Abstract
Pesticides applied in agriculture can affect the structure and function of nontarget populations at lower doses and for longer timespans than predicted by the current risk assessment frameworks. We identified a mechanism for this observation. The populations of an aquatic invertebrate (Culex pipiens) exposed over several generations to repeated pulses of low concentrations of the neonicotinoid insecticide (thiacloprid) continuously declined and did not recover in the presence of a less sensitive competing species (Daphnia magna). By contrast, in the absence of a competitor, insecticide effects on the more sensitive species were only observed at concentrations 1 order of magnitude higher, and the species recovered more rapidly after a contamination event. The underlying processes are experimentally identified and reconstructed using a simulation model. We conclude that repeated toxicant pulse of populations that are challenged with interspecific competition may result in a multigenerational culmination of low-dose effects.
Introduction
A rapid decline in biodiversity has been observed in many ecosystems.1 The responsible stressors identified to date for aquatic systems include morphological degradation, high nutrient concentrations, pesticides, suspensions, and organic pollution, which threaten biota in the majority of streams worldwide.2,3 The long-term field relevance of pulsed pesticide exposure in affecting aquatic biodiversity on a landscape level was first identified in central Europe4 and was then validated globally.5,6 These investigations show that population effects in the field are a factor of 10 to 100 lower than those predicted to be safe, even by the most conservative “first tier” risk characterization in the EU.7 Such a discrepancy between laboratory ecotoxicity tests and field observations on wildlife has recently also been identified for ionizing radiation.8 Additionally recovery from toxicant exposure takes unexpectedly long in the field.9 These mismatches between toxicant effects observed in the laboratory and the field raise the question of the actual degree of conservativeness of the risk assessment scheme.
A potential mechanism for these mismatches may be that the individual sensitivity and the recovery time of the population after toxicant stress are increased by environmental stressors present in the field. Examples include the increased sensitivity of marine crustaceans to copper in the presence of UV radiation,10 the increased sensitivity of amphibians to agrochemicals under the pressure of trematode infection11 and the increased recovery time of aquatic invertebrates under the pressure of interspecific competition.12,13 In some situations, more than one environmental stressor may interact with toxicant exposure. For example, the combined action of predation and parasitism has been shown to result in dramatic impacts on the growth rate of Daphnia magna populations exposed to the pesticide carbaryl.14 Such investigations highlight the need to account for stressor interactions in risk assessment of toxicant effects and conservation planning.15,16 However, the effects of combined stressors, such as toxicants and environmental parameters, have usually been studied within the timespan of one generation. In the present investigation, we aimed at identifying the transgenerational effects of such combined stressors, as they represent realistic scenarios in most field situations.
Materials and Methods
Test system
The experiment comprised 36 test systems of 5.5 L (so-called nanocosms) with populations of the mosquito Culex pipiens. In 12 nanocosms, Culex populations were left to develop alone. In 24 nanocosms, Culex populations developed in the presence of the water flea Daphnia magna as competitor. The populations were exposed to five pulses of the insecticide thiacloprid over a period of 277 days (details of the exposure see below). The populations in the nanocosm systems were monitored at least twice a week by noninvasive image analysis. The populations were photographed using a digital camera (Camedia C-4000 Zoom; Olympus, Melville, NY). In order to obtain a high-quality image that was free from reflections, the camera was fixed to one end of a rectangular lightproof box (length, 0.7 m), whereas the opposite end of the box was fitted against the front surface of the test vessel. The organisms were illuminated from above (light intensity below net cover, ∼46 400 lx). To increase the contrast of the illuminated organisms, a black plastic film was taped to the back of the test vessels. The digital camera had the following settings: image resolution 2048 × 1536 pixels, shutter speed 1/30 s, aperture F2.8, photosensitivity ISO 400, 3× optical zoom, and focal depth in the middle of the test vessel. The photographs were evaluated by an image analysis technique that consisted of two steps. In the first step, Daphnia and Culex larvae were detected as moving objects during swimming with algorithms adapted from Liess et al. (2006). In the second step, mosquito larvae that had gathered in a motionless state below the water surface for breathing were detected.
The nanocosm system enables a reliable measurement of the abundance and size structure of populations of Daphnia and Culex larvae. A comparison between true abundance and the abundance monitored by the nanocosm system showed residual variances of 0.3% for Daphnia (r2 = 0.997, n = 13) and 11% for Culex larvae (r2 = 0.89, n = 11). A detailed description of the nanocosm test system including its construction as well as the procedure and validation of the image analysis technique is described elsewhere.17
Aquatic Populations - Feeding and Water Quality
The one-species nanocosms were initiated with 15 first-instar Culex larvae (obtained from the Federal Environment Agency, UBA, Berlin, Germany). The two-species nanocosms were initiated with 15 first-instar Culex larvae and 15 neonates of Daphnia magna, clone B (obtained from Bayer CropScience, Monheim, Germany). The populations were cultured in 5.5 L cylindrical glass vessels (Harzkristall, Derenburg, Germany). The glass vessels were filled with 4 L of Elendt M7 medium.18 Each glass vessel contained 500 g of washed aquarium sand (diameter 1–2 mm) at the bottom, which served as a support for bacteria to promote self-purification of the test system. In both setups, the populations were fed three times a week with an equal amount of food. The food was given as a suspension of ground dog biscuits (Hd-H biscuits, obtained from ssniff Spezialitäten GmbH, Germany), stinging nettle (Folia urticae) and batch-cultured green algae (Desmodesmus subspicatus). The total carbon content of the food suspension was 0.9 mg/L. In the first two weeks, the double amount of food was given to support the establishment of the populations. The test vessels were covered with a net (polyester, 0.5 mm mesh size, obtained from Brettschneider, Heimstetten, Germany) to prevent the escape of adult mosquitoes. Two holes of 1 cm in diameter were made in the net to enable access to the populations. One opening was used to feed adult mosquitoes above the water surface. We closed this opening with a rolled-up pad of cotton wool that was soaked in a saturated solution of glucose and was replaced three times a week. Another opening was used to aerate the culture water three times a day for 15 min via silicone tubing (14 cm below the surface of the water; diameter, 4 mm; tapered end, 0.5 mm). The studies were performed at 20 °C. The photoperiod was controlled (16:8 h light:dark), and lighting was provided by a 70 W, cool-white fluorescent tube that was situated 10 cm above the test vessels. The biofilm on the front window of the test vessel was removed once a week with a magnetic aquarium cleaner.
Water quality monitoring showed that the concentrations of ions were such that no negative effects on Daphnia or Culex larvae would be expected (mean ± S.E., n = 102; nitrite, 0.002 ± 0.001 mg/L; ammonium, 0.027 ± 0.02; phosphate, 0.19 ± 0.04; nitrate, 6.4 ± 0.4 mg/L - based on five measurements). Other parameters, such as conductivity (920 ± 11.8 μS/cm), temperature (20.7 ± 0.5 °C), pH (7.7 ± 0.05), and oxygen levels (95 ± 2.3%) were regularly checked with instruments of WTW (Weilheim, Germany) and Knick (Berlin, Germany).
Exposure to Thiacloprid
The neonicotinoid insecticide thiacloprid (CAS: 111988–49–9) was obtained from Agrar-Handel and Transport (Schafstädt, Germany) as the commercial product Calypso (suspension concentrate) with 480 g/L of the active ingredient (Bayer CropScience Deutschland, Monheim, Germany). We prepared a toxicant stock solution of 10 g/L in distilled water with continuous stirring during the 24 h before exposure. The stock solution was diluted with M7 medium and appropriate quantities were added to the nanocosm test systems. The populations of Culex larvae and Daphnia were not fed a day before and on the day of contamination to reduce sorption of the toxicant to particulate organic matter. Twenty-four hours after each pulse contamination, 80% of the water in all the vessels was replaced with fresh, uncontaminated Elendt M7 medium. Thiacloprid pulses were carried out at the following nominal concentrations: control, 3.3 μg/L, 10 μg/L and 33 μg/L. Each treatment concentration was replicated three times in the one-species setup with Culex larvae, and six times in the two-species setup with Culex and Daphnia populations. Prior to the first pulse, the replicates were assorted to the treatment concentrations in such a way that the population abundance at each concentration level was similar with respect to the mean and the variance. In the two-species setup with Culex and Daphnia populations, three replicates of Daphnia populations went to extinct (two replicates at 10 μg/L and 1 replicate at 33 μg/L). Because our focus was to test the influence of competition on the recovery of Culex larvae, these vessels were removed from the analysis. Pulse contaminations took place at day 25, 66, 144, 214, and 277 from test start. In the two-species setup, the second pesticide pulse was delayed to day 81.
For validation of exposure concentration of thiacloprid in the nanocosm test systems, samples of 200 mL volume were taken. Thiacloprid was extracted by solid-phase extraction with Chromabond Easy 6 mL columns (Macherey-Nagel & Company KG, Düren, Germany), eluted in acetonitril and concentrated to a volume of 1 mL. Measurements were performed with RP-HPLC according to the norm DIN EN ISO 11369 (high-performance liquid chromatography system with Diodenarray Detektor II Series 2000, binary pump, autosampler, column oven [30 °C], Perkin-Elmer, Wellesley, MA). The injection volume was 100 μL, dissolved in acetonitril/water solution with gradient-grade pump program (from 20% up to 80% of acetonitril). The detection limit was 0.02 μg/L. The columns were LiChrospher 60 RP-Select B (250 × 4 mm, 5 μm particle size, Merck KGaA, Darmstadt, Germany). The measurements were performed by Kommunale Wasserwerke Leipzig (Leipzig, Germany).
The pulse contamination was terminated by a water exchange after 24 h following each pulse. To measure the actual thiacloprid concentration during the pulse, water samples were taken directly before this water change. On average, the measured concentrations were 6.1% less than the nominal concentrations and reached 98.2% of the nominal concentration at 3.3 μg/L, 95.7% at 10 μg/L, 87.6% at 33 μg/L with a coefficient of variation of less than 16%. The measured concentrations were not increasing with the number of pulse contamination (one-way ANOVA, n = 48, F = 1.2, df =4, p = 0.32). Due to the fact that the measured exposure concentrations did not differ strongly from the nominal concentrations, all exposure concentrations are given as nominal values. To quantify the reduction of thiacloprid concentration after the 24 h pulse, the highest two concentrations 10 μg/L and 33 μg/L were randomly sampled with increasing time lag to the water change. On average, the nominal concentrations were reduced to percentages of 13.5, 3.4, 0.5, and 0.1% at 2, 15, 30, and 58 days after the pesticide pulse, respectively.
Data Analyses
All analyses and construction of plots were conducted with the statistical software R.19 A level of p = 0.05 was used to define significance for statistical analyses unless otherwise stated. The abundance of Culex larvae and biomass of Daphnia is given as mean values between two pulse contaminations. Differences between the means of the control and the exposed treatments were calculated using one-way analysis of variance followed by Dunnett’s post hoc multiple-comparison test (time-by-time ANOVA, adapted from Diggle et al.20 Data were square root-transformed to fulfill the conditions of data normality and homoscedasticity.
The long-term response of Culex populations was given as mean population density after the third contamination (day 144) and was analyzed with respect to possible interactive effects between the two factors of concentration and competition level. In order to distinguish the main effect from interaction effects, the population density was normalized to the control level of the two competition setups. Before normalization, the population density was square root-transformed to fulfill the conditions of data normality and homoscedasticity. After performing a two-way ANOVA, the interaction effects were analyzed as main effects of one factor at fixed values of the other factor. For each competition setup, significant differences between the means of the control and the exposed treatments were determined by Dunnett’s post hoc multiple-comparison test. For each treatment concentration, the means of the two competition setups were compared by Student’s t test; a level of p = 0.017 was used to define significance (Bonferroni correction in case of three pairwise comparisons).
The relevance of the competitive pressure between the two species was analyzed by linear regression. We related the abundance of Culex pipiens larvae with the biomass of Daphnia magna. The biomass of Daphnia population was used as abundance and individual size is integrated to an integrated measure of competitive challenge. Biomass was calculated as the sum of individual dry weights W (μg). These individual dry weights W were calculated on the basis of the detected body lengths L (mm). For Daphnia, we used the relationship W = 1.5 × 10–8 L2.84.21 The abundance of Culex pipiens and biomass of Daphnia populations were given as the mean values of each replicate after the first contamination (day 25). To eliminate the long-term population dynamic from the analysis, both variables were normalized to the control.
Individual-Based Simulation Model
To verify the mechanism proposed from the experimental results, we applied an individual-based simulation model of two competing species. The aim of this modeling exercise was not to tailor the model exactly to the experimental setup but to approximate the system and the most relevant mechanisms by simplified assumptions. We compared the pattern in population dynamic observed in the experiment with those produced by a simple mechanism for competition (i.e., the reduction of reproduction). As the model includes only the mechanism proposed in the current paper, comparable pattern of experimental observations and modeling results would demonstrate that the proposed mechanism is plausible.
Two generic species were modeled, one sensitive (representing Culex) and the other insensitive (representing Daphnia), using an agent-based model. The model includes the entire life cycle, comprising maturation, reproduction and mortality. For Culex, female individuals reproduce sexually, whereas female Daphnia reproduces by cloning. Competition for a common resource affects reproduction (eq 1). Additionally we assume that some individuals are more strongly affected by inter- and intraspecific competition resulting in individual differences of reduced reproduction. Hence, individuals vary in their susceptibility to competition. The parameter values characterizing Culex and Daphnia (Table 1) were estimated based on average values from previous experimental experience with these two species. This also relates to the variation of individual parameters around the species-specific values in the model with σ = 10%.
Table 1. Parameter Values Used in the Simulationsa.10,12,22.
| parameter | Culex | Daphnia |
|---|---|---|
| size of starting population [ind.] | 10 | 10 |
| carrying capacity (K) | 75 | 150 |
| age at maturity [d] | 25 | 10 |
| baseline mortality [d–1] | 0.025 | 0.025 |
| time steps between reproductive events [d] | 25 | 4 |
| potential number of offspring (bpot) | 30 | 20 |
| competitive strength (c) | 0.325 | 0.325 |
| acute toxic effect on mortality [%] | 10, 20, 40 | |
| chronic toxic effect on mortality [%] | 1, 5, 20 |
The same values were used for Culex in the scenario without Daphnia present. Parameter values characterizing both species were estimated from previous experiments with the two species.
The direct effect of the toxicant was modeled as an increase in acute mortality at the time of contamination. For the concentrations tested in the experiment (3.3, 10, and 33 μg/L thiacloprid), the probability of each sensitive individual dying was increased for one modeling time step (1°day) by 10%, 20% and 40%, respectively, after the successive pulse contamination (Table 1). Additionally, we assumed a chronic effect on the mortality of all the sensitive individuals that were present at the time of contamination. For the remaining lifetime of the surviving individuals, the chronic mortality was increased by 1%, 5%, and 20%, respectively. The increased short- and long-term mortality of the sensitive species was estimated based on toxicity experiments conducted with <24 h old larvae.10 To account for the lower sensitivity of older individuals in our experiments, we adjusted the mortality to the observed time course after the first contamination. For the less sensitive species, no direct effects of the toxicant were assumed. This scenario resembles the situation in the experiment because the less sensitive Daphnia magna is characterized by an acute (24 h) and long-term (14 day) LC50 that is about 3 orders of magnitude higher than that of Culex pipiens.10 The contamination events were modeled at the same time as in the experiment. The results of 2000 replicate simulation runs were averaged. To identify a variation of means that is comparable between the experiment and the model, we calculated the standard error by applying a bootstrapping procedure on all replications by sampling 6 random model runs with 10 000 repetitions.
| 1 |
bix,act: actual number of offspring of individual x of species i, bix,pot: potential number of offspring of individual x of species i, i: population size of species i in the previous time step, Nj: population size of species j in the previous time step, cji: competitive strength of species j (i.e., the effect of species j on species i), Ki: carrying capacity of the system for species i, aix: susceptibility to competition of individual x of species i (∼norm, μ = 1, σ = 0.1).
Results
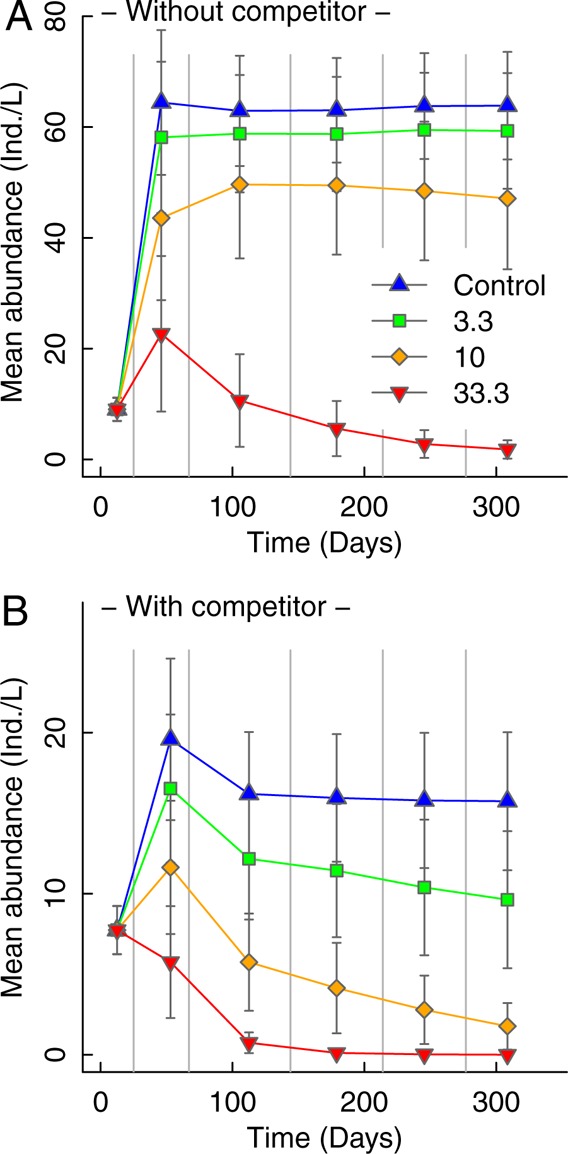
We experimentally investigated the process of the culmination of low-dose effects of successive pulse applications of pesticides on populations under competitive pressure. In the absence of a toxicant, larval populations of the mosquito Culex pipiens approached the carrying capacity of the test system after approximately 50 days in the absence of the competing crustacean Daphnia magna and after approximately 100 days in the presence of competing Daphnia (Figure 1). The Daphnia population developed from low density at the beginning of the experiment and reached carrying capacity after approximately 100 days in the absence of pesticides (Figure 1).
Figure 1.
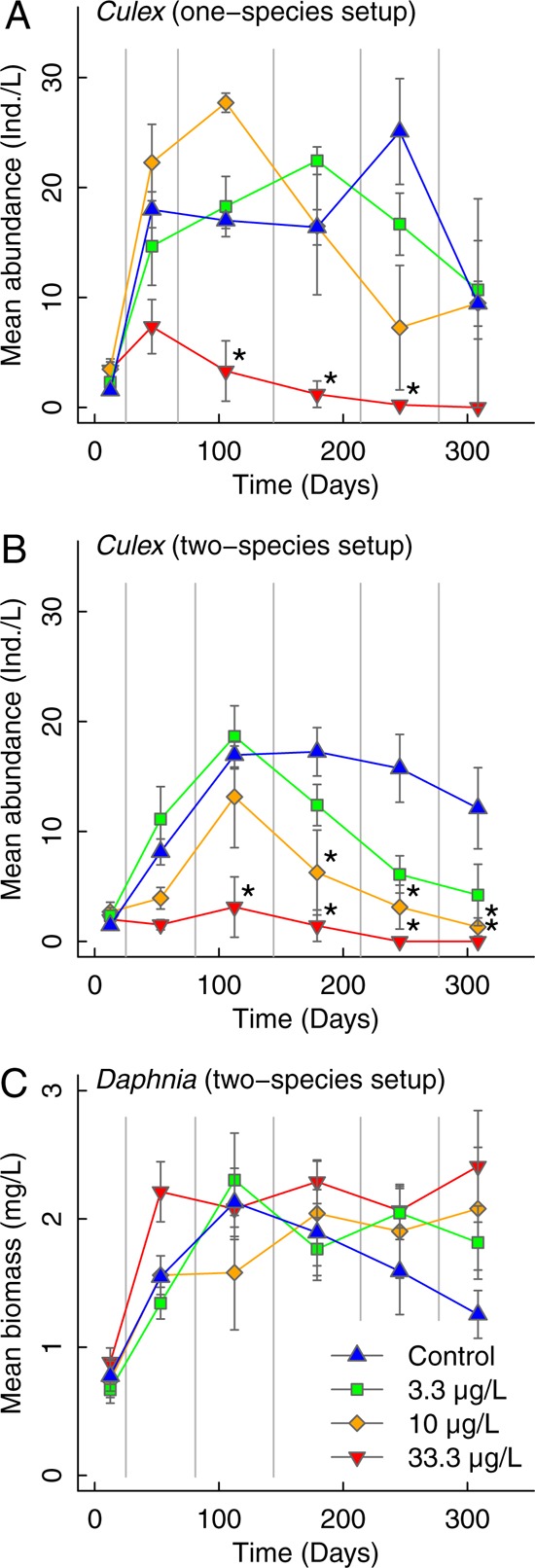
Time courses for the abundance of populations of Culex pipiens larvae in the absence of the competitor Daphnia magna (A) and in the presence of the competitor Daphnia magna (B) and the biomass of Daphnia in the presence of Culex pipiens (C), presented as the mean values between two successive pesticide pulses. Biomass of Daphnia is shown as measure for competition strength. Test organisms were exposed to five successive pulses of different thiacloprid concentrations at days 25, 67, 144, 214, and 277 for (A) and at days 25, 81, 144, 214, and 277 for (B and C). Asterisks indicate a significant difference from the control (p < 0.05, ANOVA followed by a Dunnett’s post hoc multiple-comparison test).
Once the Culex population reached carrying capacity, the combination of successive pulse applications of pesticide and the presence of a competitor resulted in a decline of the Culex pipiens populations that continued over multiple generations at all the pesticide concentrations tested. This pattern of population dynamics strongly differed from the dynamics of the Culex populations in the absence of competitors, a situation in which only the highest pesticide concentration resulted in a decline of population abundance (Figure 1). Hence, the long-term concentration–response relationship depends strongly on the presence of competition with another species.
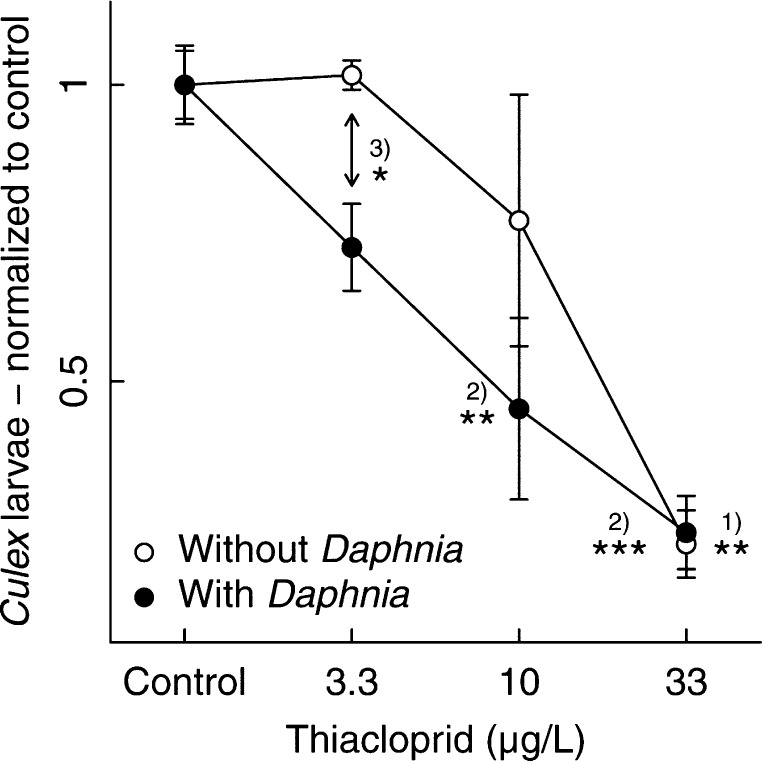
The culmination of low-dose effects under competitive pressure began to develop after approximately 100 days, a period when both populations (Culex pipiens and Daphnia magna) reached their carrying capacity and the pressure of competition was high (Figure 1B). Hence, we averaged the mean Culex larval abundance after reaching carrying capacity (days 144 to 339). During that time period, we identified a significant effect of interspecific competition by Daphnia magna on the population density of Culex larvae at the lowest concentration tested (3.3 μg/L). With increasing concentrations, the relevance of competition for the characteristics of the concentration–response relationship decreases, and the sole effect of the pesticide dominates (Figure 2).
Figure 2.
Normalized concentration–response curve of Culex pipiens larvae exposed to thiacloprid with and without competition by Daphnia magna. The population density after the third contamination (day 144) was averaged and normalized to the control. Comparisons with the mean of the respective control indicated a significant difference at 10 μg/L and 33 μg/L when Daphnia are present (comparison 1), and at 33 μg/L when Daphnia are not present (comparison 2; ANOVA followed by Dunnett’s post hoc multiple-comparison test, *p < 0.05, **p < 0.01, and ***p < 0.001); comparison between the setups with and without Daphnia revealed a difference at 3.3 μg/L thiacloprid (Student’s t test, df =6, p < 0.03, after Bonferroni correction, comparison 3).
The relevance of the competitive pressure between the two species in our experimental system is indicated by a negative relationship between the abundance of Culex pipiens larvae and the biomass of Daphnia magna (Figure 3). This relation shows that the chemical is not inherently more toxic when a competitor is present but that competitive processes are responsible for the culmination of toxicant effects.
Figure 3.
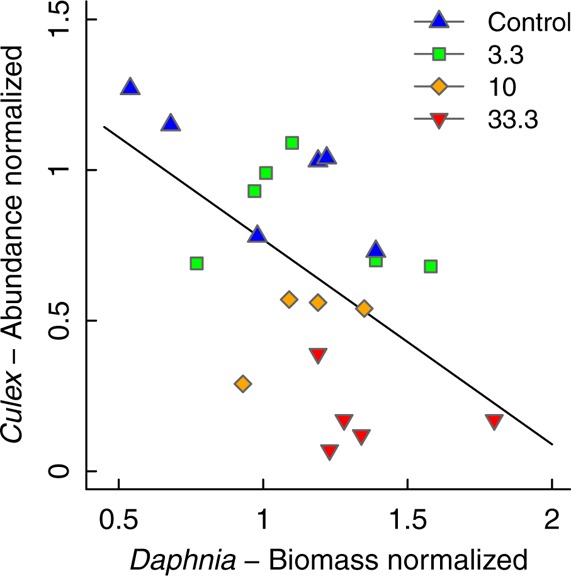
Abundance of Culex pipiens larvae plotted against the biomass of Daphnia magna. Linear regression: intercept = 1.4, slope = −0.68, adjusted r2 = 0.26, df = 19, p = 0.01. Each data point represents the mean average of a replicate normalized to the control.
Discussion
Mechanisms Responsible for the Culmination of Low-Dose Effects
We assume three sequential processes operating under repeated toxicant pulse exposure and competitive pressure. First, the resource availability increases due to lethal and sublethal impairments of the more sensitive species (Culex in this study). For example, accidental insecticide contamination can reduce stream invertebrate abundance, allowing high growth rates of algae.23 Additionally, an increased resource availability might also occur due to sublethal impairments of the reproductive success or a decreased feeding activity.24
Second, these resources are consumed to a great extent by the less sensitive species (here Daphnia), thus inducing an increased population growth and thus an increased biomass compared to the control. Such processes have been previously observed when competing populations with contrasting sensitivities co-occur.12,13 In our experiment, this is shown by the negative relationship between the abundance of Culex pipiens larvae and the biomass of Daphnia magna (Figure 3).
Third, the resources consumed by the less sensitive species (Daphnia) are not available for the more sensitive species (Culex) which, in turn, reduces its potential for recovery. By contrast in a scenario without competitors, the surviving individuals of an affected population benefit from increased resource availability. These processes have been demonstrated for competing individuals within a population22 and competing species within a community.12,13
Finally, we want to highlight that initial negligible effects, may culminate and produce relevant long-term population effects when exposure is repeated. The important role of interspecific competition for the evaluation of chemical effects has also been hypothesized by Crow and Taub,25 suggesting that “the presence of a competitor may have a dramatic effect on the response of a system...”
Verification of Proposed Mechanisms
We applied an individual-based simulation model of two competing species to ascertain whether repeated toxicant exposure of a sensitive population that is challenged with interspecific competition may result in multigenerational culmination of low-dose effects. The model is highly simplified and not tailored to exactly match the experimental setup but to approximate the system and the most relevant mechanisms. It represents two competing generic aquatic species with similar life history characteristics as the species in the experiment (e.g., Culex and Daphnia). The modeled population dynamics were comparable to those observed in the experiment: a fast decline in the abundance of the more sensitive species (Culex) with and without the presence of the less insensitive competitor (Daphnia). In the scenario without Daphnia, the population of Culex recovered before the next pulse of contamination, except at the highest concentrations tested, due to the strong effects on mortality (Figure 4a). In the scenario with competing individuals, there was an increase in the abundance of Daphnia after each contamination event due to the alleviation of resource competition, hindering the full recovery of Culex populations until the next contamination and resulting in a steady decline of these populations at all three concentrations modeled (Figure 4b).
Figure 4.
Modeled population dynamics of (A): More sensitive species (i.e., Culex) without interspecific competition and (B): More sensitive species (Culex) in presence of the less sensitive competing species (Daphnia). Effects of five successive pulse contaminations. The variation around the means is given as standard error (bootstrapping procedure). Vertical lines indicate exposure events matching those in the experiment.
The characteristics of the population dynamics observed in the experiment and simulated in the model are highly similar: repeated pesticide pulses in combination with competition resulted in the long term culmination of low-dose effects. This outcome strongly increased our confidence in the processes proposed to mechanistically explain the observed competition-induced culmination effect for the sensitive Culex population.
Implications for the Assessment of Reoccurring Stress Events
″The potential threat to ecosystems by multiple stressors″ increasingly raises concerns.26 Here we show that the magnitude and duration of the population effects exerted by repeated toxicant pulses is strongly related to the strength of interspecific competition. Considering this effect culmination enables to link long-term effects of repeated low-dose toxicant pulses at concentrations that should have no effect on the number of individuals within a population (i.e., below the no observed effect concentration for each individual pulse). Hence, effect culmination contributes to a mechanistic understanding of sustainable pesticide effects in the field that are far below those concentrations predicted to be safe within the risk assessment process.4−6 In such cases, repeated toxicant pulses are coupled with strong interspecific competition in complex stream communities. Additionally, these mechanisms also allow to explain that populations can rapidly recover from high concentrations of toxicants in the absence of interspecific competition, this is illustrated, for example, by the fast recovery observed in mosquito populations in the absence of competing species.27
Here we identified the mechanism of effect culmination for toxicant stress. We assume that this mechanism is also relevant for nontoxic reoccurring stress events acting lethally or sublethally on individuals that are under competitive challenge.
Acknowledgments
We thank the Helmholtz Association for long-term funding enabling this work. Additional support was provided by the EU, seventh Framework Programme (CREAM, PITN-GA-2009-238148). Additionally we thank three anonymous reviewers for their valuable comments that helped to improve the paper.
Author Contributions
Study design: M.L.; experimental design and implementation: all; data analysis: K.F.; simulation model: M.K., M.L.; discussion and interpretation of results: all; drafting of manuscript: M.L.; revising manuscript: all.
The authors declare no competing financial interest.
References
- Butchart S. H. M.; Walpole M.; Collen B.; van Strien A.; Scharlemann J. P. W.; Almond R. E. A.; Baillie J. E. M.; Bomhard B.; Brown C.; Bruno J.; Carpenter K. E.; Carr G. M.; Chanson J.; Chenery A. M.; Csirke J.; Davidson N. C.; Dentener F.; Foster M.; Galli A.; Galloway J. N.; Genovesi P.; Gregory R. D.; Hockings M.; Kapos V.; Lamarque J. F.; Leverington F.; Loh J.; McGeoch M. A.; McRae L.; Minasyan A.; Morcillo M. H.; Oldfield T. E. E.; Pauly D.; Quader S.; Revenga C.; Sauer J. R.; Skolnik B.; Spear D.; Stanwell-Smith D.; Stuart S. N.; Symes A.; Tierney M.; Tyrrell T. D.; Vie J. C.; Watson R. Global biodiversity: Indicators of recent declines. Science 2010, 32859821164–1168. [DOI] [PubMed] [Google Scholar]
- Vorosmarty C. J.; McIntyre P. B.; Gessner M. O.; Dudgeon D.; Prusevich A.; Green P.; Glidden S.; Bunn S. E.; Sullivan C. A.; Liermann C. R.; Davies P. M. Global threats to human water security and river biodiversity. Nature 2010, 4677315555–561. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach R. P.; Escher B. I.; Fenner K.; Hofstetter T. B.; Johnson C. A.; von Gunten U.; Wehrli B. The challenge of micropollutants in aquatic systems. Science 2006, 31357901072–1077. [DOI] [PubMed] [Google Scholar]
- Liess M.; von der Ohe P. C. Analyzing effects of pesticides on invertebrate communities in streams. Environ. Toxicol. Chem. 2005, 244954–965. [DOI] [PubMed] [Google Scholar]
- Schäfer R. B.; von der Ohe P. C.; Rasmussen J.; Kefford B. J.; Beketov M. A.; Schulz R.; Liess M. Thresholds for the effects of pesticides on invertebrate communities and leaf breakdown in stream ecosystems. Environ. Sci. Technol. 2012, 4695134–5142. [DOI] [PubMed] [Google Scholar]
- Rasmussen J. J.; Wiberg-Larsen P.; Baattrup-Pedersen A.; Friberg N.; Kronvang B. Stream habitat structure influences macroinvertebrate response to pesticides. Environ. Pollut. 2012, 164, 142–149. [DOI] [PubMed] [Google Scholar]
- EU: Placing of plant protection products on the market, PPP 1107/2009. Placing of plant protection products on the market, PPP 1107/2009 2009.
- Garnier-Laplace J.; Geras’kin S.; Della-Vedova C.; Beaugelin-Seiller K.; Hinton T. G.; Real A.; Oudalova A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [DOI] [PubMed] [Google Scholar]
- Peterson C. H.; Rice S. D.; Short J. W.; Esler D.; Bodkin J. L.; Ballachey B. E.; Irons D. B. Long-term ecosystem response to the Exxon Valdez oil spill. Science 2003, 30256532082–2086. [DOI] [PubMed] [Google Scholar]
- Beketov M. A.; Liess M. Acute and delayed effects of the neonicotinoid insecticide thiacloprid on seven freshwater arthropods. Environ. Toxicol. Chem. 2008, 272461–70. [DOI] [PubMed] [Google Scholar]
- Rohr J. R.; Schotthoefer A. M.; Raffel T. R.; Carrick H. J.; Halstead N.; Hoverman J. T.; Johnson C. M.; Johnson L. B.; Lieske C.; Piwoni M. D.; Schoff P. K.; Beasley V. R. Agrochemicals increase trematode infections in a declining amphibian species. Nature 2008, 45572171235–U50. [DOI] [PubMed] [Google Scholar]
- Foit K.; Kaske O.; Liess M. Competition increases toxicant sensitivity and delays the recovery of two interacting populations. Aquat. Toxicol. 2012, 106–107, 25–31. [DOI] [PubMed] [Google Scholar]
- Knillmann S.; Stampfli N. C.; Beketov M. A.; Liess M. Intraspecific competition increases toxicant effects in outdoor pond microcosms. Ecotoxicology 2012, 1–10. [DOI] [PubMed] [Google Scholar]
- Coors A.; De Meester L. Synergistic, antagonistic and additive effects of multiple stressors: Predation threat, parasitism and pesticide exposure in Daphnia magna. J. Appl. Ecol. 2008, 4561820–1828. [Google Scholar]
- Crain C. M.; Kroeker K.; Halpern B. S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008, 11121304–1315. [DOI] [PubMed] [Google Scholar]
- Relyea R.; Hoverman J. Assessing the ecology in ecotoxicology: A review and synthesis in freshwater systems. Ecol. Lett. 2006, 9101157–71. [DOI] [PubMed] [Google Scholar]
- Foit K.; Kaske O.; Wahrendorf D. S.; Duquesne S.; Liess M. Automated Nanocosm test system to assess the effects of stressors on two interacting populations. Aquat. Toxicol. 2012, 109, 243–9. [DOI] [PubMed] [Google Scholar]
- OECD. Report of the Final Ring Test of the Daphnia Magna Reproduction Test; Organization for Economic Cooperation and Development: Pariso, 1997. [Google Scholar]
- R Development Core Team, R: A language and environment for statistical computing, reference index version 2.15.2., Vienna, Austria, www.r-procect.org
- Diggle P. J., Liang K. Y., Zeger S. L.. Analysis of Longitudinal Data; Oxford University Press: New York, 1994. [Google Scholar]
- Dumont H. J.; van de Velde I.; Dumont S. The dry weight of biomass in a selection of cladocera, copepoda and rotifera from plankton, periphyton and benthos of continental waters. Oecologia 1975, 19, 75–97. [DOI] [PubMed] [Google Scholar]
- Liess M.; Foit K. Intraspecific competition delays recovery of population structure. Aquat. Toxicol. 2010, 97, 15–22. [DOI] [PubMed] [Google Scholar]
- Zwick P. Insecticides as a threat to flowing waters. Naturwissenschaften 1992, 79, 437–442. [Google Scholar]
- Reynaldi S.; Duquesne S.; Jung K.; Liess M. Linking feeding activity and maturation of Daphnia magna following short-term exposure to fenvalerate. Environ. Toxicol. Chem. 2006, 2571826–1830. [DOI] [PubMed] [Google Scholar]
- Crow M. E.; Taub F. B. Designing a microcosm bioassay to detect ecosystem level effects. Intern. J. Environ. Studies 1979, 13, 141–147. [Google Scholar]
- Eggen R. I.; Behra R.; Burkhardt-Holm P.; Escher B. I.; Schweigert N. Challenges in ecotoxicology. Environ. Sci. Technol. 2004, 38358A–64A. [DOI] [PubMed] [Google Scholar]
- Chase J. M.; Knight T. M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003, 6111017–1024. [Google Scholar]