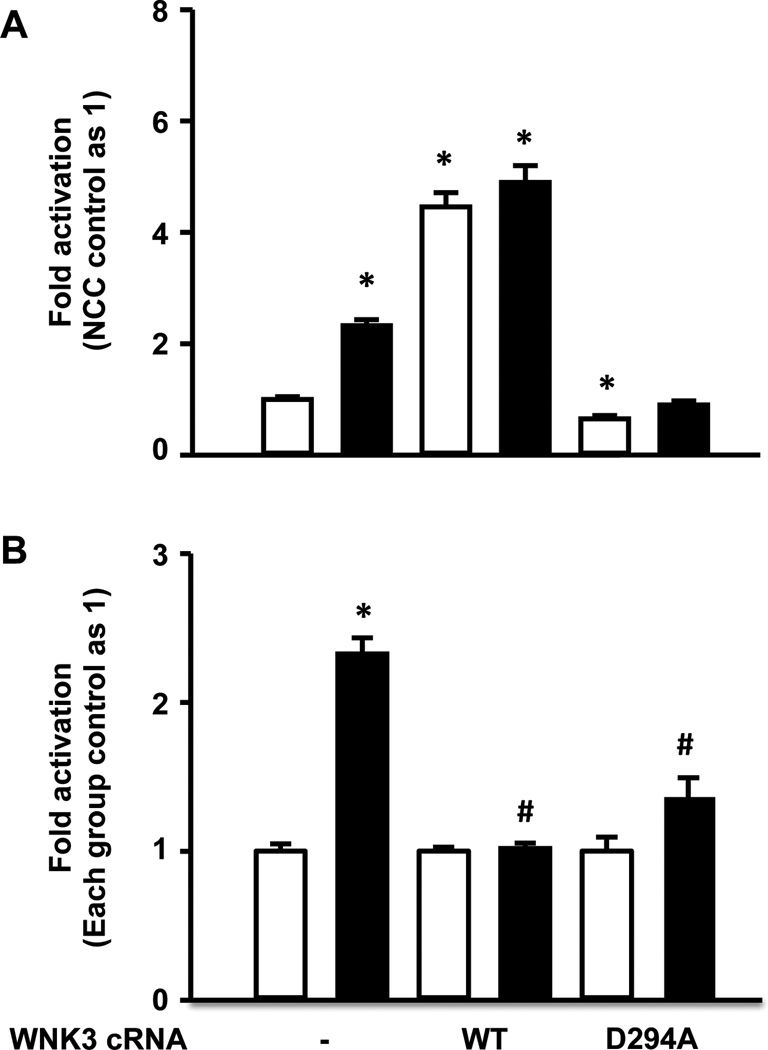
NCC activation by insulin and WNK3 share the same pathway
Since it is known that oocytes endogenously express the WNK isoforms and NCC activation by insulin or by WNK3 [17] results in phosphorylation of the same threonine (T-58), we tested the hypothesis that WNK3 and insulin could share a common pathway for NCC activation. To further explore this possibility, we assessed the effect of insulin on Na+ uptake in the presence of wild type or mutant versions of WNK3. In the upper panel of figure 4, the thiazide-sensitive Na+ uptake observed in control oocytes injected with NCC alone in the absence of insulin was taken as 100% (1-fold) and all other groups, were normalized accordingly, while in the lower panel (Fig. 4B), for each group of injected oocytes, the uptake observed in the absence of insulin was taken as 100% (1-fold), and in the presence of insulin was normalized accordingly. As previously shown [17], wild type WNK3 induced a significant increase in NCC activity (Fig. 4A). Addition of insulin, however, had no further effect on NCC activity. As also shown before [16], the catalytically inactive WNK3-D294A induced a dominant negative effect, reducing NCC activity to 64 ± 6% of the control group (Fig. 4A). In the presence of this mutant, the effect of insulin on NCC was dramatically reduced (Fig 4B).
Figure 4. Effect of wild type and mutant WNK3 on the NCC basal activity and response to insulin.
Xenopus laevis oocytes were injected with rNCC cRNA alone or together with wild type WNK3 or the dominant negative WNK3-D294A, as stated. Three days later tracer Na+ uptake was assessed in the absence (white bar) or presence (black bar) of insulin. (A) The Na+ uptake observed in oocytes injected with rNCC alone in the absence of insulin was taken as 1 and all other groups were normalized accordingly. *p<0.01 vs rNCC control. (B) The Na+ uptake observed for each group in the absence of insulin was taken as 1 and in the presence of insulin was normalized to its own control group. *p<0.0001 to control group. #p<0.0001 vs. rNCC alone in the presence of insulin. Figures show compiled results from at least five different experiments of each group.
Discussion
In the present study we show that insulin is a powerful activator of NCC and that increased activity of the cotransporter is associated with augmented phosphorylation of threonine 58 that has been previously shown to be associated with activation of NCC [15, 17, 20, 34]. The increased activity and/or phosphorylation were observed in several models including: Xenopus laevis oocytes, mDCT15 cells, and in ex-vivo perfused rat kidneys.
Our data suggest that insulin activation of NCC occurs via PI3K-mTORC2-Akt1, but not through the MAP kinases or mTORC1 (Figures 2 and S1). In contrast, our data does not support that SGK1 is implicated, as occurs with ENaC [33, 35], because overexpression of wild type or dominant negative version of SGK1 together with NCC did not change the level of response to insulin. Because DCT1 is not considered to be part of the aldosterone sensitive distal nephron owing to the absence of 11-β-hydroxysteroid dehydrogenase (type II) [36], it is believed that modulation of NCC in this region does not occur through SGK1, in contrast to DCT2, in which the aldosterone-SGK1-Nedd4-2 pathways is proposed to regulate NCC [19]. Thus, SGK1-independent modulation of NCC would provide an interesting pathway for differential regulation between NCC and ENaC.
In a recent study, Sohara et al. [37] suggested that insulin increases NCC phosphorylation in a WNK4-dependent manner in mice and mpkDCT cells. Mice were treated with a single intraperitonal dose of insulin and NCC phosphorylation at threonine residues 53 and 58, and serine residue 71, was studied. They observed that NCC phosphorylation increased in the wild type, but not in a hypomorphic WNK4 mouse. This approach, however, is not useful to discriminate between a direct action of insulin versus the consequences of systemic effects of the hormone. For instance, if the mice had experienced some degree of hypoglycemia, this could have activated the adrenergic system, which in turn would have stimulated renin release, increasing the production of angiotensin II that is known to induce NCC phosphorylation by a WNK4-SPAK dependent mechanism [31, 38]. Our study does not support that kinases WNK4 and SPAK are implicated in NCC activation by insulin because we observed that co-expression in oocytes of NCC with wild type WNK4 or kinase inactive WNK4 did not affect the insulin-induced activation of NCC (Fig 3A and 3B). Additionally, reducing WNK4 expression in mDCT15 cells with a WNK4 knockdown strategy [26], did not prevent the insulin activation of NCC.
Our data suggest that WNK3, another with no lysine kinase with known activating effects on NCC [16, 17] is implicated in the insulin activation of NCC (Fig. 4). Increased activity of NCC by WNK3 o by insulin is associated with phosphorylation of the same threonine residue [17]. In oocytes co-injected with NCC and WNK3, no further effect of insulin was observed, supporting the possibility that insulin and WNK3 stimulate NCC by a similar mechanism. Furthermore, knocking down WNK3 activity by co-injecting oocytes with the dominant negative, catalytically inactive WNK3 precluded the positive effect of insulin on NCC. Supporting this conclusion, a recent study shows that activity of the Na+:K+:2Cl− cotransporter NKCC1 in glioblastoma cells is increased by epidermal growth factor by a PI3K-WNK3 dependent mechanism [39]. Taken together, these observations implicate WNK3 in insulin activation of NCC, but we don’t yet know the catalytically active sites or the mechanisms mediating insulin’s action via WNK3. Further investigation will be necessary to clarify them.
In this study most of the experiments were done using a concentration of insulin around 20 U/ml, which is higher than regular concentration in plasma, even during hyperinsulinism. However, we observed in oocytes a significant effect with as low as 1 U/ml. It is possible that affinity of human insulin for the insulin receptor in oocytes or mDCT15 cells is lower that in human cells in vivo. In addition, the experiments on the kidney ex vivo perfusion system were done using much more lower concentrations (0.06 to 0.125 U/ml). Being the kidney the most perfused organ of the body, it is quite sensitive to small changes in hormone concentrations in plasma own to the amount of plasma passing per minute. In addition, although it is known that insulin receptors are expressed in the kidney, all along the nephron, most studies show an intracellular location [3, 40, 41]. Thus, although is known that DCT expresses insulin receptor, it is not clear if it is present in the basolateral side, location at which will be affected only by plasma concentration of the hormone, or if it is also expressed in the apical side and thus, could be affected by insulin molecules in tubular fluid. It is worth mention that during insulin resistant states it has been suggested that insulin receptor expression is also decreased in the kidney [41]. Therefore, the role of NCC activation on the developing of hypertension in animal models with hyperinsulinism requires further studies. In this regard, however, supporting this possibility, a recent study in hyperinsulinemic rats suggest that indeed NCC phosphorylation is increased [42].
One of the many actions of insulin is to modulate the renal handling of salt and potassium [4]. Initial studies demonstrated that insulin-induced salt retention is due to the effect of this hormone in the distal nephron [4, 5]. It is now known that insulin activates the epithelial sodium channel ENaC in the collecting duct, through a mechanism involving SGK1 and Akt1 [9, 10, 33, 35]. On the other hand, it has been observed that insulin induces an increase in expression of NCC [6, 8, 9] and that hyperinsulinemic states are associated with increased response to thiazide diuretics [6]. We now provide evidence for a direct effect of insulin on NCC activity.
There are at least two physiological reasons for insulin to activate NCC in the distal convoluted tubule. One is the fact that insulin promotes growth and this needs to be accompanied by salt and water retention. Additionally, increased sodium reabsorption at a time when the kidney is experiencing a higher filtered load of sodium from the dietary bolus, would help to modulate salt excretion during and after meal ingestion. Increasing activity of both, NCC and ENaC help to accomplish this goal. The second reason is the reduction of potassium excretion. Insulin induces hypokalemia due to its systemic effect of promoting cellular K+ uptake, together with glucose [43]. In consequence, to avoid hypokalemia due to the systemic effect of insulin after a meal, it has been suggested that insulin simultaneously prevents potassium excretion by the kidney [44]. One condition required to reduce potassium excretion is to decrease the delivery of salt to the collecting duct. This is achieved by increasing the activity of NCC in the early portion of DCT [36]. It is known, for instance, that low potassium diet increases phosphorylation and surface expression of NCC in DCT1 [45, 46].
Our data supports that insulin activates NCC. This effect of insulin could be one of the mechanisms to explain the strong association between obesity, diabetes, and metabolic syndrome with arterial hypertension. Hyperinsulinism is a common feature of these syndromes due to peripheral resistance to insulin. Increased circulating levels of insulin could amplify the hormone’s effects in non-classical target organs for the hormone. Because the kidney is the most perfused organ of the body, it is expected to be highly sensitive to the increased circulating levels of insulin. Thus, stimulation of both, expression [3, 6, 8] and activity (this study) of NCC by insulin could be an important mechanism linking obesity to arterial hypertension.
Supplementary Material
Acknowledgments
We thank Dr. Dario Alessi from the MRC phosphorylation unit, Dundee University for providing us with the phosphor-T58-NCC antibody.
M.C-C was supported by a scholarship from CONACYT-Mexico and is a graduate student in the Biomedical Science Ph.D. programs of the Universidad Nacional Autónoma de México.
Supported in part by the Leducq Foundation Transatlantic Network on Hypertension and CONACYT Grant 165815 (to G.G.), the NIH K08 DK081728 (to B.K.) and R01 DK-085097 (to R.S.H.), and the Research Service, Atlanta VA Medical Center (R.S.H.).
Footnotes
Part of this work was presented during the 2010 Annual Meeting of the American Society of Nephrology in Denver, CO and published as an abstract (J. Am. Soc. Nephrol 21:64A, 2010).
Disclosures: None.
References
- 1.Bogaert YE, Linas S. The role of obesity in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2009;5:101–111. doi: 10.1038/ncpneph1022. [DOI] [PubMed] [Google Scholar]
- 2.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286:R803–R813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 3.Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol. 2007;293:F974–F984. doi: 10.1152/ajprenal.00149.2007. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21:165–171. doi: 10.1007/BF00252649. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Goldberg M, Agus ZS. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976;58:83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of the Renal Thiazide-Sensitive Na-Cl Cotransporter, Blood Pressure, and Natriuresis in Obese Zucker Rats Treated with Rosiglitazone. Am J Physiol Renal Physiol. 2005;289:F442–F450. doi: 10.1152/ajprenal.00335.2004. [DOI] [PubMed] [Google Scholar]
- 7.Riazi S, Tiwari S, Sharma N, Rash A, Ecelbarger CM. Abundance of the Na-K-2Cl cotransporter NKCC2 is increased by high-fat feeding in Fischer 344 X Brown Norway (F1) rats. Am J Physiol Renal Physiol. 2009;296:F762–F770. doi: 10.1152/ajprenal.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of Blood Pressure, the Epithelial Sodium Channel (ENaC), and other Key Renal Sodium Transporters by Chronic Insulin Infusion in Rats. Am J Physiol Renal Physiol. 2006;290:F1055–F1064. doi: 10.1152/ajprenal.00108.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and beta-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol. 2001;281:F639–F648. doi: 10.1152/ajprenal.2001.281.4.F639. [DOI] [PubMed] [Google Scholar]
- 10.Blazer-Yost BL, Esterman MA, Vlahos CJ. Insulin-stimulated trafficking of ENaC in renal cells requires PI 3-kinase activity. Am J Physiol Cell Physiol. 2003;284:C1645–C1653. doi: 10.1152/ajpcell.00372.2002. [DOI] [PubMed] [Google Scholar]
- 11.Simon DB, Nelson-Williams C, Johnson-Bia M, Ellison D, Karet FE, Morey-Molina A, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nature Genetics. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 12.Acuna R, Martinez-de-la-Maza L, Ponce-Coria J, Vazquez N, Ortal-Vite P, Pacheco-Alvarez D, et al. Rare mutations in SLC12A1 and SLC12A3 protect against hypertension by reducing the activity of renal salt cotransporters. J Hypertens. 2011;29:475–483. doi: 10.1097/HJH.0b013e328341d0fd. [DOI] [PubMed] [Google Scholar]
- 13.Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. Journal of the American Medical Association. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.Pacheco-Alvarez D, San Cristobal P, Meade P, Moreno E, Vazquez N, Munoz E, et al. The Na-Cl cotransporter is activated and phosphorylated at the amino terminal domain upon intracellular chloride depletion. J Biol Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 16.Rinehart J, Kahle KT, De Los Heros P, Vazquez N, Meade P, Wilson FH, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacheco-Alvarez D, Vazquez N, Castaneda-Bueno M, los Heros P, Cortes-Gonzalez C, Moreno E, et al. WNK3-SPAK interaction is required for the modulation of NCC and other members of the SLC12 family. Cell Physiol Biochem. 2012;29:291–302. doi: 10.1159/000337610. [DOI] [PubMed] [Google Scholar]
- 18.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, et al. Molecular pathogenesis of inherited hypertension with hyperkalemia: The Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arroyo JP, Lagnaz D, Ronzaud C, Vazquez N, Ko BS, Moddes L, et al. Nedd4-2 Modulates Renal Na+-Cl− Cotransporter via the Aldosterone-SGK1-Nedd4-2 Pathway. J Am Soc Nephrol. 2011;22:1707–1719. doi: 10.1681/ASN.2011020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, et al. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 21.Castaneda-Bueno M, Vazquez N, Bustos-Jaimes I, Hernandez D, Rodriguez-Lobato E, Pacheco-Alvarez D, et al. A Single Residue in Transmembrane Domain 11 defines the different affinity for thiazides between Mammalian and Flounder NaCl transporter. Am J Physiol Renal Physiol. 2010;299:F1111–F1119. doi: 10.1152/ajprenal.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno E, San Cristobal P, Rivera M, Vazquez N, Bobadilla NA, Gamba G. Affinity defining domains in the Na-Cl cotransporter: different location for Cl− and thiazide binding. J Biol Chem. 2006;281:17266–17275. doi: 10.1074/jbc.M602614200. [DOI] [PubMed] [Google Scholar]
- 23.Monroy A, Plata C, Hebert SC, Gamba G. Characterization of the thiazide-sensitive Na(+)-Cl(−) cotransporter: a new model for ions and diuretics interaction. Am J Physiol Renal Physiol. 2000;279:F161–F169. doi: 10.1152/ajprenal.2000.279.1.F161. [DOI] [PubMed] [Google Scholar]
- 24.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Science signaling. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 25.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 26.Ko B, Hansen L, Mistry A, Mallick R, Hoover R. Acute angiotensin II effects on NCC are depedent on WNK4. FASEB Journal. 2012:26. [Google Scholar]
- 27.Ko B, Kamsteeg EJ, Cooke LL, Moddes LN, Deen PM, Hoover RS. RasGRP1 stimulation enhances ubiquitination and endocytosis of the sodium-chloride cotransporter. Am J Physiol Renal Physiol. 2010;299:F300–F309. doi: 10.1152/ajprenal.00441.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bautista R, Sanchez A, Hernandez J, Oyekan A, Escalante B. Angiotensin II type AT(2) receptor mRNA expression and renal vasodilatation are increased in renal failure. Hypertension. 2001;38:669–673. doi: 10.1161/hy09t1.096186. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Mendoza A, Hong E, Escalante B. The role of nitric oxide in angiotensin II-induced renal vasoconstriction in renovascular hypertension. J Hypertens. 1998;16:697–703. doi: 10.1097/00004872-199816050-00018. [DOI] [PubMed] [Google Scholar]
- 30.Scavo L, Shuldiner AR, Serrano J, Dashner R, Roth J, de Pablo F. Genes encoding receptors for insulin and insulin-like growth factor I are expressed in Xenopus oocytes and embryos. Proc Natl Acad Sci USA. 1991;88:6214–6218. doi: 10.1073/pnas.88.14.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.San Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko B, Mistry AC, Hanson LN, Mallick R, Cooke LL, Hack BK, et al. A New Model of the Distal Convoluted Tubule. Am J Physiol Renal Physiol. 2012;303:F700–F710. doi: 10.1152/ajprenal.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng CJ, Huang CL. Activation of PI3-kinase stimulates endocytosis of ROMK via Akt1/SGK1-dependent phosphorylation of WNK1. J Am SocNephrol. 2011;22:460–471. doi: 10.1681/ASN.2010060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, et al. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79:66–76. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Barbry P, Maiyar AC, Rozansky DJ, Bhargava A, Leong M, et al. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am J Physiol Renal Physiol. 2001;280:F303–F313. doi: 10.1152/ajprenal.2001.280.2.F303. [DOI] [PubMed] [Google Scholar]
- 36.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G. Aldosterone paradox: diferential regulation of ion transport in distal nephron. Physiology (Bethesda) 2011;26:115–123. doi: 10.1152/physiol.00049.2010. [DOI] [PubMed] [Google Scholar]
- 37.Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, et al. Acute insulin stimulation induces phosphorylation of the Na-Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoSOne. 2011;6:e24277. doi: 10.1371/journal.pone.0024277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, et al. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109:7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garzon-Muvdi T, Schiapparelli P, Ap Rhys C, Guerrero-Cazares H, Smith C, Kim DH, et al. Regulation of Brain Tumor Dispersal by NKCC1 Through a Novel Role in Focal Adhesion Regulation. PLoS biology. 2012;10:e1001320. doi: 10.1371/journal.pbio.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, et al. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA. 2008;105:6469–6474. doi: 10.1073/pnas.0711283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari S, Halagappa VK, Riazi S, Hu X, Ecelbarger CA. Reduced expression of insulin receptors in the kidneys of insulin-resistant rats. J Am Soc Nephrol. 2007;18:2661–2671. doi: 10.1681/ASN.2006121410. [DOI] [PubMed] [Google Scholar]
- 42.Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, et al. Enhanced phosphorylation of Na-Cl cotransporter in experimental metabolic syndrome - role of insulin. Clin Sci (Lond) 2012;123:635–647. doi: 10.1042/CS20120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faletti CJ, Perrotti N, Taylor SI, Blazer-Yost BL. sgk: an essential convergence point for peptide and steroid hormone regulation of ENaC-mediated Na+ transport. Am J Physiol Cell Physiol. 2002;282:C494–C500. doi: 10.1152/ajpcell.00408.2001. [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol. 2010;299:F890–F897. doi: 10.1152/ajprenal.00323.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na-Cl-cotransporter NCC in vivo is regulated by dietary salt, potassium and SGK1. Am J Physiol Renal Physiol. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.