Abstract
Magnetic resonance plays a leading role in the management of oncology patients, providing superior contrast resolution and greater sensitivity compared with other techniques, which enables more accurate tumor identification, characterization and staging. Contrast agents are widely used in clinical magnetic resonance imaging; approximately 40–50% of clinical scans are contrast enhanced. Most contrast agents are based on the paramagnetic gadolinium ion Gd3+, which is chelated to avoid the toxic effects of free gadolinium. Multiple factors such as molecule structure, molecule concentration, dose, field strength and temperature determine the longitudinal and transverse relaxation rates (R1 and R2, respectively) and thus the T1- and T2-relaxivities of these chelates. These T1- and T2-relaxivities, together with their pharmacokinetic properties (i.e. distribution and concentration in the area of interest), determine the radiologic efficacy of the gadolinium-based contrast agents.
Keywords: Magnetic resonance, contrast media, relaxivity, gadolinium
Introduction
Magnetic resonance plays a leading role in the management of oncology patients, providing superior contrast resolution and greater sensitivity compared with other techniques, which enables more accurate tumor identification, characterization and staging. Contrast agents are widely used in clinical magnetic resonance (MR) imaging; approximately 40–50% of clinical scans are contrast enhanced[1]. In general use, contrast agents are useful to detect and enhance differences in tissue vascularization, which is very helpful in the field of oncological imaging. Most contrast agents are based on the paramagnetic gadolinium ion Gd3+, which is chelated to avoid the toxic effects of free gadolinium. The paramagnetic centre of the metal chelate interacts directly with the protons of the surroundings, the consequences of which are increased signal intensity on T1-weighted images, and ultimately improved diagnostic confidence[2]. Currently, 10 intravenous gadolinium-based contrast agents (GBCA) are approved, although not all GBCAs are available in all countries. Most approved GBCAs are purely extracellular agents meaning that they distribute exclusively within the extracellular space and are excreted via glomerular filtration through the kidneys with a biological half-life of approximately 1.5–2.0 h in patients with normal renal function[3].
Multiple factors such as molecule structure, concentration, dose, field strength and temperature determine the longitudinal and transverse relaxation rates (r1 and r2, respectively) and thus the T1- and T2-relaxivities of these chelates. These T1- and T2-relaxivities, together with their pharmacokinetic properties (i.e. distribution and concentration in the area of interest), determine the radiologic efficacy of the GBCA[4,5].
The radiologic efficacy of contrast media can be expressed by the increase in signal intensity (enhancement). The extent of the enhancement is proportional to the local concentration of contrast agent and the relaxivity of the contrast agent. Relaxivity reflects the capability to shorten the water proton relaxation rates T1 and T2/T2* and is an inherent property of the contrast molecule. It can be considered as a parameter to compare the efficacy of different contrast agents.
Paramagnetic contrast agents can be classified according to their molecular structure (acyclic [linear]; macrocyclic), charge (ionic; non-ionic), and distribution (non-specific extracellular, i.e. vascular-interstitial distribution; liver-specific; blood pool)[3].
While molecular structure mostly influences the stability of the molecule, with macrocyclic GBCA being more stable than acyclic linear GBCA; the in vivo relaxivity of the contrast agent depends on the physiological environment (i.e., blood, interstitial fluids, intracellular space), as well as the capacity of the contrast agent to interact with macromolecules in the blood (protein binding)[5]. In this regard, certain GBCAs (i.e. gadobenate dimeglumine, MultiHance; gadoxetic acid, Primovist) have a hydrophobic moiety in their structure, which enables the agent to interact reversibly with serum proteins. These agents have higher in vivo relaxivities than GBCAs that do not have this substituent and do not interact with serum proteins. Thus, even at the same concentration and dose, different GBCAs have different T1 (r1)- and T2 (r2)-relaxivities. A third agent with a hydrophobic moiety is the blood-pool agent gadofosveset (Vasovist/Ablavar). However, this agent has limited availability in the worldwide market (United States) and its role is limited to the imaging of vessels. Thus, it is not discussed here.
In the case of MultiHance and Primovist, this substituent also permits these agents to be taken up by functioning hepatocytes and eliminated via the hepatobiliary pathway (3–5% for MultiHance; 50% for Primovist)[3]. However, whereas in Europe MultiHance is approved both for extrahepatic and hepatic imaging, Primovist is only approved for hepatic imaging. In the United States, MultiHance is approved only for the central nervous system (CNS) and magnetic resonance angiography (MRA), Primovist is only approved for hepatic imaging.
Another way to obtain higher relaxivity at equivalent total administered dose is to use highly concentrated contrast agent. The molar agent gadobutrol (Gadovist 1.0) contains an identical amount of active ingredient in only half the volume and thus results in a higher concentration in the first pass if injected at identical dose and flow rate. However, during the equilibrium phase, the concentration, and thus the local tissue relaxivity, is similar to that of conventional extracellular GBCAs.
Non-specific GBCAs that distribute in the extracellular fluid space are currently the most widely used contrast agents, also in the field of oncological imaging. These contrast agents are most effective during the dynamic phase of contrast enhancement when differential blood flow between tumor and normal parenchyma leads to characteristic lesion enhancement patterns, which helps in lesion detection and characterization[6]. Unfortunately, for focal liver lesions, dynamic phase imaging alone can prove unsatisfactory at times for the accurate diagnosis of hepatic lesions[7,8]. The use of contrast agent with liver-specific properties increases the accuracy of MR for the identification and characterization of both benign and malignant focal liver lesions[8–10].
Of critical importance currently is the dose of GBCA administered. This reflects concerns over the safety of GBCA, particularly in patients with severe renal impairment who may be at increased risk for nephrogenic systemic fibrosis (NSF). The recommended dose of non-specific GBCA for most clinical indications is 0.1 mmol/kg of body weight, although higher doses (0.2–0.3 mmol/kg), may be required for certain applications (e.g. MRA and CNS imaging). However, higher than standard doses of GBCA are not universally approved by regulatory authorities. Only the non-specific macrocyclic agent ProHance is currently approved for use at a high dose (>1 mmol/kg); all other GBCAs used at higher than standard dose are off-label applications. Most GBCAs are approved at a dose of 0.1 mmol/kg of body weight for most applications. Variations occur in the case of the liver-specific agents MultiHance (approved at a dose of 0.05 mmol/kg for liver imaging but at 0.1 mmol/kg for CNS, breast and MRA) and Primovist (approved at a dose of 0.025 mmol/kg).
Due to the different concentrations of the various GBCAs (0.25 M for Primovist, 1.0 M for Gadovist, 0.5 M for all other GBCAs), care should be taken to administer the correct volume to achieve the approved dose. For liver imaging, the volume administered for MultiHance should be half that conventional GBCAs and for Primovist a quarter of the volume should be used.
Clinical applications
Comparison of the efficiency of different GBCAs can be made in a variety of clinical settings, using both quantitative and qualitative analyses. In oncological imaging, the imaging protocol differs for different body parts and clinical situations. Whereas dynamic imaging during the first pass is essential for the detection and characterization of focal lesions in certain organs (breast, abdominal and pelvic organs), in other organs (brain, musculoskeletal), imaging is primarily performed during the equilibrium phase.
For practical purposes, GBCAs are categorized as high relaxivity (HR-GBCA: gadobenate dimeglumine, MultiHance), high concentration (HC-GBCA: gadobutrol, Gadovist), liver-specific (LS-GBCA: gadobenate dimeglumine, MultiHance, and gadotexate, Primovist) and conventional (C-GBCA: all other contrasts).
Brain
In the surgical planning for gliomas, MR contrast-enhanced T1-weighted images are essential to better define the volume of the surgically resectable mass, as the prognosis after surgery is better and survival is longer if macroscopically complete removal of a glioma is achieved[11]; the survival time for patients for whom only partial removal is achieved is no longer than for patients who undergo stereotactic biopsy[12]. Also treatment of brain metastases depends on accurate knowledge of the number, volume and location of the metastases.
Steady-state morphologic imaging
C-GBCA versus C-GBCA. Just one intra-individual crossover study has compared 2 GBCAs with similar relaxivity for brain tumor imaging[13]. In that study, 2 blinded readers compared Magnevist with ProHance in 80 subjects for the presence of disease, degree of enhancement, location and number of lesions, and additional information gained (definition of lesion borders, improved visualization, distinction of edema, disease classification, determination of recurrent tumor, other). Neither reader noted any significant differences in terms of GBCA preference (readers 1 and 2 preferred ProHance over Magnevist in 2 and 4 cases, respectively, and Magnevist over ProHance in 1 and 2 cases, respectively) and no differences were noted between agents in terms of the additional information provided on postcontrast images.
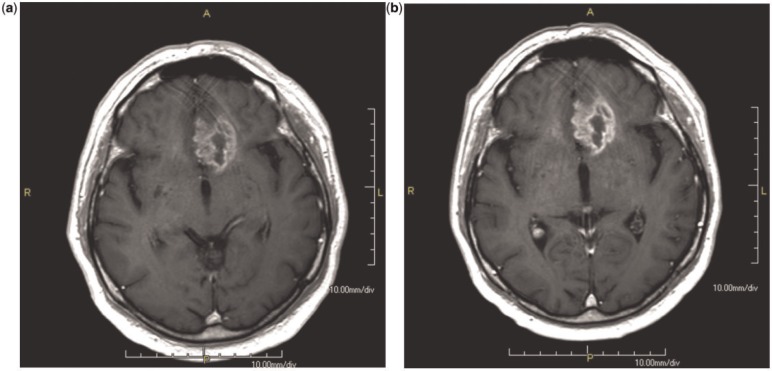
HR-GBCA versus C-GBCA. Numerous intra-individual crossover studies have compared the efficacy of HR-GBCA with C-GBCAs[14–19]. Early studies compared MultiHance with Magnevist[14] and Dotarem[15] in 27 and 23, patients, respectively, at 1.5 T. Subsequently, much larger, multicenter studies compared MultiHance with Magnevist[16,17] and Omniscan[18] in 151 and 136 patients, respectively, again at 1.5 T. These studies were designed to demonstrate superiority and all concluded that the higher relaxivity agent MultiHance at an approved dose of 0.1 mmol/kg body weight is significantly superior in terms of both qualitative (global diagnostic preference, lesion border delineation, definition of disease extent, visualization of lesion internal morphology, lesion contrast enhancement) and quantitative (contrast-to-noise ratio, lesion-to-background ratio) enhancement to conventional relaxivity agents at equivalent dose (Figs. 1 and 2). This benefit of high relaxivity also extends to imaging at 3 T, as demonstrated by Rumboldt et al. in 46 patients[19], where brain lesion depiction was significantly improved with 0.1 mmol/kg MultiHance compared with 0.1 mmol/kg Magnevist.
Figure 1.
Glioblastoma, same patient after 0.1 mmol/kg of Magnevist (a) and Multihance (b). The enhancement of the lesion is higher after Multihance, with a better delineation of the borders.
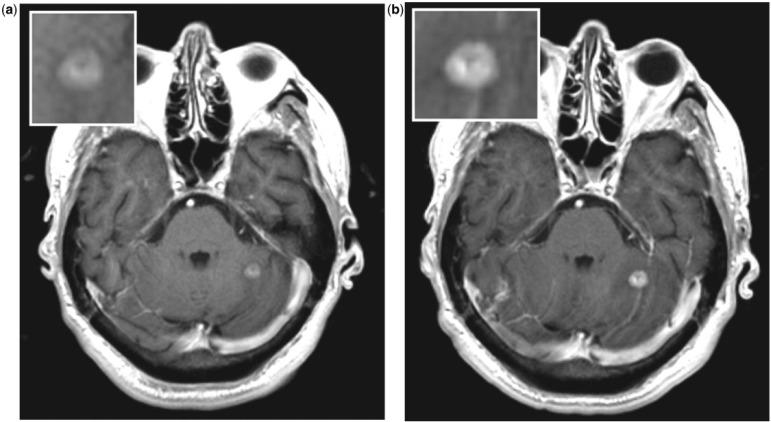
Figure 2.
Lung metastases, same patient after 0.1 mmol/kg of Magnevist (a) and Multihance (b). The enhancement of the lesion is higher after Multihance, with a better delineation of the borders.
Although these studies were performed exclusively in adult patients, Colosimo et al.[20] showed that higher relaxivity is advantageous also in paediatric patients. In a two-centre, prospective, fully blinded, randomized parallel-group study performed on 63 subjects aged 6 months to 16 years, the authors demonstrated significantly better contrast enhancement and lesion depiction with 0.1 mmol/kg MultiHance (n = 29 patients) than with 0.1 mmol/kg Magnevist (n = 34 patients).
HC-GBCA versus C-GBCA. A single-centre intra-individual comparison of Gadovist and Magnevist in 27 patients at 1.5 T suggested that the higher concentration agent might have advantages over the conventional agent for the visualization of brain metastases, particularly in terms of improved lesion conspicuity[21]. However, no quantitative assessment of lesion enhancement was performed and conclusions were based solely on the subjective assessment of 2 neuroradiologists in consensus. A more robust multicenter study subsequently compared Gadovist and Dotarem at 1.5 T in 136 patients with cerebral neoplastic enhancing lesions[22]. In this study, significant preference for Gadovist compared with Dotarem was noted by 2 of 3 blinded readers for overall reader preference. However, none of the 3 readers considered Gadovist to be superior for lesion delineation and only 1 reader noted minimally significant preference for Gadovist for the definition of lesion internal structure. Quantitatively, the percent lesion enhancement following Gadovist was approximately 9% higher than that following Dotarem as expected from the differences in their respective relaxivities, but this yielded no significant difference between the 2 agents for measured contrast-to-noise ratio. No differences in the number of lesions detected with either agent were observed.
In another prospective phase 3 study performed on 419 patients for the US Food and Drug Administration approval of Gadovist for CNS imaging, 3 blinded readers each reported similar contrast enhancement, lesion border delineation, and lesion internal morphology and a similar overall accuracy of diagnosis for Gadovist and ProHance when these 2 agents were administered at an equivalent dose of 0.1 mmol/kg body weight[23].
HR-GBCA versus HC-GBCA. Most recently, a large, multicenter, randomized, intra-individual study compared HR-GBCA MultiHance with HC-GBCA Gadovist[24]. In that study, 123 patients each underwent one examination with 0.1 mmol/kg MultiHance and one examination with 0.1 mmol/kg Gadovist. Three blinded readers consistently demonstrated highly significant (P < 0.0001) preference for MultiHance for all qualitative end points with good inter-reader agreement for all evaluations. In addition, significant superiority was noted for all quantitative assessments for MultiHance, with a mean difference of approximately 22% in percent lesion enhancement compared with Gadovist. The conclusion of the study was that gadolinium concentration has little to no practical clinical impact on steady-state morphologic imaging, and that at identical approved (0.1 mmol/kg) doses, the relaxivity of the GBCA is the dominant characteristic determining the degree of enhancement.
Perfusion imaging
Perfusion-weighted imaging (PWI) is achieved using a fast susceptibility-weighted imaging sequence that utilizes the T2* relaxing properties of the GBCA. Because T2* relaxivity is primarily a function of gadolinium concentration, more highly concentrated agents may be expected to have advantages for first-pass PWI[25]. Two intra-individual crossover clinical trials, however, revealed no significant differences between the HC-GBCA Gadovist and the HR-GBCA MultiHance when these agents were injected at an equivalent approved dose of 0.1 mmol/kg body weight[26,27]. In both studies, quantitative analysis revealed nearly identical signal intensity/time (SI/T) curves with no differences noted in terms of maximal relative signal drop, full width half maximum or signal-to-noise ratio of the concentration curve at maximum concentration. Likewise, qualitative evaluation of relative cerebral blood volume and relative cerebral blood flow maps by 2 experienced blinded radiologists revealed no differences and no advantages for either of the 2 GBCAs. Because the contrast injection rates are very high for PWI (typically 5 ml/s), the resulting injection times are very short (a dose of 0.1 mmol/kg bodyweight of 1 M Gadovist given to a 70-kg patient at a rate of 5 ml/s would be injected in just 1.5 s, whereas an equivalent dose of an 0.5 M agent would be injected in just 3 s). As a consequence, the similar behaviour on PWI for Gadovist and MultiHance might potentially be explained by contrast bolus normalization through the heart and lungs before its arrival in the tissue of interest[28].
In summary, the standard GBCA dose used in MR imaging of the CNS is 0.1 mmol/kg of body weight. For improved lesion detection, particularly of metastases, higher than standard doses (0.2–0.3 mmol/kg of body weight) may be used but the use of higher doses is off-label for all GBCAs except ProHance. Significantly better contrast enhancement and lesion conspicuity is achieved with HR-GBCA compared with C-GBCA and HC-GBCA when administered at equivalent doses. At 3 T, imaging of CNS lesions with HR-GBCA is more effective. For perfusion imaging, HC-GBCA may theoretically provide an advantage although carefully controlled intra-individual crossover studies have failed to demonstrate significant differences.
Breast
Breast MR imaging is highly accurate for delineating disease extent in patients with a recent diagnosis of breast cancer, is useful for definitive problem solving in cases of equivocal mammographic and/or ultrasonographic findings, and is superior to mammography and ultrasonography for the detection of residual cancer after neoadjuvant chemotherapy. However, it is important to avoid overtreatment due to depiction of false-positive lesions. The very high sensitivity of breast MR imaging, particularly in younger woman who are at increased risk of breast cancer, is such that it is widely incorporated into surveillance programs for high-risk women for breast cancer[29]. Dynamic contrast-enhanced imaging of the breast is the most important sequence, although it is important to strike the correct balance between temporal and spatial resolution[30]. The use of GBCAs is fundamental for breast MR imaging.
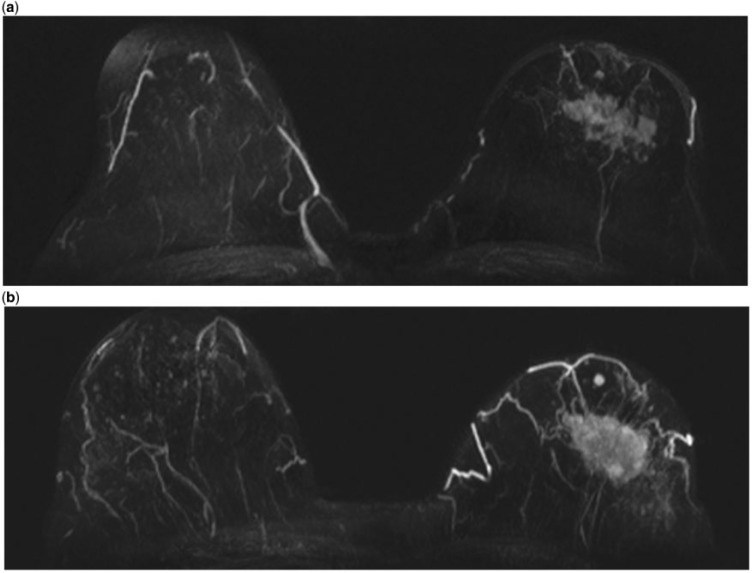
HR-GBCA versus C-GBCAs. Two early intra-individual comparisons of MultiHance and Magnevist demonstrated unequivocally that higher relaxivity is advantageous in terms of the detection of malignant breast lesions and the overall diagnostic performance of breast MR imaging (Fig. 3)[31,32]. That diagnostic performance is strongly influenced by the choice of MR contrast agent has subsequently been confirmed in a large-scale intra-individual comparison of 151 patients at 1.5 T[33]. In that study, significant superiority for MultiHance over Magnevist was noted independently by 3 blinded readers in terms of malignant lesion detection (91.7–94.4% vs 79.9–83.3%; P ≤ 0.0003) and diagnostic performance (sensitivity, 91.1–95.2% vs 81.2–84.6%; specificity, 96.9–99.0% vs 93.8–97.8%; accuracy, 96.7–98.2% vs 92.8–96.1%; P ≤ 0.0094). Similarly, a significantly superior positive predictive value (77.2–91.1% vs 60.9–80.7%; P ≤ 0.0002) and negative predictive value (99.0–99.4% vs 97.8–98.1%; P ≤ 0.0003) was noted with MultiHance. These results were achieved using identical acquisition and image interpretation parameters. Studies have shown that diagnostic performance and breast lesion characterization with MultiHance can be improved still further if higher initial enhancement thresholds are used than are used with conventional GBCA[34,35]. This reflects the higher T1-relaxivity of MultiHance and the resulting greater signal intensity enhancement.
Figure 3.
Breast carcinoma, same patient after 0.1 mmol/kg of Magnevist (a) and Multihance (b). The enhancement of the lesion is higher after Multihance, with a better delineation of the borders as well as of spiculated margins.
HR-GBCA versus HC-GBCA. A recent intra-individual comparison of 72 patients at 1.5 T suggested that Gadovist may be non-inferior to MultiHance for breast lesion detection when administered at a dose of 0.1 mmol/kg body weight[36]. However, further studies in a larger and more representative patient cohort using a more appropriate and objective study design are needed to confirm these findings[37].
In summary, for MR breast imaging, HR-GBCA allows more malignant lesions to be detected and improves overall diagnostic performance. However, the greater enhancement achieved with higher relaxivity agents should be taken into account when evaluating breast MR images in order to improve diagnostic performance still further. Although a preliminary study suggested minimal differences between HR-GBCA and HC-GBCA for breast MR imaging, further studies are needed to confirm these preliminary results.
Bone and soft tissue tumors
MR imaging of bone and soft tissue tumors is usually performed during the equilibrium phase, when GBCA is distributed through the entire volume of distribution (about 32 l). In this phase, HC-GBCAs do not provide superior enhancement compared with C-GBCAs at an equivalent dose of 0.1 mmol/kg body weight[38]. No comparisons have been made between HR-GBCA and C-GBCA or HC-GBCA.
Liver
Contrast-enhanced MR imaging is a highly accurate non-invasive imaging modality for the detection and characterization of solid hypervascular focal liver lesions and is the imaging method of choice for improved differential diagnosis in cases of equivocal or indeterminate lesions on ultrasonography or computed tomography[39]. However, it is not always possible to accurately diagnose a given lesion on conventional T1-weighted dynamic phase imaging because of overlapping enhancement patterns between different lesion types[7]. Moreover, the frequent atypical appearance of certain lesion types might further complicate the diagnosis. The development of GBCA with liver-specific properties has markedly improved the accuracy of MR imaging for the identification and characterization of focal liver lesions[40–42].
To date, 2 different LS-GBCAs are available: gadobenate dimeglumine (MultiHance) and gadotexate acid (Primovist). Both contrast agents combine the properties of a conventional non-specific GBCA with that of an agent targeted specifically to hepatocytes[43–45]. This feature permits improved lesion detection and characterization based on the functional characteristics of lesions: lesions that contain functioning hepatocytes are able to take up the Gd-BOPTA contrast-effective molecule of MultiHance in a manner similar to that of normal liver parenchyma, thus appearing iso- to hyperintense on delayed T1-weighted hepatobiliary phase images, whereas lesions that do not contain functioning hepatocytes are generally unable to take up Gd-BOPTA and thus appear hypointense relative to enhanced normal liver parenchyma.
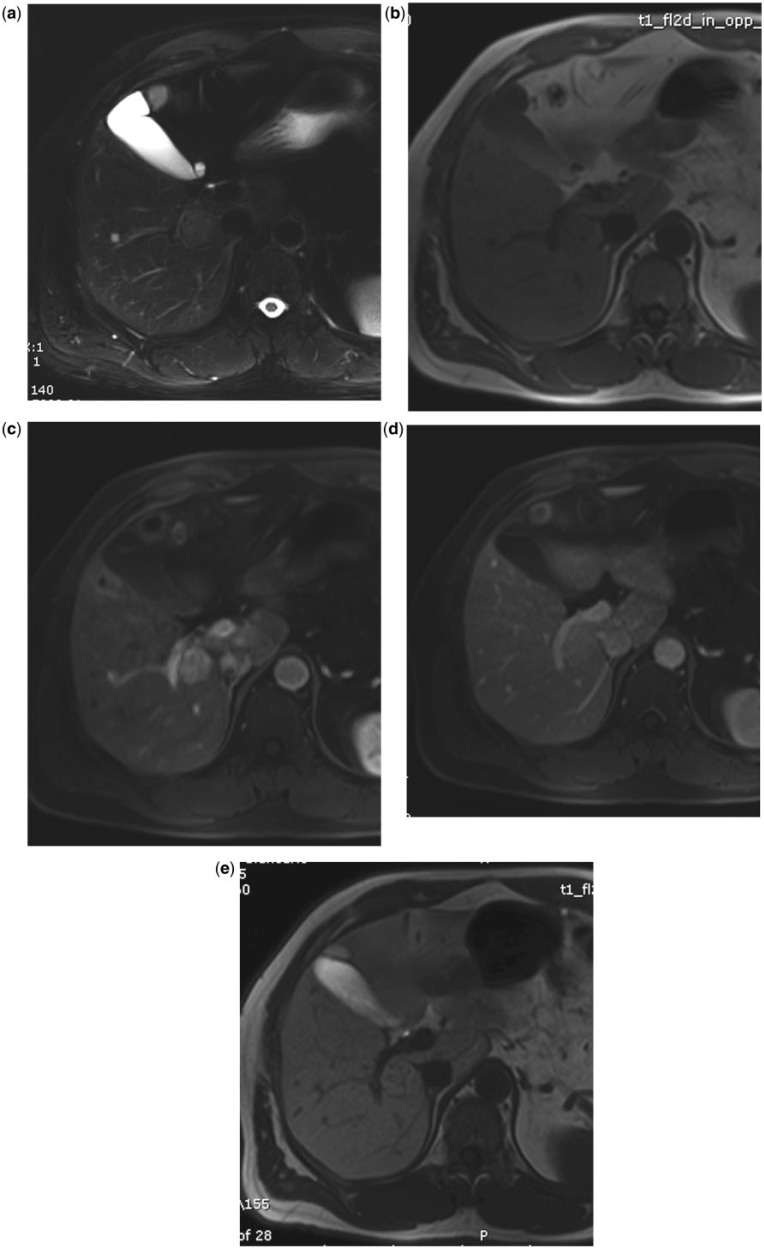
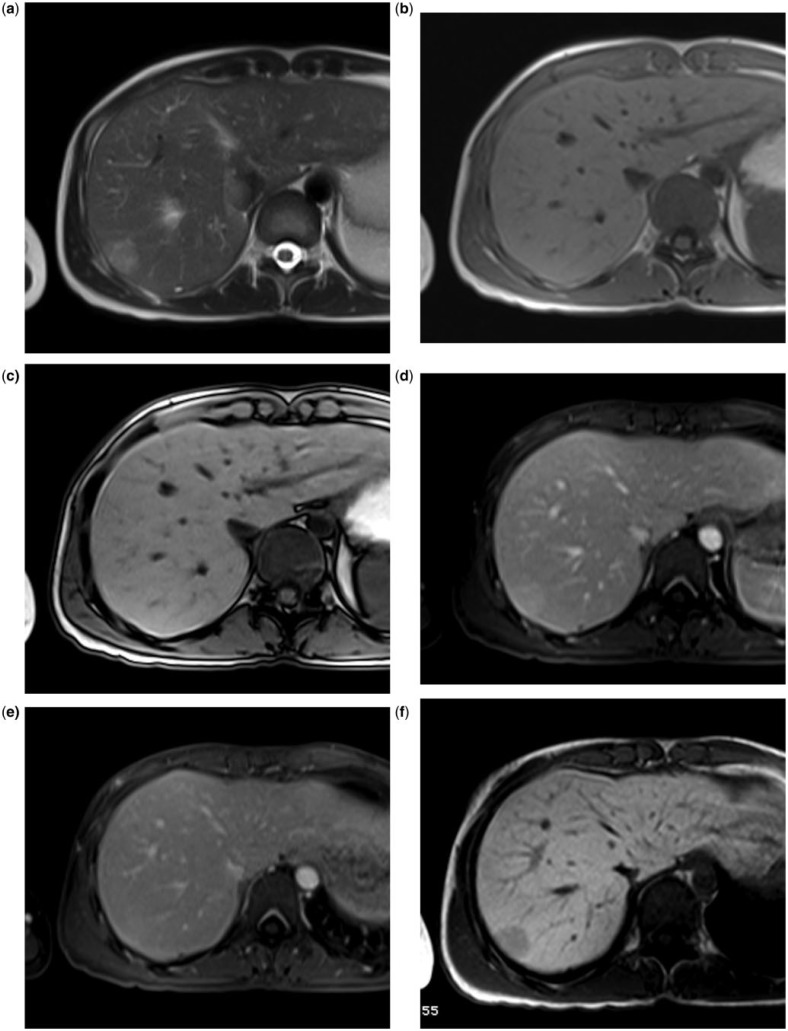
With both LS-GBCAs it is possible to reliably characterize small benign hepatocellular focal liver lesions, such as focal nodular hyperplasia (Fig. 4)[10,41,46,47] or adenoma (Fig. 5)[41,47].
Figure 4.
Small hypervascular liver lesion in a 50-year-old man. MultiHance. The lesion is slightly hyperintense on T2 (a) and hypointense on T1 (b). During dynamic imaging, the lesion is hypervascular in the arterial phase (c), without wash-out in the portal phase (d). During the hepatobiliary phase (e, 2 hours after MultiHance injection), the lesion is hyperintense due to active uptake by the hepatocytes of the lesions: focal nodular hyperplasia.
Figure 5.
Small hypervascular liver lesion in a 35-year-old woman with a history of taking oral contraceptives. MultiHance. The lesion is slightly hyperintense on T2 (a) and isointense on T1, both in phase (b) and out of phase (c). During dynamic imaging, the lesion is slight hypervascular in the arterial phase (d), without wash-out in the portal phase (e). During the hepatobiliary phase (f, 2 h after MultiHance injection), the lesion is hypointense due to lack of uptake by the hepatocytes of the lesions: adenoma.
With malignant focal liver lesions, both contrast agents increase the accuracy of MR imaging in detecting and characterizing metastases compared with dynamic imaging[8,48] and diffusion-weighted imaging (DWI)[49,50]. According to recent meta-analyses, MR with LS-GBCA has the best accuracy in detecting colorectal metastases[51,52].
In the cirrhotic liver, although dynamic imaging is the key sequence to evaluate the wash-in/wash-out enhancement pattern of hepatocellular carcinoma (HCC) (i.e. the most important suggestive feature of lesion malignancy[53]), the use of LS-GBCA allows not only the acquisition of conventional dynamic phase images but also provides additional information on the capacity of the nodule to take up the contrast agent; in the cirrhotic liver, hypointensity of a nodule in the hepatobiliary phase is a sign of malignancy[8]. Association of hepatobiliary phase images after Primovist with DWI images helps in evaluating hypovascular nodules; hyperintensity on diffusion-weighted images in hypovascular hypointense nodules on hepatobiliary phase Primovist-enhanced MR imaging in patients with cirrhosis is strongly associated with progression to hypervascular HCC[54].
Conclusions
The use of GBCAs in oncological MR imaging is fundamental for the management of patients, allowing better detection and characterization of malignant lesions. Different classes of GBCAs are available. An understanding of their mechanism of contrast enhancement allows more precise information to be obtained, according to the clinical need. According to comparative studies, HR-GBCAs allow superior enhancement and contrast delineation of lesions especially during steady-state imaging studies, whereas at first-pass imaging studies, a significant difference between HC-GBCA and HR-GBCA has not been demonstrated up to now.
Not all areas of oncological imaging have been addressed here; other important fields (e.g. prostate imaging; pelvic neoplasm) need to be explored.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This article was presented at the ICIS Society Meeting and 13th Annual Teaching Course, York, UK, 30 September to 2 October 2013.
References
- 1.Lin SP, Brown JJ. MR contrast agents: physical and pharmacologic basics. J Magn Reson Imaging. 2007;25:884–899. doi: 10.1002/jmri.20955. [DOI] [PubMed] [Google Scholar]
- 2.Caravana O, Farrara CT, Frullanoa L, Uppala R. Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T1 contrast agents. Contrast Media Mol Imaging. 2009;4:89–100. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellin FM, Van Der Molen AJ. Extracellular gadolinium-based contrast media: an overview. Eur J Radiol. 2008;66:160–167. doi: 10.1016/j.ejrad.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Giesel FL, von Tengg-Kobligk F, Wilkinson LD, et al. Influence of human serum albumin on longitudinal and transverse relaxation rates (R1 and R2) of magnetic resonance contrast agents. Invest Radiol. 2006;41:222–228. doi: 10.1097/01.rli.0000192421.81037.d5. [DOI] [PubMed] [Google Scholar]
- 5.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann H-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 6.Semelka RC, Shoenut JP, Kroeker MA, et al. Focal liver disease: comparison of dynamic contrast-enhanced CT and T2-weighted fat-suppressed, FLASH, and dynamic gadolinium-enhanced MR imaging at 1.5 T. Radiology. 1992;184:687–694. doi: 10.1148/radiology.184.3.1324509. [DOI] [PubMed] [Google Scholar]
- 7.Hamm B, Thoeni RF, Gould RG, et al. Focal liver lesions: characterization with nonenhanced and dynamic contrast material-enhanced MR imaging. Radiology. 1994;190:417–423. doi: 10.1148/radiology.190.2.8284392. [DOI] [PubMed] [Google Scholar]
- 8.Morana G, Grazioli L, Kirchin MA, et al. Solid hypervascular liver lesions: accurate identification of true benign lesions on enhanced dynamic and hepatobiliary phase magnetic resonance imaging after gadobenate dimeglumine administration. Invest Radiol. 2011;46:225–239. doi: 10.1097/RLI.0b013e3181feee3a. [DOI] [PubMed] [Google Scholar]
- 9.Caudana R, Morana G, Pirovano GP, et al. Focal malignant hepatic lesions: MR imaging enhanced with gadolinium benzyloxypropionictetra-acetate (BOPTA)–preliminary results of phase II clinical application. Radiology. 1996;199:513–520. doi: 10.1148/radiology.199.2.8668804. [DOI] [PubMed] [Google Scholar]
- 10.Grazioli L, Morana G, Federle MP, et al. Focal nodular hyperplasia: morphologic and functional information from MR imaging with gadobenate dimeglumine. Radiology. 2001;221:731–739. doi: 10.1148/radiol.2213010139. [DOI] [PubMed] [Google Scholar]
- 11.Warmuth-Metz Post-operative imaging after brain tumor resection. Acta Neurochir Suppl. 2003;88:13–20. doi: 10.1007/978-3-7091-6090-9_4. [DOI] [PubMed] [Google Scholar]
- 12.Berger MS. Malignant astrocytomas: surgical aspects. Semin Oncol. 1995;21:172–184. [PubMed] [Google Scholar]
- 13.Greco A, Parker JR, Ratcliffe CG, Kirchin MA, McNamara MT. Phase III, randomized, double blind, crossover comparison of gadoteridol and gadopentetate dimeglumine in magnetic resonance imaging of patients with intracranial lesions. Australas Radiol. 2001;45:457–463. doi: 10.1046/j.1440-1673.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 14.Knopp MV, Runge VM, Essig M, et al. Primary and secondary brain tumors at MR imaging: bicentric intraindividual crossover comparison of gadobenate dimeglumine and adopentetate dimeglumine. Radiology. 2004;230:55–64. doi: 10.1148/radiol.2301021085. [DOI] [PubMed] [Google Scholar]
- 15.Colosimo C, Knopp MV, Barreau X, et al. A comparison of Gd-BOPTA and Gd-DOTA for contrast-enhanced MRI of intracranial tumours. Neuroradiology. 2004;46:655–665. doi: 10.1007/s00234-003-1128-4. [DOI] [PubMed] [Google Scholar]
- 16.Maravilla KR, Maldjian JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology. 2006;240:389–400. doi: 10.1148/radiol.2402051266. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn MJ, Picozzi P, Maldjian JA, et al. Evaluation of intraaxial enhancing brain tumors on magnetic resonance imaging: intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for visualization and assessment, and implications for surgical intervention. J Neurosurg. 2007;106:557–566. doi: 10.3171/jns.2007.106.4.557. [DOI] [PubMed] [Google Scholar]
- 18.Rowley HA, Scialfa G, Gao PY, Maldjian JA, Hassell D, Kuhn MJ. Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol. 2008;29:1684–1691. doi: 10.3174/ajnr.A1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rumboldt Z, Rowley HA, Steinberg F, et al. Multicenter, double-blind, randomized, intra-individual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine in MRI of brain tumors at 3 tesla. J Magn Reson Imaging. 2009;29:760–767. doi: 10.1002/jmri.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colosimo C, Demaerel P, Tortori-Donati P, et al. Comparison of gadobenate dimeglumine (Gd-BOPTA) with gadopentetate dimeglumine (Gd-DTPA) for enhanced MR imaging of brain and spine tumours in children. Pediatr Radiol. 2005;35:501–510. doi: 10.1007/s00247-004-1392-4. [DOI] [PubMed] [Google Scholar]
- 21.Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P. Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol. 2009;50:933–940. doi: 10.1080/02841850903095385. [DOI] [PubMed] [Google Scholar]
- 22.Anzalone N, Scarabino T, Venturi C, et al. Cerebral neoplastic enhancing lesions: Multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0 M) and gadoterate meglumine (0.5 M) at 0.1 mmolGd/kg body weight in a clinical setting. Eur J Radiol. 2013;82:139–145. doi: 10.1016/j.ejrad.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Center for Drug Evaluation and Research. Application number 201277Orig1s000; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/201277. Accessed 8 February 2012. [Google Scholar]
- 24.Seidl Z, Vymazal J, Mechl M, Goyal M, Herman M, Colosimo C. Does higher gadolinium concentration play a role in the morphologic assessment of brain tumors? Results of a multicenter intraindividual crossover comparison of gadobutrol versus gadobenate dimeglumine (the MERIT Study) AJNR Am J Neuroradiol. 2012;33:1050–1058. doi: 10.3174/ajnr.A3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giesel FL, Mehndiratta A, Risse F, Rius M, Zechmann CM, von Tengg-Kobligk H. Intraindividual comparison between gadopentetate dimeglumine and gadobutrol for magnetic resonance perfusion in normal brain and intracranial tumors at 3 Tesla. Acta Radiol. 2009;50:521–530. doi: 10.1080/02841850902787685. [DOI] [PubMed] [Google Scholar]
- 26.Essig M, Lodemann KP, Le-Huu M, Brüning R, Kirchin M, Reith W. Intraindividual comparison of gadobenate dimeglumine and gadobutrol for cerebral magnetic resonance perfusion imaging at 1.5 T. Invest Radiol. 2006;41:256–263. doi: 10.1097/01.rli.0000191333.19068.6b. [DOI] [PubMed] [Google Scholar]
- 27.Thilmann O, Larsson EM, Björkman-Burtscher IM, Ståhlberg F, Wirestam R. Comparison of contrast agents with high molarity and with weak protein binding in cerebral perfusion imaging at 3 T. J Magn Reson Imaging. 2005;22:597–604. doi: 10.1002/jmri.20420. [DOI] [PubMed] [Google Scholar]
- 28.Heiland S, Erb G, Ziegler S, Krix M. Where contrast agent concentration really matters - a comparison of CT and MRI. Invest Radiol. 2010;45:529–537. doi: 10.1097/RLI.0b013e3181ea703d. [DOI] [PubMed] [Google Scholar]
- 29.Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;244:672–691. doi: 10.1148/radiol.2443051661. [DOI] [PubMed] [Google Scholar]
- 30.Kuhl CK. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 31.Pediconi F, Catalano C, Occhiato R, et al. Breast lesion detection and characterization at contrast-enhanced MR mammography: gadobenate dimeglumine versus gadopentetate dimeglumine. Radiology. 2005;237:45–56. doi: 10.1148/radiol.2371041369. [DOI] [PubMed] [Google Scholar]
- 32.Pediconi F, Catalano C, Padula S, et al. Contrast-enhanced MR mammography: improved lesion detection and differentiation with gadobenate dimeglumine. AJR Am J Roentgenol. 2008;191:1339–1346. doi: 10.2214/AJR.07.3533. [DOI] [PubMed] [Google Scholar]
- 33.Martincich L, Faivre-Pierret M, Zechmann CM, et al. Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for breast MR imaging (DETECT Trial) Radiology. 2011;258:396–408. doi: 10.1148/radiol.10100968. [DOI] [PubMed] [Google Scholar]
- 34.Sardanelli F, Fausto A, Esseridou A, Di Leo G, Kirchin MA. Gadobenate dimeglumine as a contrast agent for dynamic breast magnetic resonance imaging: effect of higher initial enhancement thresholds on diagnostic performance. Invest Radiol. 2008;43:236–242. doi: 10.1097/RLI.0b013e318160678d. [DOI] [PubMed] [Google Scholar]
- 35.Carbonaro LA, Verardi N, Di Leo G, Sardanelli F. Handling a high relaxivity contrast material for dynamic breast MR imaging using higher thresholds for the initial enhancement. Invest Radiol. 2010;45:114–120. doi: 10.1097/RLI.0b013e3181cc2929. [DOI] [PubMed] [Google Scholar]
- 36.Pediconi F, Kubik-Huch R, Chilla B, Schwenke C, Kinkel K. Intra-individual randomised comparison of gadobutrol 1.0 M versus gadobenate dimeglumine 0.5 M in patients scheduled for preoperative breast MRI. Eur Radiol. 2013;23:84–92. doi: 10.1007/s00330-012-2557-4. [DOI] [PubMed] [Google Scholar]
- 37.Schneider G, Fries P. Letter to the Editor re: intra-individual randomised comparison of gadobutrol 1.0 M versus gadobenate dimeglumine 0.5 M in patients scheduled for preoperative breast MRI. Eur Radiol. 2013;23:2095–2096. doi: 10.1007/s00330-013-2879-x. [DOI] [PubMed] [Google Scholar]
- 38.Pennekamp W, Roggenland D, Hering S, et al. Intra-individual, randomised comparison of the MRI contrast agents gadobutrol and gadoterate in imaging the distal lower limb of patients with known or suspected osteomyelitis, evaluated in an off-site blinded read. Eur Radiol. 2011;21:1058–1067. doi: 10.1007/s00330-010-2008-z. [DOI] [PubMed] [Google Scholar]
- 39.Elsayes KM, Leyendecker JR, Menias CO, et al. MRI characterization of 124 CT-indeterminate focal hepatic lesions: evaluation of clinical utility. HPB (Oxford) 2007;9:208–215. doi: 10.1080/13651820701216950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morana G, Grazioli L, Testoni M, Caccia P, Procacci C. Contrast agents for hepatic magnetic resonance imaging. Top Magn Reson Imaging. 2002;13:117–150. doi: 10.1097/00002142-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Grazioli L, Morana G, Kirchin MA, Schneider G. Accurate differentiation of focal nodular hyperplasia from hepatic adenoma at gadobenate dimeglumine – enhanced MR imaging: prospective study. Radiology. 2005;236:166–177. doi: 10.1148/radiol.2361040338. [DOI] [PubMed] [Google Scholar]
- 42.Ichikawa T, Saito K, Yoshioka N, et al. Detection and characterization of focal liver lesions: a Japanese phase III, multicenter comparison between gadoxetic acid disodium-enhanced magnetic resonance imaging and contrast-enhanced computed tomography predominantly in patients with hepatocellular carcinoma and chronic liver disease. Invest Radiol. 2010;45:133–141. doi: 10.1097/RLI.0b013e3181caea5b. [DOI] [PubMed] [Google Scholar]
- 43.Kirchin MA, Pirovano G, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA): an overview. Invest Radiol. 1998;33:798–809. doi: 10.1097/00004424-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Hamm B, Staks T, Muhler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 45.Morana G, Salviato E, Guarise A. Contrast agents for hepatic MRI. Cancer Imaging. 2007;7(Spec No A):S24–27. doi: 10.1102/1470-7330.2007.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta RT, Iseman CM, Leyendecker JR, Shyknevsky I, Merkle EM, Taouli B. Diagnosis of focal nodular hyperplasia with MRI: multicenter retrospective study comparing gadobenate dimeglumine to gadoxetate disodium. AJR Am J Roentgenol. 2012;199:35–43. doi: 10.2214/AJR.11.7757. [DOI] [PubMed] [Google Scholar]
- 47.Mohajer K, Frydrychowicz A, Robbins JB, Loeffler AG, Reed TD, Reeder SB. Characterization of hepatic adenoma and focal nodular hyperplasia with gadoxetic acid. J Magn Reson Imaging. 2012;36:686–696. doi: 10.1002/jmri.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YK, et al. Detection of liver metastases: Gd BOPTA-enhanced dynamic phases and delayed phase MR imaging versus SPIO-enhanced MR imaging. Eur Radiol. 2005;15:220–228. doi: 10.1007/s00330-004-2570-3. [DOI] [PubMed] [Google Scholar]
- 49.Shimada K, Isoda H, Hirokawa Y, Arizono S, Shibata T, Togashi K. Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. Eur Radiol. 2010;20:2690–2698. doi: 10.1007/s00330-010-1842-3. [DOI] [PubMed] [Google Scholar]
- 50.Lowenthal D, Zeile M, Lim WY, et al. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011;21:832–840. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 51.Floriani I, Torri V, Rulli E, et al. Performance of imaging modalities in diagnosis of liver metastasis from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging. 2010;31:19–31. doi: 10.1002/jmri.22010. [DOI] [PubMed] [Google Scholar]
- 52.Neikel MC, Bipat S, Stoker S. Diagnostic imaging of CLM with CT, MRI, FDG PET/PET-CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 53.EASL–EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Kim YK, Lee WJ, Park MJ, Kim SH, Rhim H, Choi D. Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology. 2012;265:104–114. doi: 10.1148/radiol.12112649. [DOI] [PubMed] [Google Scholar]