Abstract
Hydroxylated polychlorinated biphenyls (OH-PCBs) were measured in surficial sediment from Indiana Harbor and Ship Canal (IHSC), East Chicago, IN and five original Monsanto Aroclors. These compounds were measured using gas chromatography with tandem mass spectrometry (GC-MS/MS) and certified standards that allowed us to identify 65 individual or co-eluting congeners. Concentrations in the sediment ranged from 0.20 to 26 ng/g dry weight. Profiles of most samples were similar and were dominated by mono- to penta-chlorinated OH-PCBs. Interestingly, most of the samples strongly resembled the OH-PCB profiles of Aroclors 1221, 1242, 1248 and 1254, yet 25% of OH-PCBs measured in the sediment were not detected in Aroclors. A strong positive correlation was found between ΣOH-PCB and ΣPCB (p < 0.0001) and also between many individual OH-PCB:PCB pairs (p < 0.05). Analysis of OH-PCB:PCB pairs suggest PCB degradation is unlikely as a source of OH-PCBs in IHSC sediment. We are the first to report levels of OH-PCBs in sediment and Aroclors, and our discovery is significant because it is likely that OH-PCB contamination exists in sediment anywhere that PCB contamination from Aroclors is present.
INTRODUCTION
Polychlorinated biphenyls (PCBs) are persistent organic pollutants that were used in industrial applications around the world. In the United States mixtures of PCBs were sold as Aroclors until production stopped in the 1970s.1 It is well-known that PCBs are metabolized to the hydroxylated form (OH-PCBs) by humans and other mammals via cytochrome P450 (CYP450)-mediated oxidation.2 Although the octanol-water partition coefficient (Kow) of OH-PCBs is theoretically slightly lower than their parent PCBs,3 OH-PCBs are still likely to bioaccumulate due to their relative hydrophobicity and also because of their binding affinity with the transport protein transthyretin (TTR) thought to be due to the structural similarity of some OH-PCBs with the hormone thyroxine.2 PCB sulfates, which are metabolites of OH-PCBs, also have binding affinity to TTR.4 OH-PCBs can affect brain development and function5–7 and the endocrine system.8–13 Certain OH-PCBs such as 4-OH-PCB 52 and 5-OH-PCB 66, have been shown to be more toxic than their parent PCBs in cell toxicity assays and LD50 studies in mice, respectively.14, 15
While levels of OH-PCBs have been reported in humans and non-laboratory animals,2, 16–23 OH-PCBs were reported in abiotic matrices for the first time by Ueno et al. in surface water, rainwater, and snow samples collected 2002–2004 from Ontario, Canada.24 To our knowledge, there have been no further publications of levels of OH-PCBs in soil or sediment matrices from natural systems.
Microbes degrade PCBs anaerobically through dechlorination and aerobically through the upper biphenyl pathway, and microbial degradation of PCBs in sediment has been well-studied as a bioremediation strategy.25–27 One intermediary metabolite of the upper biphenyl pathway is dihydroxylated-PCB. A further metabolite of the upper biphenyl pathway is chlorinated benzoic acid, which has been measured in Hudson River sediment.28 While microbes have CYP450 enzymes implicated in animals’ PCB metabolism to monohydroxylated-PCBs, there is no published research showing microbes are capable of metabolizing PCBs to monohydroxylated-PCBs, and neither monohydroxylated- nor dihydroxylated-PCBs have been directly identified in sediment samples.
In addition to the possibility of microbial transformation as a source, OH-PCBs are potentially present as contaminants in the original Aroclors. Although banned from production, PCBs produced as Aroclors remain in use throughout the world. Contaminants such as chlorinated naphthalenes, dibenzofurans, and chlorinated dibenzofurans have already been reported in Aroclors.29–32
In North America, the urban areas of the Great Lakes region in particular are known to be contaminated with elevated PCB levels. The Indiana Harbor and Ship Canal (IHSC) branches off the southwestern edge of Lake Michigan in East Chicago, IN and has been designated an Area of Concern by the International Joint Commission.33 In a previously-published study we measured concentrations of PCBs in surficial sediment from IHSC that ranged from 53 to 35,000 ng/g dry weight (dw).34 PCB congener profiles in these sediments closely resemble Aroclor 1248,34 and IHSC is a major source of PCBs to Lake Michigan and to the air.35
The aims of this study were to develop an OH-PCB analytical method and determine OH-PCBs in the PCB-contaminated IHSC surficial sediment and in original Aroclors. In this paper we describe our extraction and GC-MS/MS analytical methods for 65 OH-PCBs and report congener-specific results from analysis of 20 sediment samples and 5 Aroclors. This is the first published report of OH-PCB concentrations in sediment and relative levels in Aroclors. We compare the OH-PCB sediment results to PCBs already reported in the same samples. Given their toxic properties and unknown environmental abundance, our study’s identification and quantification of OH-PCBs in sediment is a first approach toward understanding the fate of OH-PCBs in the environment.
METHODS AND MATERIALS
Sample Collection and Extraction
Surficial sediment samples were collected in 2006 from IHSC in East Chicago, Indiana (Figure 1). The sampling campaign is described elsewhere.34 In preparation for analysis, 20 sediment samples were weighed (~ 3 g), mixed with a known amount of combusted diatomaceous earth, and spiked with 100 ng surrogate standard 4′-OH-PCB 159 (4′-hydroxy-2,3,3′,4,5,5′-hexachlorobiphenyl, AccuStandard, New Haven, CT, USA). Samples were extracted using pressurized solvent extraction according to Martinez et al.34 Original Monsanto Aroclors 1016, 1221, 1242, and 1254 in their original containers were obtained from Dr. Larry Robertson at The University of Iowa. Aroclor 1248 was purchased from AccuStandard. A known amount of Aroclor (6 to 23 mg) was dissolved in 4 mL hexane and spiked with 5 ng of the same surrogate standard as used for the sediment samples.
Figure 1.
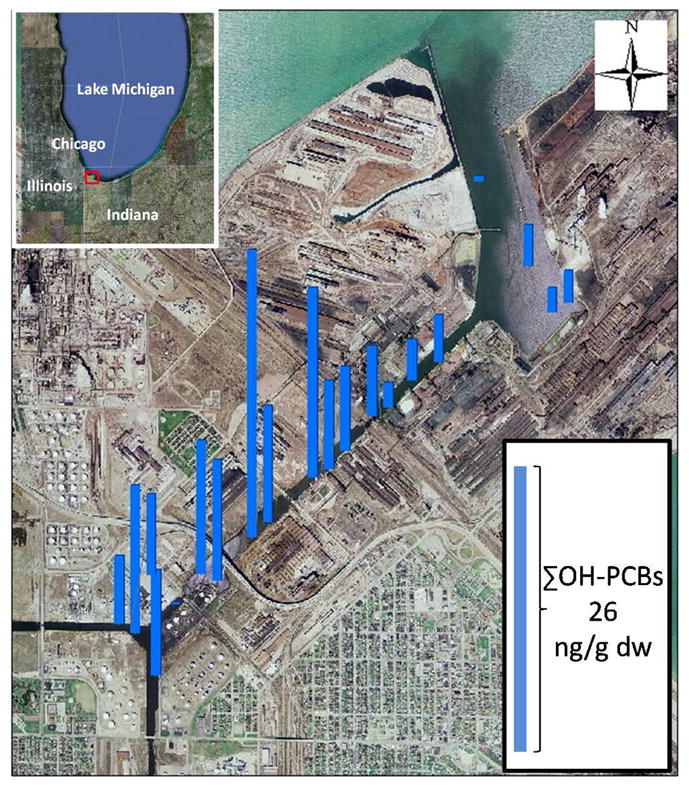
Spatial distribution of ΣOH-PCBs in IHSC. ΣOH-PCB58 in the 20 samples ranged from 0.20 to 26 ng/g dw.
In both sediment samples and Aroclors, the OH-PCB fraction was separated from the PCB fraction by liquid-liquid partitioning, derivatized, and cleaned as described in detail in Marek et al.20 Briefly, the ASE extract containing PCBs and OH-PCBs were mixed with a solution of potassium hydroxide in ethanol to deprotonate the OH-PCBs and move them to the aqueous layer. After the PCB fraction was extracted with hexane, OH-PCBs were re-protonated using hydrochloric acid (2M) and extracted from the aqueous layer with 9:1 hexane:MTBE. OH-PCBs were derivatized to their methoxylated form (MeO-PCBs) using diazomethane. Maintenance of yellow sample color after 3 hours indicated presence of excess diazomethane and was interpreted as an indication of complete derivatization. Interferences were removed by mixing the OH-PCB extracts with sulfuric acid (H2SO4) and then by passing the sample through H2SO4 silica gel columns. Immediately prior to analysis on the instrument, sediment samples were spiked with 30 ng and Aroclors were spiked with 25 ng internal standard PCB 209 (2,2′,3,3′,4,4′,5,5′,6,6′-decachlorobiphenyl, AccuStandard). All solvents were pesticide residue analysis quality (ThermoFisher Scientific).
Instrument Analysis
A GC with tandem MS (Agilent 7000) was employed for identification and quantification. The GC program operated as follows: The GC was equipped with a Supelco SPB-Octyl capillary column (5% phenyl methyl siloxane, 30 m × 250 μm ID, 0.25 μm film thickness) with helium as the carrier gas flowing at 0.8 mL/min and nitrogen/argon as the collision gas. The GC operated in solvent vent injection mode at the following injection conditions: initial temperature 45 °C, initial time 0.06 min, ramp 600 °C min−1 to inlet temperature 325 °C at 4.4 psi. The GC oven temperature program was 45 °C for 2.56 min, 45 to 75 °C at 100 °C/min and hold at 75 °C for 5 min, 75 to 150 °C at 15 °C/min and hold at 150 °C for 1 min, 150 to 280 at 2.5 °C/min and final hold 5 min (total run time 70.86 min). The triple quadrupole MS Electron Ionization source was set to 260 °C.
Samples were quantified for 65 OH-PCBs as 59 individual or co-eluting MeO-PCB chromatographic peaks. Although there are 837 theoretically-possible OH-PCBs,36 these 65 (Table S1) were the commercially available standards at the time of analysis. Calibration standards were purchased from Wellington Laboratories (Guelph, ON, Canada) and AccuStandard. Sixty-three of the targeted OH-PCBs were mono-hydroxylated and two were di-hydroxylated. The 65 standards ranged from mono-to nona-chlorinated and were assigned an abbreviation by using the BZ PCB number and then assigning the position of the OH group according to that PCB’s primed or unprimed ring.2, 37 Full congener names with their corresponding abbreviations are listed in the Supporting Information.
The MS-MS method was optimized for each standard. Elution time of each standard was determined in Select Ion Mode (SIM). The dominant product ion was then determined using product scan experiments. Congeners within the same homologue displayed different product ions, usually depending on the position of the methoxy group. In general, congeners with an ortho-substituted MeO group lost a Cl or a Cl plus a CH3 group, and congeners with a meta- or para-substituted MeO group lost the MeO group plus another C. In some cases the 2nd-most predominant ion was used in order to reduce the overall total number of mass scans required in the final Multiple Reaction Monitoring (MRM) method. The final MS-MS method was confirmed in MRM mode using the precursor and product ions for each standard (Table S1).
Congener identity in IHSC sediment samples were confirmed using GC-HRMS equipped with a DB5 column by Canada Centre for Inland Waters, Environment Canada (Burlington, ON). Congeners were identified with GC-HRMS when chromatographic peaks in the samples matched the retention times and isotopic ratios of primary and secondary ions of the pure, analytical standards.
Congener mass was calculated by applying a relative response factor calculated from each calibration standard and the internal standard recovery in each sample. Surrogate standard recoveries were used to evaluate extraction efficiency, but sample mass was not corrected according to surrogate recovery.
Statistics
Distribution of the ΣOH-PCB sample concentrations were skewed to the right, and concentration data was approximately normalized following logarithm transformation. Statistical analysis comparing sample groups was performed on the transformed data.
Concentration of sum and individual OH-PCBs was modeled by simple linear regression with sum and individual PCBs as the explanatory variable. The overall linear relationship between ΣOH-PCBs and ΣPCBs was assessed by an F-test using α = 0.05. Analysis was carried out in R 2.13.138 (ΣOH-PCBs) and MATLAB R2012a (7.14.0.739)(individual OH-PCBs).
The Welch two sample t-test was employed for determining significant differences of ΣOH-PCBs in harbor, channel, and branches of IHSC and the ratios of OH-PCBs/PCBs. Analysis was carried out in R 2.13.1.38
Principal component analysis (PCA) was performed on the congener profiles of the samples and Aroclors using MATLAB R2012a (7.14.0.739).
Cosine theta (cosθ) was calculated for each combination of sediment and Aroclor pairs for the purpose of comparing OH-PCB profiles, 39 where cosθ = 0 describes two completely different profiles and cosθ = 1 describes two identical profiles. Prior to cosine theta analysis, each congener was normalized to the sum of all congeners in that sample.
Quality Control
A full suite of Quality Control samples were assessed using surrogate standards, instrument blanks, method blanks, and replicates of Standard Reference Material (SRM) from National Institute of Standards and Technology (NIST SRM 1944: New York, New Jersey Waterway sediment).
OH-PCB surrogate standard recoveries ranged from 61 to 144% (106 ± 27%) in sediment samples and from 95 to 160% (119 ± 29%) in Aroclor samples. Instrument blanks consisting of hexane were analyzed with each instrument run. OH-PCBs detected in instrument blanks were always below the limit of quantification (LOQ). LOQ was calculated for each congener as the average plus 2 times the standard deviation in the method blanks (Table S3), and congener mass below LOQ was given a value of zero. Method blanks consisting of combusted diatomaceous earth were extracted and analyzed along with the samples. Six OH-PCB congeners were removed from the sediment sample data set because they contributed the majority of contamination in the OH-PCB method blanks (70–89% of the total mass). Prior to their removal these six OH-PCB congeners represented 17–55% (29 ± 10%) of the total identified OH-PCB mass in the sediment samples. OH-PCBs are not certified in the NIST sediment SRM; however, analysis of PCBs in the SRM using the same extraction and quantification procedure resulted in 15 ± 15 % average difference between measured and certified values.34
RESULTS AND DISCUSSION
Concentration of OH-PCBs in surficial sediment
ΣOH-PCB58 in the 20 samples ranged from 0.20 to 26 ng/g dw with a mean of 8.5 ± 5.9 ng/g dw not including two outliers. Individual congener concentrations ranged from below LOQ to 10.24 ng/g dw (Table S4). The spatial extent of measured ΣOH-PCB58 in IHSC is shown in Figure 1. There is a general trend of increasing OH-PCBs from the harbor to the branches of the canal. Concentrations of ΣOH-PCB58 were higher in the main channel (p = 0.089) and branches (p = 0.036) than in the harbor.
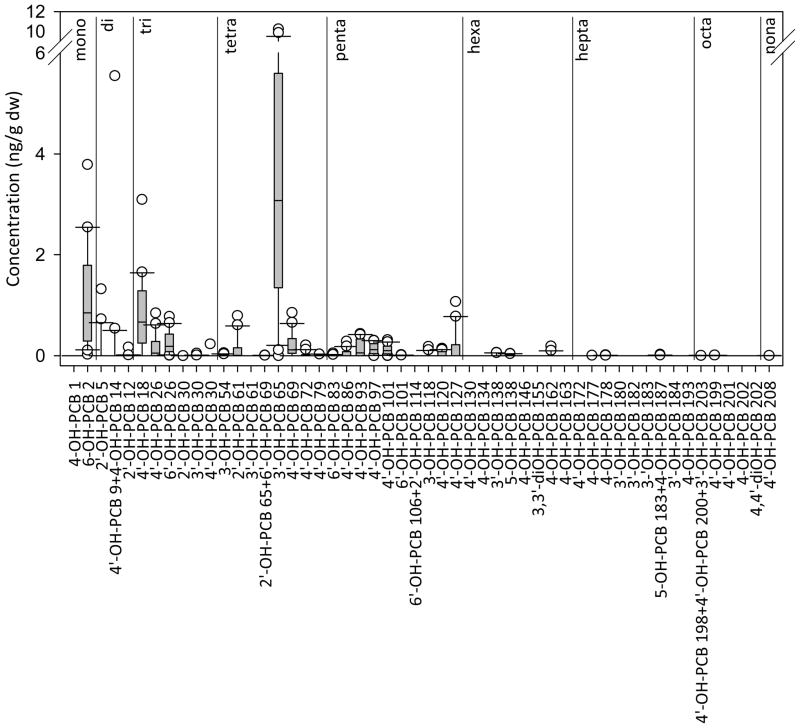
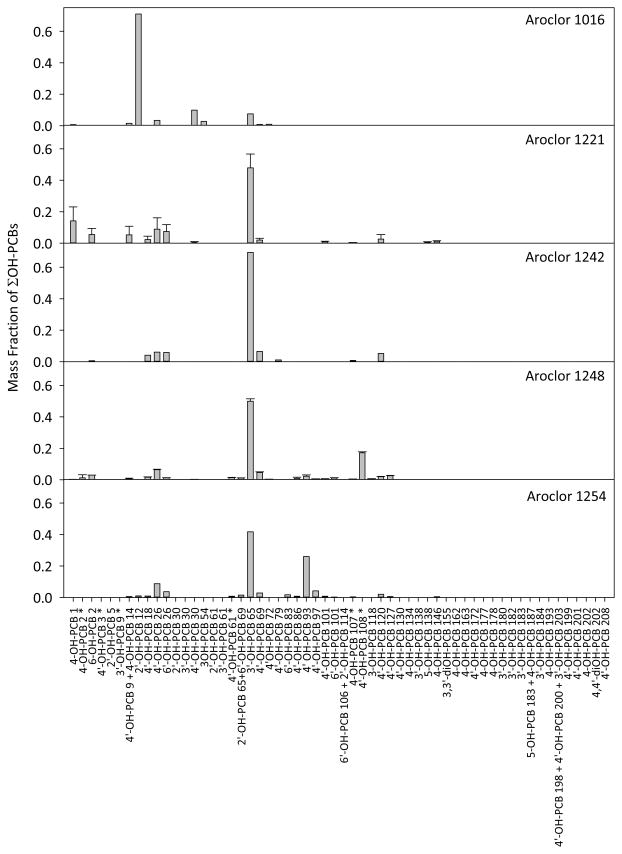
Of the 58 mono- and di-hydroxylated PCBs analyzed, 40 mono-hydroxylated PCBs were detected in the sediment samples. Neither di-hydroxylated PCB was detected. Several peaks in the chromatograms were unidentifiable due to lack of availability of standards. The most prominent identifiable congeners measured were 3′-OH-PCB 65, 6-OH-PCB 2, and 4′-OH-PCB 18 which comprised an average of 46%, 16%, and 9%, respectively, of the sum concentrations in the 20 samples. Though mono- to nona-chlorinated congeners were detected in the samples, the most prominent congeners had five or fewer chlorines (Figure 2).
Figure 2.
Distribution of concentration of OH-PCBs in sediment samples (n = 20). Congeners are listed from low to high PCB number and are grouped according to homologue. Data are plotted as box plots with the median indicated by the bold horizontal line, the two middle quartiles shown as polyhedrons above and below the median and the 95th percentiles shown as the horizontal lines connected by a solid vertical line. Outlier points are indicated by open circles. Congener mass below LOQ was given a value of zero. The most prominent congener, 3′-OH-PCB 65, is also the most prominent congener in Aroclors 1221, 1242, 1248, and 1254. Data are in Table S4.
ΣOH-PCB concentrations show a weak positive relationship with total organic carbon (TOC) in the sediment (R2 = 0.20, p = 0.048, Figure S1). Concentrations of OH-PCBs were normalized to TOC and ranged from 5.0 to 440 ng/g TOC dw (160 ± 110 ng/g TOC dw). These TOC-normalized concentrations are much higher than concentrations (0.23 and 0.99 ng/g TOC) reported by Ueno et al. in particulates from two surface water samples.24
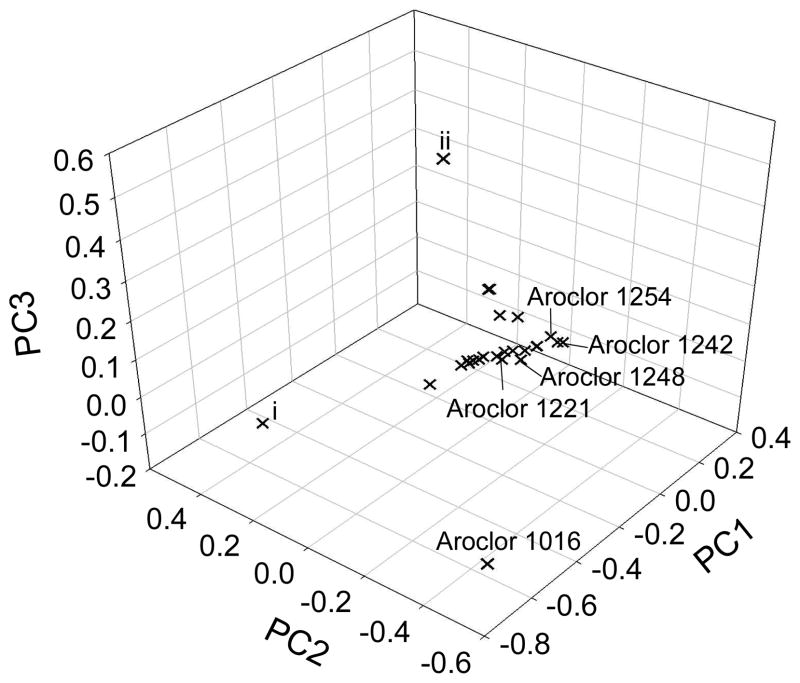
Evaluation of the variability in the OH-PCB congener profiles is limited by the lack of availability of all OH-PCB standards, yet comparisons among samples are still illuminating. PCA shows that most samples are similar (Figure 3). Indeed, the OH-PCB congener profiles of 18 of the 20 samples are very similar (cosθ = 0.9 ± 0.07). One sample in the harbor and one sample in the main channel have very different profiles (cosθ = 0.3 ± 0.2). Those two samples also have OH-PCB concentrations much lower than the rest of the samples. The difference in profiles and concentration is due to the low number of congeners detected in these samples and because the few congeners detected in those samples were at lower concentration than in the other samples.
Figure 3.
Principal Component Analysis of congener profiles of sediment (n=20) and Aroclors (n=5). The first 3 principal components explained 84% of the variance. Samples i and ii are different from the average sediment profile and have lower concentrations of OH-PCBs than the other samples.
Comparison of OH-PCBs with PCBs in surficial sediment
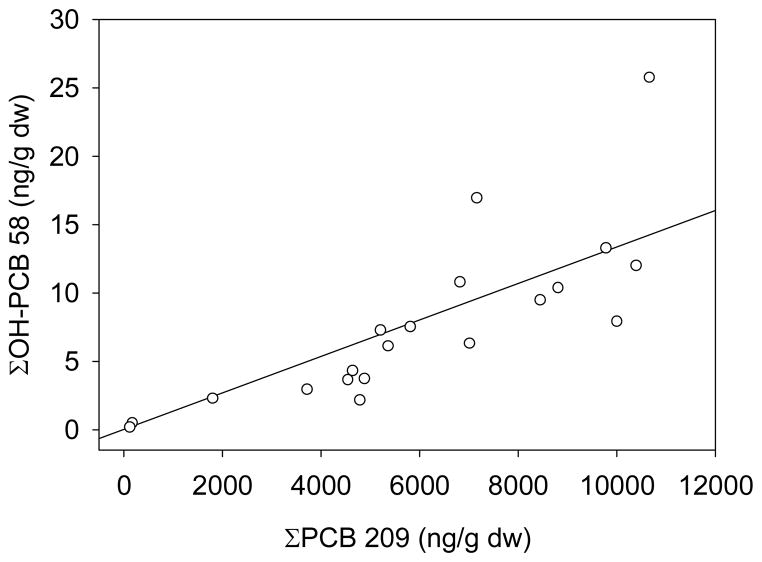
There was a significant increase in ΣOH-PCB concentration with increasing ΣPCB concentration (R2 = 0.61, p < 0.0001, Figure 4). Individual congeners were also investigated to determine whether there was evidence of PCB degradation to OH-PCBs in the sediment. Every combination of OH-PCB and PCB was analyzed to determine whether the relationship was significant and whether it was a positive or negative relationship. There are 8268 theoretically-possible pairs from 52 OH-PCB and 159 PCB congeners or co-eluting congeners. However, each pair was only evaluated if both congeners in the pair were measured in at least 3 samples, and 2764 pairs met these criteria. The Pearson’s Correlation Coefficient and p-value were determined for each congener pair (Figure S2), and 713 (26%) had significant relationships (p < 0.05).
Figure 4.
Comparison of ΣPCB209 with ΣOH-PCB58 with regression. Each open circle represents one sample. The line represents the regression (R2 = 0.61, p < 0.0001).
Of those significant pairs, almost all correlations (705 pairs) were positive. Of the pairs with a statistically-significant relationship, 99 could theoretically be a degradation pair and 614 could not We identified a degradation pair as an OH-PCB with equal or fewer chlorines as the PCB and chlorines in the same positions around the biphenyl as the PCB. By this definition we are assuming degradation would take the form of dechlorination but not chlorination or rearrangement of the chlorines.
There are 120 statistically significant pairs involving OH-PCBs detected in sediment but not Aroclors, and of those pairs only four could theoretically be a degradation pair. Thus it is unlikely that the presence of OH-PCBs in the sediment is solely due to PCB degradation in-situ. Figure S3 shows the relationships and structures of four example pairs.
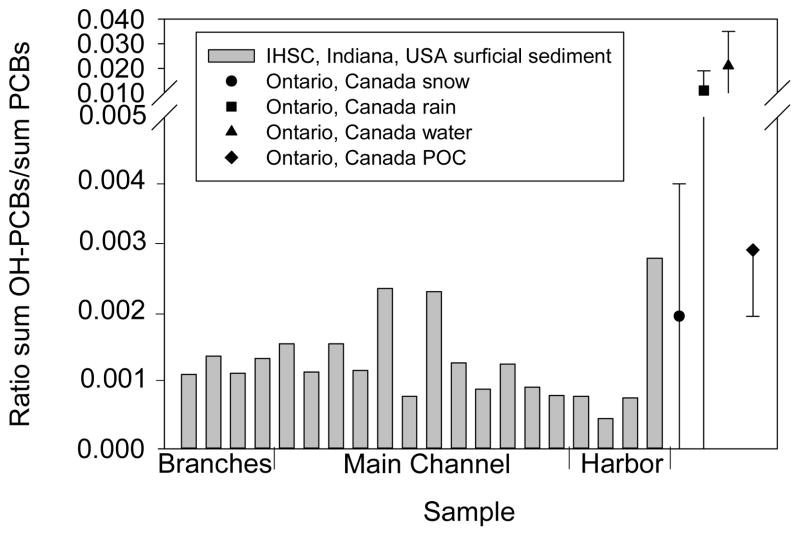
The ratio of ΣOH-PCBs to ΣPCBs was calculated for each sample in order to investigate whether the source of OH-PCBs was the same from sample to sample. Ratios ranged an order of magnitude from 4.5×10 −4 to 2.9×10 −3, but there is no clear trend from harbor to branches of IHSC (Figure 5). Higher ratios were detected in one sample from the harbor and two samples from the main channel (p < 0.0001). The ratios in IHSC samples are mostly lower than those determined by Ueno et al. from snow, rain, surface water, and particulate organic carbon samples (Figure 5).24 This difference could be from Ueno et al.’s inclusion of unidentified OH-PCBs for determining their ratios or from differences in the source of OH-PCB contamination.
Figure 5.
Ratio of ΣOH-PCBs/ΣPCBs in sediment from IHSC and snow, rain, surface water, and particulate organic carbon (POC) from Ontario, Canada.24 Sediment samples are ordered from the branches to the harbor of IHSC.
Aroclor analysis and discussion of potential sources
There are several possible sources of OH-PCBs in the sediment, including their presence in the original Aroclors. We analyzed five original Monsanto Aroclors (1016, 1221, 1242, 1248, and 1254) and detected OH-PCBs in every Aroclor (Table S5). The most prominent OH-PCB congener identified in sediment, 3′-OH-PCB 65, is also the most prominent congener in Aroclors 1221, 1242, 1248, and 1254. In total, 75% of detected congeners in sediment were also detected in at least one Aroclor. PCA shows most Aroclors are similar to each other and to the samples (Figure 3). The congener profiles (Figure 6) of Aroclors 1221, 1242, 1248, and 1254 were similar to the samples (cosθ = 0.8 ± 0.2) and each other (cosθ = 0.9 ± 0.07), while the congener profile of Aroclor 1016 was dissimilar to the samples (cosθ = 0.1 ± 0.03) and the other Aroclors (cos = 0.1 ± 0.0). The difference in congener profiles between Aroclor 1016 and the other Aroclors could be explained based on the process that Monsanto used to produce Aroclor 1016. Aroclor 1016 was distilled from Aroclor 1242 in order to remove higher chlorinated PCBs.1 This process presumably also removed some OH-PCBs.
Figure 6.
Congener profiles of OH-PCBs in original commercial Aroclors. Aroclor 1221 and Aroclor 1248 profiles represent the average and error bars represent the standard deviation of three replicates. Asterisks indicate congeners not included in the sediment data set. The profile of Aroclor 1016 is different from the samples and other Aroclors. Aroclor data are in Table S5.
OH-PCBs could form through degradation of PCBs in the sediment; however, we did not detect di-hydroxylated PCBs and there is no known pathway for the microbial formation of mono-hydroxhylated PCBs. Effluent from sewage treatment plants (STPs) could also contain OH-PCBs from human excretion of OH-PCBs in feces or as disinfection by-products from the reaction of PCBs with hydroxyl radicals used in treating the effluent immediately prior to discharge. In the only published study of OH-PCBs in abiotic matrices, Ueno et al. concluded that STPs are a likely source of OH-PCBs into the aquatic environment after measuring the highest concentrations in water samples collected within 0.5–2 km of STPs.24 An East Chicago STP with a permit to treat municipal, industrial, commercial, and agricultural waste discharges its effluent into the East Branch of the Grand Calumet River, 5.5 km upstream of IHSC. We note that the OH-PCBs we detected in IHSC sediment are not the same OH-PCBs commonly detected in Americans’ human blood serum.20, 40 OH-PCBs could also be industrial byproducts or formed in the atmosphere or in natural water through reaction of PCBs and OH radicals.41–45 Because a quarter of identified detected OH-PCBs could not be accounted for by Aroclor contamination, these additional sources require further investigation.
In this paper we reported levels of OH-PCBs in sediment and relative levels in Aroclors for the first time. These measurements suggest that the presence of OH-PCBs in sediment is at least partly due to OH-PCB contamination of the original Aroclors. Our discovery of OH-PCBs in Aroclors is significant because it is likely that OH-PCB contamination exists in sediment anywhere that PCB contamination from Aroclors is present.
Supplementary Material
Acknowledgments
We are grateful to the U.S. EPA Great Lakes National Program Office for donating the sampling ship and support staff. At the University of Iowa, we thank Dr. Larry Roberston for the Aroclors, Dr. Hans Joachim-Lehmler for synthesizing the diazomethane, Jon Durst and Collin Just for their contributions to instrument analysis, and Dr. Kai Wang for statistics advice. We thank Grazina Pacepavicius and Dr. Mehran Alaee at Environment Canada for confirmation analysis of OH-PCBs in sediment. This work was funded as part of the Iowa Superfund Research Program, NIH P42 ES013661, and a GAANN fellowship from the Department of Education, P200A09035011.
Footnotes
Supporting Information Available. Supporting Information includes figures of correlations and tables of analytical details, nomenclature, and congener-specific sample and Aroclor data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Erickson MD, Kaley RG. Applications of polychlorinated biphenyls. Environ Sci Pollut R. 2011;18(2):135–151. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- 2.Letcher RJ, Klasson-Wehler E, Bergman A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In: Paasivirta J, editor. New Types of Persistent Halogenated Compounds. Vol. 3. Springer-Verlag; Berlin-Heidelberg: 2000. pp. 315–359. [Google Scholar]
- 3.Hilal SH, Karickhoff SW, Carreira LA. Prediction of the solubility, activity coefficient and liquid/liquid partition coefficient of organic compounds. Qsar Comb Sci. 2004;23(9):709–720. [Google Scholar]
- 4.Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect. 2013;121(6):657–62. doi: 10.1289/ehp.1206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodavanti PRS, Ward TR, Derr-Yellin EC, McKinney JD, Tilson HA. Increased [H-3]phorbol ester binding in rat cerebellar granule cells and inhibition of Ca-45(2+) buffering in rat cerebellum by hydroxylated polychlorinated biphenyls. Neurotoxicology. 2003;24(2):187–198. doi: 10.1016/S0161-813X(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 6.Londono M, Shimokawa N, Miyazaki W, Iwasaki T, Koibuchi N. Hydroxylated PCB induces Ca2+ oscillations and alterations of membrane potential in cultured cortical cells. J Appl Toxicol. 2010;30(4):334–342. doi: 10.1002/jat.1501. [DOI] [PubMed] [Google Scholar]
- 7.Park HY, Park JS, Sovcikova E, Kocan A, Linderholm L, Bergman A, Trnovec T, Hertz-Picciotto I. Exposure to Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in the Prenatal Period and Subsequent Neurodevelopment in Eastern Slovakia. Environ Health Persp. 2009;117(10):1600–1606. doi: 10.1289/ehp.0900611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braathen M, Mortensen AS, Sandvik M, Skare JU, Arukwe A. Estrogenic Effects of Selected Hydroxy Polychlorinated Biphenyl Congeners in Primary Culture of Atlantic Salmon (Salmo salar) Hepatocytes. Arch Environ Con Tox. 2009;56(1):111–122. doi: 10.1007/s00244-008-9163-0. [DOI] [PubMed] [Google Scholar]
- 9.Gabrielsen KM, Villanger GD, Lie E, Karimi M, Lydersen C, Kovacs KM, Jenssen BM. Levels and patterns of hydroxylated polychlorinated biphenyls (OH-PCBs) and their associations with thyroid hormones in hooded seal (Cystophora cristata) mother-pup pairs. Aquat Toxicol. 2011;105(3–4):482–491. doi: 10.1016/j.aquatox.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Meerts IATM, Assink Y, Cenijn PH, van den Berg JHJ, Weijers BM, Bergman A, Koeman JH, Brouwer A. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci. 2002;68(2):361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- 11.Otake T, Yoshinaga J, Enomoto T, Matsuda M, Wakimoto T, Ikegami M, Suzuki E, Naruse H, Yamanaka T, Shibuya N, Yasumizu T, Kato N. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ Res. 2007;105(2):240–246. doi: 10.1016/j.envres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Ptak A, Ludewig G, Robertson L, Lehmler HJ, Gregoraszczuk EL. In vitro exposure of porcine prepubertal follicles to 4-chlorobiphenyl (PCB3) and its hydroxylated metabolites: Effects on sex hormone levels and aromatase activity. Toxicol Lett. 2006;164(2):113–122. doi: 10.1016/j.toxlet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Schuur AG, Brouwer A, Bergman A, Coughtrie MWH, Visser TJ. Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem-Biol Interact. 1998;109(1–3):293–297. doi: 10.1016/s0009-2797(97)00140-3. [DOI] [PubMed] [Google Scholar]
- 14.Stadnicki SS, Allen JR. Toxicity of 2,2′,5,5′-Tetrachlorobiphenyl and Its Metabolites, 2,2′,5,5′-Tetrachlorobiphenyl-3,4-Oxide and 2,2′,5,5′-Tetrachlorobiphenyl-4-O1 to Cultured-Cells Invitro. B Environ Contam Tox. 1979;23(6):788–796. doi: 10.1007/BF01770043. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto HA, Yoshimur H. Metabolic Studies on Polychlorinated Biphenyls .3. Complete Structure, Acute Toxicity Metabolites of 2, 4, 3′4′-Tetrachlorobiphenyl. Chem Pharm Bull. 1973;21(10):2237–2242. doi: 10.1248/cpb.21.2237. [DOI] [PubMed] [Google Scholar]
- 16.Fernie KJ, Letcher RJ. Historical Contaminants, Flame Retardants, and Halogenated Phenolic Compounds in Peregrine Falcon (Falco peregrinus) Nestlings in the Canadian Great Lakes Basin. Environ Sci Technol. 2010;44(9):3520–3526. doi: 10.1021/es100400n. [DOI] [PubMed] [Google Scholar]
- 17.Gilroy EA, Muir DG, McMaster ME, Darling C, Campbell LM, de Solla SR, Parrott JL, Brown SB, Sherry JP. Polychlorinated biphenyls and their hydroxylated metabolites in wild fish from wheatley Harbour Area of Concern, Ontario, Canada. Environ Toxicol Chem. 2012;31(12):2788–2797. doi: 10.1002/etc.2023. [DOI] [PubMed] [Google Scholar]
- 18.Jorundsdottir H, Lofstrand K, Svavarsson J, Bignert A, Bergman A. Organochlorine Compounds and Their Metabolites in Seven Icelandic Seabird Species - a Comparative Study. Environ Sci Technol. 2010;44(9):3252–3259. doi: 10.1021/es902812x. [DOI] [PubMed] [Google Scholar]
- 19.Letcher RJ, Gebbink WA, Sonne C, Born EW, McKinney MA, Dietz R. Bioaccumulation and biotransformation of brominated and chlorinated contaminants and their metabolites in ringed seals (Pusa hispida) and polar bears (Ursus maritimus) from East Greenland. Environ Int. 2009;35(8):1118–1124. doi: 10.1016/j.envint.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Marek RF, Thorne PS, Wang K, DeWall J, Hornbuckle KC. PCBs and OH-PCBs in Serum from Children and Mothers in Urban and Rural U.S. Communities. Environ Sci Technol. 2013;47:3353–3361. doi: 10.1021/es304455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomiyama K, Murata S, Kunisue T, Yamada TK, Mizukawa H, Takahashi S, Tanabe S. Polychlorinated Biphenyls and Their Hydroxylated Metabolites (OH-PCBs) in the Blood of Toothed and Baleen Whales Stranded along Japanese Coastal Waters. Environ Sci Technol. 2010;44(10):3732–3738. doi: 10.1021/es1003928. [DOI] [PubMed] [Google Scholar]
- 22.Nomiyama K, Yonehara T, Yonemura S, Yamamoto M, Koriyama C, Akiba S, Shinohara R, Koga M. Determination and Characterization of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in Serum and Adipose Tissue of Japanese Women Diagnosed with Breast Cancer. Environ Sci Technol. 2010;44(8):2890–2896. doi: 10.1021/es9012432. [DOI] [PubMed] [Google Scholar]
- 23.Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ. Analysis of hydroxylated metabolites of PCBs (OH-PCBs) and other chlorinated phenolic compounds in whole blood from Canadian Inuit. Environ Health Persp. 2000;108(7):611–616. doi: 10.1289/ehp.00108611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno D, Darling C, Alaee M, Campbell L, Pacepavicius G, Teixeira C, Muir D. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ Sci Technol. 2007;41(6):1841–1848. doi: 10.1021/es061539l. [DOI] [PubMed] [Google Scholar]
- 25.Borja J, Taleon DM, Auresenia J, Gallardo S. Polychlorinated biphenyls and their biodegradation. Process Biochem. 2005;40(6):1999–2013. [Google Scholar]
- 26.Mackova M, Uhlik O, Lovecka P, Viktorova J, Novakova M, Demnerova K, Sylvestre M, Macek T. Bacterial Degradation of Polychlorinated Biphenyls. In: Barton LL, Mandl M, Loy A, editors. Geomicrobiology: Molecular and Environmental Perspective. Springer Netherlands; pp. 347–366. [Google Scholar]
- 27.Pieper DH, Seeger M. Bacterial metabolism of polychlorinated biphenyls. J Mol Microb Biotech. 2008;15(2–3):121–138. doi: 10.1159/000121325. [DOI] [PubMed] [Google Scholar]
- 28.Flanagan WP, May RJ. Metabolite Detection as Evidence for Naturally-Occurring Aerobic Pcb Biodegradation in Hudson River Sediments. Environ Sci Technol. 1993;27(10):2207–2212. [Google Scholar]
- 29.Albro PW, Parker CE. Comparison of the Compositions of Aroclor-1242 and Aroclor-1016. J Chromatogr. 1979 Feb;169:161–166. doi: 10.1016/0021-9673(75)85041-2. [DOI] [PubMed] [Google Scholar]
- 30.Bowes GW, Mulvihill MJ, Decamp MR, Kende AS. Gas-Chromatographic Characteristics of Authentic Chlorinated Dibenzofurans - Identification of 2 Isomers in American and Japanese Polychlorinated Biphenyls. J Agr Food Chem. 1975;23(6):1222–1223. doi: 10.1021/jf60202a045. [DOI] [PubMed] [Google Scholar]
- 31.Bowes GW, Mulvihill MJ, Simoneit BRT, Burlingame AL, Risebrough RW. Identification of Chlorinated Dibenzofurans in American Polychlorinated Biphenyls. Nature. 1975;256(5515):305–307. doi: 10.1038/256305b0. [DOI] [PubMed] [Google Scholar]
- 32.Vos JG, Koeman JH, van der Maas HL, ten Noever de Brauw MC, de Vos RH. Identification and toxicological evaluation of chlorinated dibenzofuran and chlorinated naphthalene in two commercial polychlorinated biphenyls. Food and Cosmetics Toxicology. 1970;8(6):625–633. doi: 10.1016/s0015-6264(70)80451-5. [DOI] [PubMed] [Google Scholar]
- 33.Status of restoration activities in Great Lakes areas of concern: A special report. International Joint Commission; 2003. [Google Scholar]
- 34.Martinez A, Norstrom K, Wang K, Hornbuckle KC. Polychlorinated biphenyls in the surficial sediment of Indiana Harbor and Ship Canal, Lake Michigan. Environ Int. 2010;36(8):849–854. doi: 10.1016/j.envint.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez A, Wang K, Hornbuckle KC. Fate of PCB congeners in an industrial harbor of Lake Michigan. Environ Sci Technol. 2010;44(8):2803–2808. doi: 10.1021/es902911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buser HR, Zook DR, Rappe C. Determination of Methyl Sulfone-Substituted Polychlorobiphenyls by Mass-Spectrometric Techniques with Application to Environmental-Samples. Anal Chem. 1992;64(10):1176–1183. [Google Scholar]
- 37.Maervoet J, Covaci A, Schepens P, Sandau CD, Letcher RJ. A reassessment of the nomenclature of polychlorinated biphenyl (PCB) metabolites. Environ Health Persp. 2004;112(3):291–294. doi: 10.1289/ehp.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Development Core Team. R: A language and environment for statistical computing, version 2.13.1. Vienna, Austria: 2011. http://www.R-project.org/ [Google Scholar]
- 39.Morrison RD, Murphy BL. Environmental Forensics: Contaminant specific guide. Elsevier Academic Press; 2006. Polychlorinated Biphenyls. [Google Scholar]
- 40.Patterson DG, Wong LY, Turner WE, Caudill SP, Dipietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL. Levels in the US Population of those Persistent Organic Pollutants (2003–2004) Included in the Stockholm Convention or in other Long-Range Transboundary Air Pollution Agreements. Environ Sci Technol. 2009;43(4):1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- 41.Anderson PN, Hites RA. OH radical reactions: The major removal pathway for polychlorinated biphenyls from the atmosphere. Environ Sci Technol. 1996;30(5):1756–1763. [Google Scholar]
- 42.Brubaker WW, Hites RA. Gas phase oxidation products of biphenyl and polychlorinated biphenyls. Environ Sci Technol. 1998;32(24):3913–3918. [Google Scholar]
- 43.Mandalakis M, Berresheim H, Stephanou EG. Direct evidence for destruction of polychlorobiphenyls by OH radicals in the subtropical troposphere. Environ Sci Technol. 2003;37(3):542–547. doi: 10.1021/es020163i. [DOI] [PubMed] [Google Scholar]
- 44.Sedlak DL, Andren AW. The Effect of Sorption on the Oxidation of Polychlorinated-Biphenyls (Pcbs) by Hydroxyl Radical. Water Res. 1994;28(5):1207–1215. [Google Scholar]
- 45.Totten LA, Eisenreich SJ, Brunciak PA. Evidence for destruction of PCBs by the OH radical in urban atmospheres. Chemosphere. 2002;47(7):735–746. doi: 10.1016/s0045-6535(01)00326-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.