Abstract
Aims
The aim of the present study was to evaluate a mobile health (mHealth) based remote medication adherence measurement system (mAMS) in elderly patients with increased cardiovascular risk treated for diabetes, high cholesterol and hypertension. Cardiovascular risk was defined as the presence of at least two out of the three risk factors: type 2 diabetes, hypercholesterolaemia and hypertension.
Methods
For treatment of diabetes, hypercholesterolaemia and hypertension, four predefined routinely used drugs were selected. Drug adherence was investigated in a controlled randomized doctor blinded study with crossover design. The mAMS was used to measure and improve objectively the adherence by means of closed-loop interactions.
Results
The mean age of the 53 patients (30 female) was 69.4 ± 4.8 years. A total of 1654 electronic blisters were handed out. A statistically significant difference (P = 0.04) between the monitoring and the control phase was observed for the diabetes medication only. In a post-study questionnaire twenty-nine patients appreciated that their physician knew if and when they had taken their medications and 13 asked for more or automated communication with their physicians. Only one subject withdrew from the study because of technical complexity.
Conclusions
The results indicate that mHealth based adherence management is feasible and well accepted by patients with increased cardiovascular risk. It may help to increase adherence, even in patients with high baseline adherence and, subsequently, lead to improved control of indicators including blood pressure and cholesterol concentrations. Electronic blisters can be used in a multi-medication regimen but need to be carefully designed for day-to-day application.
Keywords: adherence, diabetes, e-blister, mHealth, telemedicine
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Adherence to medication regimens is crucial for effective treatment of chronic disease but is often low.
A variety of adherence management systems have been developed to improve this situation.
mHealth based e-blister technology had not previously been evaluated in a real world clinical environment.
WHAT THIS STUDY ADDS
mHealth based adherence management is feasible and well accepted by patients with increased cardiovascular risk.
It may help to increase adherence, even in patients with high baseline adherence.
e-blisters can be used in a multi-medication regimen but need to be improved for day-to-day application.
Introduction
The demographic change in the 21st century demands new strategies in health care for the elderly. Allowing people to stay in their home environment as long as possible is an important goal in all European societies. Regular intake of prescribed medication is of significant importance especially for aged subjects and technical tools might help. Innovative information and communication technologies are generally supposed to play an important role in retaining elderly patients in their home environment for longer [1].
Providing continuous medical care for elderly subjects living in their own homes represents a major challenge. An essential prerequisite for successful therapy is the insight and co-operation of patients as indicated by regular intake of prescribed drugs (adherence). In order to inform patients about their drug intake and to enhance their adherence we have developed an innovative mobile health (mHealth) based medication adherence management system (mAMS).
The prevalence of type 2 diabetes is increasing due to both a diabetogenic lifestyle and the growing life expectancy of diabetics [2]. In a population-based study in Germany in 2001, the prevalence of diabetes was estimated at 8.8% in the total population and was 25% in the age group of 70+ years [3]. Multifactorial and intense treatment of diabetes reduces the number of micro and macrovascular complications [4] as well as mortality [5].
Another major risk factor for coronary heart disease (CHD) is diabetic dyslipidaemia [6]. A third major cardiovascular risk factor is arterial hypertension. Due to the greatly increased CHD risk of type 2 diabetes, diabetes is regarded as a CHD risk equivalent in current guidelines for lipid management [7].
The aim of the present study was to evaluate a mHealth based remote mAMS in elderly patients with increased cardiovascular risk and treated with medications for diabetes, high cholesterol and hypertension. Cardiovascular risk was defined as the presence of at least two out of the three risk factors: type 2 diabetes, hypercholesterolaemia and hypertension. Adherence was investigated in a crossover design by pill count and by a mAMS.
Methods
mHealth based mAMS
A mAMS was set up to monitor objectively a patient's behaviour in taking the prescribed medication and hence to measure and, if insufficient, to improve his/her adherence by means of closed loop interactions. The system utilized common information and communication technologies (Web and mobile phone applications) as well as embedded microelectronic components (including printed electronics) to record the timestamp and amount of pills taken. The architecture of the system (Figure 1) and the components used by patients and physicians have been described previously [8]. Briefly, the system comprised of the following elements:
Figure 1.
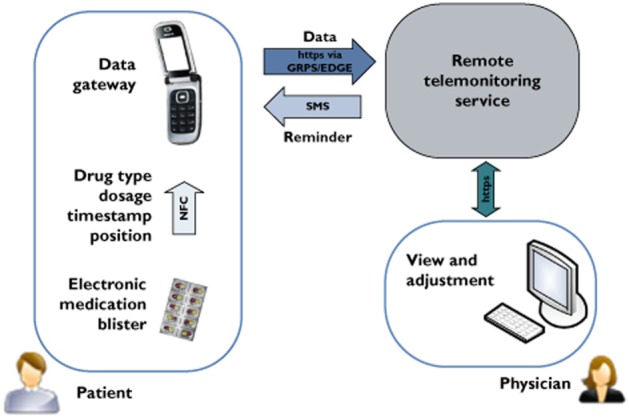
Architecture of mHealth based medication adherence measurement system; NFC enabled mobile phone acts as data gateway to transmit the blister data to a remote telemonitoring service, where it can be seen by a physician
Electronic medication blister
To track objectively the dosage and timing of medication intake, we used electronic medication blisters (OtCM, DSM TCG B.V., Mauritslaan, the Netherlands) as add-ons to standard medication blisters. Printed circuitry labels were applied to the aluminium foil cover of the blister (bottom side) and linked to a small printed circuit board (positioned on the blister's upper side) where some electronic parts were mounted. Taking out a pill broke the conductive track inside the label directly underneath the respective position. This event was recognized and led to storage of the corresponding data (position and time stamp) in the microcontroller's internal memory. These data could be interrogated wirelessly by a near field communication (NFC) enabled reader device.
Mobile phone based data gateway
In order to read and process these data NFC enabled mobile phones (Nokia 6131 NFC, Nokia, Espoo, Finland) running a dedicated software application were used. The application was designed to launch automatically after touching a patient specific user card based on radio frequency identification (RFID) technology. This card also acted as security token and stored a unique user ID which was used to authenticate the patients against the backend system. Once the application was launched a graphical user interface asked the user to interrogate the data stored on the blister. By touching it with the mobile phone (Figure 2) the data were read automatically and immediately transmitted to a remote database via a mobile Internet connection based on General Packet Radio Service (GPRS) or Enhanced Data Rates for GSM Evolution (EDGE) technology. If an acknowledged transmission could not be performed, e.g. due to a temporary lack of mobile network connectivity, data were stored on the mobile phone to be synchronized during the next transmission session together with the data from the subsequent blister readout.
Figure 2.

Gesture of touching the blister with the mobile phone to read out the event data
Remote telemonitoring service
A web-based telehealth system received the data sent from the mobile phone and analyzed aspects including timing and number of pills taken. Processed data were used to remind the patients automatically via SMS messages that, for privacy reasons, did not contain any medical content. Data were presented to the study physician numerically and graphically via browser based user interface.
Study design
A randomized single-blinded (doctor blinded), controlled, single centre study with crossover design was started with the aim to include 150 patients with a defined risk for cardiovascular conditions. These conditions were characterized by the presence of at least two out of three health risks:
Type 2 diabetes
Hypertension
Hypercholesterolaemia
Medication adherence of the participating patients was measured based on four defined standard drugs for treating diabetes (metformin 1000 mg, Arcana), hypercholesterolaemia (simvastatin 40 mg, Bayer and rosuvastatin 10 mg, AstraZeneca) and hypertension (ramipril 5 mg, Sandoz).
After signing informed consent the participants were registered to the mAMS and randomized to one of two study groups (group 1 or group 2). Initially all participants had to pass a 4 week run-in period with standard medication blisters and handwritten medication intake diaries. Afterwards, participants randomized to group 1 started with a monitoring phase (MON) using the mAMS. Patients randomized to group 2 started with a control phase (CON) characterized by standard medication blisters, routine care and handwritten medication intake diaries.
After a period of 20 weeks all patients changed to the crossover phase (group 1: MON changed to CON and group 2: CON changed to MON). This complementary phase lasted for another 20 weeks. This crossover design was chosen to assess the differences in medication adherence in both groups. After the complementary phase it was intended that all patients had to run through a 4 week control phase followed by a 4 week monitoring phase. The idea behind these two phases at the end of the study was to examine the sustainable effect of given prescriptions. Figure 3 shows the time flow of the study phases in both groups.
Figure 3.
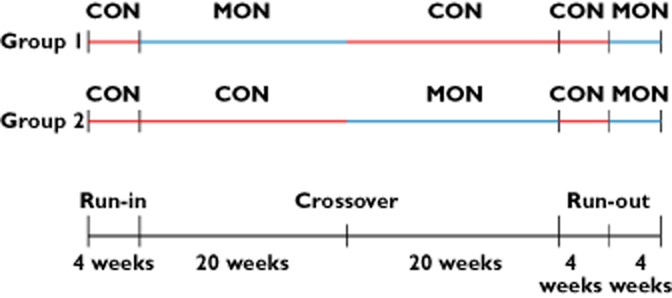
Time flow of study phases in group 1 and group 2
In the monitoring phase the prescription of medication intake (dosage and timeframe to be taken: morning, noon, evening, night) was entered into the system by the study physician. Patients were equipped with a user ID card, the mobile phone and the electronic blisters and were trained in using them.
Each time the patient took out a pill from a blister (according to the defined scheme) he/she had to perform the following procedure for each blister:
touch the user ID card with the mobile phone (to automatically launch the application and login);
readout the event data by touching the blister with the mobile phone;
wait until data were transmitted successfully (indicated on display) and the application was closed automatically.
If the patients did not transmit their recorded events as defined, the system automatically triggered an SMS to be sent to the patients' study phones on the following day reminding them to transmit the data regularly and on time. A study co-ordinator regularly observed the transmitted data and contacted all subjects with minor adherence (<70% of prescribed drugs taken) via phone call once a week, trying to increase the adherence motivation of patients.
Throughout the entire study medication adherence was verified with respect to the four defined drugs. Standard medication blisters as returned after the control phases were used for pill counting. Documented pill counts of these blisters of each patient were compared with electronically documented data acquired by the mAMS in the monitoring phase.
Statistical analysis
The primary study end point was the intake rate in both 20 weeks phases (MON and CON). The secondary end point was the comparison of laboratory data (fasting blood glucose concentration, HbA1c, blood cholesterol concentration) and blood pressure, measured during routinely scheduled checks at the beginning and at the end of the study.
Since this was a pilot project, no power calculations were done to compute sample size. The planned sample size of 150 subjects was determined by clinical considerations.
In the analysis, only data of subjects who entered the crossover phase were included. Baseline patient characteristics were not different between drop-out and finally analyzed subjects (data not shown). The distribution of data was tested with a Kolmogorov-Smirnov test. Normally distributed data at the beginning and at the end of the study were compared using a paired t-test and for non-normally distributed data, Wilcoxon test and Kruskal–Wallis test were used. Frequencies were compared using the Chi-square test. A two-sided value of P < 0.05 was considered statistically significant. Analyses were performed using Medcalc 12.3 (http://www.medcalc.be).
A questionnaire consisting of eight questions to assess the usability, benefit, as well as potential design improvements of the components, was handed out at the end of the monitoring phase. In addition to that the patients' behaviour was examined by analyzing the recorded data in terms of time delay between the takeout of a pill and the corresponding data transmission.
The study was submitted to the ethics committee of the city of Vienna. The ethics committee responded that an appraisal was not necessary since this study was neither classified as a clinical trial nor as trial to examine a new medical methodology. Nevertheless the study was registered at the Austrian data privacy committee due to the fact that personal data were recorded and processed.
Results
Course of the study and characteristics
The study was conducted at the diabetes outpatient clinic, Health Centre South, Vienna, Austria. Due to a decision by the study sponsor, i.e. the company that provided the electronic blisters, the study was terminated prematurely after a period of 13 months. Within this period 77 patients had been registered in the study. At the time of termination 24 drop outs were counted due to different reasons. Six participants stopped participation immediately after the first follow-up without specifying a reason while seven participants recalled their consent. Six had to stop due to medical reasons which were not linked with the study, while four participants showed adverse reactions to the study medication. A single patient stopped using the system because of difficulties to operate it. Because of the premature termination no participant reached the two 4 week study phases at the end of the study. Thus, no results about sustainable effect of given prescriptions could be obtained.
The data of the 53 patients who entered the crossover phase before termination were considered for final statistics. These 53 patients (30 female) had a mean age of 69.4 ± 4.75 years and participated for a mean period of 296 ± 35 days in the study. All of them had been equipped with the mobile phone, the user ID card and electronic blisters to be monitored by the mAMS for a mean period of 125.7 ± 27.4 days per patient. A total of 1654 electronic blisters were handed out, with an average of 30.6 ± 13.1 blisters per patient. Table 1 summarizes these data. Metformin was prescribed the most with a proportion of 57.8% (957 blisters). The other three drugs were handed out less frequently with a quantity of 332 (ramipril), 193 (simvastatin) and 172 (rosuvastatin) blisters.
Table 1.
Characteristics of patients who finished the crossover phase; values are presented as mean ± SD
| Participants until termination of study | 53 |
| Female/male (n) | 24/29 |
| Age (years) | 69.4 ± 4.8 |
| Study period (days) | 296 ± 35 |
| Monitoring period (days) | 125.6 ± 27.7 |
| Used electronic blisters (n) | 30.9 ± 13.0 |
| Waist circumference (cm) | 112 ± 15 |
| Body mass index (kg m−2) | 32.9 ± 5.9 |
Primary end point – medication adherence
A comparison of the medication adherence in the monitoring and control phase related to the four different medications showed a significant difference in intake of metformin (P = 0.04) in favour of the MON phase. This outcome did not consider the two study groups individually. Regarding the other three medications no significant difference was found. Table 2 shows the comparison of the intake of these four medications in both study phases. These numbers are based on the pill count of standard medication blisters in the control phase and the documented events of the mAMS in the monitoring phase, respectively.
Table 2.
Medication compliance of all study phases independent of cross-over direction
| Monitoring phase (electronic blisters) | Control phase (standard blisters) | ||||||
|---|---|---|---|---|---|---|---|
| median (IQR) | min | max | median (IQR) | min | max | P | |
| Metformin | 1 (1 – 1) | 0,93 | 1 | 1 (1 – 1) | 0,89 | 1 | 0.04 |
| Simvastatin | 1 (1 – 1) | 0,93 | 1 | 1 (1 – 1) | 1 | 1 | 0.50 |
| Rosuvastatin | 1 (1 – 1) | 0,97 | 1 | 1 (1 – 1) | 0,91 | 1 | 0.23 |
| Ramipril | 1 (1 – 1) | 0,96 | 1 | 1 (1 – 1) | 0,93 | 1 | 0.50 |
IQR, interquartile range.
Considering both groups individually a direct comparison of the crossover paths (group 1 P = 0.52 vs. group 2 P = 0.23) revealed that there were no significant differences in medication adherence rates.
A comparison of adherence in matters of time by comparing manual written diary data in the control phase and automatically documented data in the control phase was not carried out.
During the monitoring phase a lack of adherence was detected in seven participants. Consequently, these patients were contacted by the study coordinator in order to increase their adherence. On average, each of them was contacted twice.
Secondary end point – change in laboratory parameters
At the time of inclusion into the study and during a follow-up meeting at the end of the observation period each participant had a routinely scheduled laboratory check to assess the target vital parameters. These data were obtained from 49 of the 53 participants who entered the crossover phase. As shown in Table 3, the P values indicate significant improvements in blood pressure (systolic and diastolic values) as well as in total and LDL cholesterol concentrations.
Table 3.
Secondary end points measured at the beginning of the study and at the end of the crossover phase; Values are presented as mean ± SD or median (lower – upper quartile)
| Beginning | End | P value | |
|---|---|---|---|
| Fasting plasma glucose (mg dl–1) | 141 ± 35 | 139 ± 33 | 0.87 |
| HbA1c (%) | 7,2 ± 0,8 | 7,1 ± 0.9 | 0.06 |
| Body weight (kg) | 92 ± 18 | 91 ± 17 | 0.21 |
| Blood pressure sys/dia (mmHg) | 133/75 | 128/70 | 0.02/0.0003 |
| Total cholesterol (mg dl–1) | 166 (147–183) | 155 (141–167) | 0.02 |
| LDL cholesterol (mg dl–1) | 87 (68–102) | 80 (67–92) | 0.06 |
| HDL cholesterol (mg dl–1) | 48 ± 12 | 47 ± 13 | 0.14 |
Usability and user behaviour
The questionnaire was completed by 52 of the 53 participants, who had finished the monitoring phase. Table 4 shows the questions and the summary of the evaluation.
Table 4.
Results of the questionnaire consisting of eight questions about usability, feasibility and system design
| Questions | Possible answers (number of selections) | Number of respondents (of 52) who answered this question |
|---|---|---|
| Q1: For me, the size of the trial mobile phone, display and keyboard is: | Too small (n = 2) Appropriate (n = 50) Too big (n = 0) | 52 |
| Q2: Is it easy for you to position the antenna of the mobile phone above the sender on the blister in order to start the data transmission? | Yes (n = 44) No (n = 5) | 49 |
| Q3: According to your experience, how reliable is the data transmission from the blister via the trial mobile phone? | Very reliable (n = 18) Reliable (n = 24) So-so (n = 10) Not reliable (n = 0) | 52 |
| Q4: Does the electronic equipment affixed to the medication blister disturb you? | Yes (n = 3) No (n = 48) | 51 |
| Q5: The training (using mobile phone and blister to perform the data transmission) was: | Very good (n = 41) Good (n = 7) Satisfying (n = 4) Poor (n = 0) | 52 |
| Q6: How do you assess the overall usability of the system? | It is easy to use (n = 47) I have difficulties using it (n = 3) I can't really use it (n = 2) | 52 |
| Q7: What is your opinion towards the benefit of the system? | I am glad that my physician knows if and when I have to take my medication (n = 29) I feel reassured as my blood sugar values are transmitted and checked at the study centre (n = 4) I wish more electronic or automated communication with my physician (n = 13) I do not see any benefit (n = 5) I feel watched (n = 1) | 52 |
| Q8: How do you assess the time-wise effort and the use in your daily routine? | The system works well and fast (n = 39) The system works well but it takes too long (n = 3) The system does not yet work properly to use it in my daily routine (n = 7) I tried, but it is too time consuming (n = 1) I tried, but it is technologically too complex (n = 0) | 50 |
User behaviour was assessed after termination of the study based on the data recorded by the mAMS. The backend of the mAMS was operating 24/7 and counted a cumulative monitoring period of 6788 days. In this period a total of 10 359 data transmission sessions with database entries were recognized. These transmissions carried over 14 203 pill takeout events recognized by the electronic blisters. Six hundred and twenty-seven out of these 14 203 recorded pill intakes did not fall into the defined time range and have been labelled by the system as ‘non-adherent’. Thus, the time adherence of all taken pills was calculated to be 95.59%. Each participant in the monitoring phase recorded and transmitted a mean quantity of 263.0 ± 123.4 pill takeout events. The analysis of the time stamps of takeout events and transmissions revealed that only 56% of the detected events were transmitted immediately after the pill was taken out (<5 min) and 88% of the data were transmitted within 24 h after the takeout event. The distribution of time delays between detection and the corresponding transmission of the events is shown in Figure 4.
Figure 4.
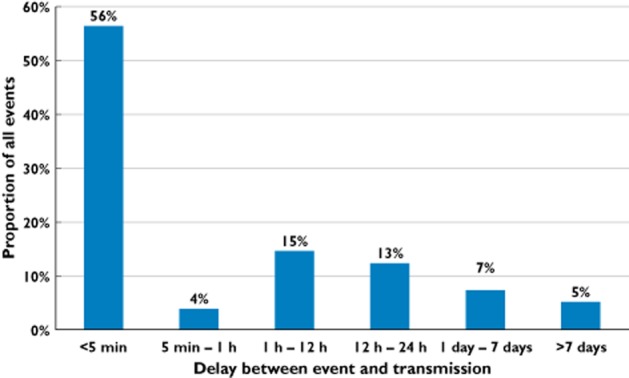
Distribution of time delays between detection and the corresponding transmission of a pill break-out event
Discussion
A variety of different mAMSs have been developed and evaluated though there is not one single nAMS considered to be a gold standard yet. They differ with respect to technology used and also to what degree they provide for ‘closed loop health care’, i.e. to what extent they facilitate interactions of the patient with the physician or pharmacist so as to initiate corrective interventions, for example by means of alerts and reminders. When applied to patients with chronic conditions and comorbidities, providing an appropriate mAMS still remains a challenge [9].
The major limitation of the current mAMS as well as other solutions to document automatically a pill takeout event is the fact that the occurrence of this event does not necessarily mean that the events have been done by the patient. As early as 2002, W. Kort et al., co-author of this article, initiated a survey in cooperation with Novartis′ R&D associate M-Labs (University of St. Gallen, Zürich, Switzerland), leading to the conclusion that, although subjects were aware of the direct (prompt) objective measurement of the pill removal, 5% of the subjects did not actually take the respective medication. Since then a correlation has been established between ‘therapy compliance event’ and ‘therapy outcome event’ by using diagnostic equipment, i.e. glucometers, blood pressure measuring devices, spirometers, body weight scales, etc. The conclusion was that 100% therapy compliance with mAMS was not achievable, and the optimum percentage was 95%. This has been reported by Novartis in May 2006 (Dr Joan Antokol, Vice President, Head, Global Privacy Office, presented at European Commission Information Society and Media).
Full adherence monitoring could only be achieved by more invasive solutions as presented by Rajagopalan et al. who proposed tagging each pill with an RFID chip and a reader necklace that recognizes the swallowing of a pill when it passes the throat [10]. Although this system may be feasible from a technical point of view, it is not likely to be widely adopted in current health care systems except in very special use cases such as medication application in addiction therapy.
Although regular and timely intake of prescribed medication following doctor's guidance lowers the risk for progression of the underlying diseases, overall adherence is assumed to be as low as about 50% [11]. Up to 20% of all prescriptions are not even picked-up from the pharmacy and about one third of patients with chronic diseases discontinue their medication in the first year without proper authorization [12].
There are previous studies dealing with similar mAMS to investigate adherence of patients to prescribed medication, e.g. the so called medication event monitoring system (MEMS). MEMS monitors consist of a conventional medicine bottle fitted with a special closure that records the time and date of each opening and closing of the container through integrated microcircuitry. Rosen et al. [13] preliminarily investigated the adherence of diabetic patients to prescribed metformin using such a MEMS in 79 subjects. Thirty-three of them had less than 80% baseline adherence; they were randomized into a group with cue-dose training over a period of 4 months or into a control group without any training. The cue-dose training resulted in a mean improvement in adhby in an improved metabolic control, measured by HbA1c [13].
In 47 non-insulin dependent diabetic veterans, adherence to sulfonylurea, i.e. oral antihyperglycaemic agents, was assessed by providers, patients′ self-report, pill counts, and a MEMS-3 device. Patients received monthly refills of sulfonylurea in vials with a MEMS cap. Providers and patients were asked to assess adherence and metabolic control. Non-adherence was observed in 47% using MEMS-3, 29% using pill counts, 29% using provider assessment and 31% using self-reporting. Assessment of medication adherence by provider, patient and pill counts did not explain metabolic control as well as assessment by MEMS-3 [14].
In another analysis in veterans, pharmacological or educational recommendations were investigated. Recommendations given by investigators were based on laboratory data and MEMS readings in the treatment group and on laboratory data and pill counts in the control group. In the MEMS group, significantly more recommendations were related to education than in the control group (47 vs. 12%). The authors concluded, that ‘MEMS data resulted in different numbers and types of recommendations than pill counts. Pharmacists then could make specific recommendations regarding patient education before resorting to pharmacologic manipulations’ [15].
Another trial investigated the impact of dosage frequency on the adherence of 91 diabetic subjects to their prescribed medication. In this study, patients received their oral antidiabetic agents from community pharmacies monthly in a MEMS container. Adherence was influenced by the frequency of doses. It was found to be 79% in the case of a single daily dose and 38% in subjects with doses to be taken three times a day. The authors also observed over consumption in more than one-third of subjects, predominantly occurring in patients with a once daily dose schedule [16].
In the current trial, a mAMS with blister-based event detection was used since in Austria drugs are mostly packed in blisters and not in bottles. We calculated the adherence without regarding the order of the study phases. A statistically significant difference (P = 0.04) between the MON and CON phase was observed only for metformin. No differences were found for simvastatin, rosuvastatin and for ramipril treatments. The reasons for only metformin adherence differences reaching statistical significance are likely to be either the higher statistical power related to the higher number of the prescribed metformin blisters or the generally lower medication adherence of several times a day regimen or both. Another important aspect of this outcome was the generally very high baseline rate of medication adherence, when compared with the data in the literature. Minimal adherence was 89% for metformin treatment in the CON phase and 93% in the MON phase and was above 90% for the other drugs in the CON as well as in the MON phase. This might be due to a selection bias that favoured highly motivated subjects to be included. It was interesting to notice that four subjects withdrew from the study due to the typical side effects of metformin, although it had been prescribed to those patients long before the start of the study, maybe indicating at least partial non-adherence before study entry.
The comparison of the laboratory values corresponding to the secondary end point as obtained at the beginning and the end of the study indicated improved values like lower values for systolic and diastolic blood pressure and total and LDL cholesterol with some of them showing statistically significant decreases (Table 3). However, most of the observed differences were probably too small to be considered clinically relevant. Also, since these values were not available at the crossover points, the comparison could be done only for the study cohort as a whole. Therefore, these improvements cannot be attributed to an increased adherence but may have been caused mostly by the intensified communication between the health care team and the participants which is often observed with treatment team changes [17].
Although the two 4 week study phases at the end could not be performed due to premature termination of the study, results of primary and secondary end points were not affected by this decision.
After the study, participants were asked about the usability and the perceived benefits of the mAMS. The majority of patients were satisfied with the ease, speed and reliability of use. Although seven participants reported having some problems with the system, most of the participants, i.e. 39 in total (74%), indicated that the system worked well and could be used in daily routine. Having the electronic components attached to the blister did not disturb patient's routine behaviour. Twenty-nine (56%) of them were glad that their physician knew if and when they took their medication and 13 (25%) asked for even more or automated communication with their physicians. Only one subject withdrew from the study because of technical complexity. From an engineering point of view we came to the conclusion during the trial that the electronics for event detection (microcontroller and coin cell battery) and data transmission (NFC chip and antenna) need to be optimized in terms of form factor and a more stable communication behaviour.
At the beginning of the study a few participants were equipped with a blood glucose meter as well as an additional NFC interface to interrogate the blood glucose measurement data by the NFC enabled mobile phone. Due to the fact that this interface did not work properly quite often, these components were not handed out after a few initial cases.
We are aware of only a single report on a previous trial dealing with an event detection system based on e-blisters similar to the ones used in this trial. Fifty-three diabetes patients were provided with that system to monitor adherence with respect to simvastatin 18. The authors also concluded that blister packs equipped with electronic labels were feasible and acceptable to patients. In contrast to our study, that system was not mHealth-based and was confined to monitoring only a single type of medication.
In conclusion, the results obtained in this randomized crossover feasibility study indicate that mHealth-based adherence management is feasible and well accepted by patients with increased cardiovascular risk. It may help to increase adherence, even in patients with high baseline adherence and, subsequently, lead to improved therapy control. e-blisters can be used in a multi-medication regimen but need to be carefully designed for day-to-day application.
Acknowledgments
The authors would like to thank all volunteers in the study for participating and donating their time. Furthermore, we like to acknowledge Dr Wolfgang Waldschütz, Dr Katharina Hohenecker and Dr Martina Sonnenfeld for helping to recruit the study patients. We also thank Mr Rene Schings (DSM TCG B.V.) and Xander Kort as well as Dr Jörg Dellbrügge for strong support in the organization of the study.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Schülke AM, Plischke H, Kohls NB. Ambient Assistive Technologies (AAT): socio-technology as a powerful tool for facing the inevitable sociodemographic challenges? Philos Ethics Humanit Med. 2010;5:8. doi: 10.1186/1747-5341-5-8. doi: 10.1186/1747-5341-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2•7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Hauner H, Köster I, von Ferber L. [Prevalence of diabetes mellitus in Germany 1998–2001. Secondary data analysis of a health insurance sample of the AOK in Hesse/KV in Hesse] Dtsch Med Wochenschr. 2003;128:2632–2637. doi: 10.1055/s-2003-812396. [DOI] [PubMed] [Google Scholar]
- 4.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 6.Taskinen M. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 7.Haffner S. Rationale for new American Diabetes Association Guidelines: are national cholesterol education program goals adequate for the patient with diabetes mellitus? Am J Cardiol. 2005;96(4A):33E–36. doi: 10.1016/j.amjcard.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Morak J, Schwarz M, Hayn D, Schreier G. Feasibility of mHealth and near field communication technology based medication adherence monitoring. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:272–275. doi: 10.1109/EMBC.2012.6345922. [DOI] [PubMed] [Google Scholar]
- 9.Stegemann S, Baeyens JP, Cerreta F, Chanie E, Loefgren A, Maio M, Schreier G, Thesing-Bleck E. Adherence measurement systems and technology for medications in older patient populations. Eur Geriatr Med. 2012;3:245–260. [Google Scholar]
- 10.Rajagopalan H, Rahmat-Samii Y. Ingestible RFID bio-capsule tag design for medical monitoring. Antennas and Propagation Society International Symposium (APSURSI), 2010 IEEE, pp 1, 4, 11–17 July 2010.
- 11.World Health Organization. Adherence to long term therapies: evidence for action. 2003. Available at http://whqlibdoc.who.int/publications/2003/9241545992.pdf (last accessed 8 July 2013)
- 12.Düsing R. [Adherence to medical treatment] Dtsch Med Wochenschr. 2006;131(46 Spec No):H28–30. doi: 10.1055/s-2006-955059. [DOI] [PubMed] [Google Scholar]
- 13.Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42:409–422. doi: 10.1016/S0005-7967(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 14.Mason BJ, Matsuyama JR, Jue SG. Assessment of sulfonylurea adherence and metabolic control. Diabetes Educ. 1995;21:52–57. doi: 10.1177/014572179502100109. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama JR, Mason BJ, Jue SG. Pharmacists' interventions using an electronic medication-event monitoring device's adherence data versus pill counts. Ann Pharmacother. 1993;27:851–855. doi: 10.1177/106002809302700705. [DOI] [PubMed] [Google Scholar]
- 16.Paes AH, Bakker A, Soe-Agnie CJ. Impact of dosage frequency on patient compliance. Diabetes Care. 1997;20:1512–1517. doi: 10.2337/diacare.20.10.1512. [DOI] [PubMed] [Google Scholar]
- 17.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, Owens DK. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 18.Craven AJ, Oke JL, Griffin SJ, Sutton S, Hardeman W, Kinmonth A-L, Farmer AJ. Feasibility and acceptability of electronic blister packaging to measure medication adherence in patients with type 2 diabetes in a long-term medication group. Available at http://ecm.cachefly.net/pdf/SAPC_SAMS2v0.7.pdf (last accessed 15 October 2012)


