Abstract
This study focuses on the development of a sensitive and simple cluster-linked immunosorbent assay (CLISA) using gold colloidal cluster labeling for determination of proteins such as antigens (Ags) or antibodies (Abs). Abs for detection can be labeled with gold colloid clusters (GCCs). The Fc domain of the Abs binds to the clusters, and the Fab domain to the Ag on a nitrocellulose membrane or a microtiter plate as a support for dot-blotting. The signal of positive interaction between GCC-labeled Abs and its dotted Ag is detectable by the naked eye and can be quantified by comparison to a color scale prepared from a dilution series of known sample concentrations. The colored reaction product is stable for prolonged periods and does not fade, making this method a simple, fast, and convenient means for detection of Ag or Ab biorecognitions and an alternative to enzyme-linked immunosorbent assay. Several interactions between different Ags or Abs (eg, β-lactoglobulin) and solutions avoiding gold colloidal cluster flocculation (eg, using protein G) were studied. CLISA was tested for other analytical purposes such as detection of IgEs in patients’ sera.
Keywords: ELISA, allergen, patient sera, CLISA, immunoassay, β-lactoglobulin
Introduction
The demand for rapid, sensitive, and low-cost alternatives to immunoassay tests for a wide variety of applications in several fields (such as allergies, tumor markers, and serological tests) is likely increasing. To meet the demand, an attractive immunoassay method for detection of antibody (Ab) or antigen (Ag) in the form of a dot-blot test on nitrocellulose (NC) membranes or in a microtiter plate format using gold nanoparticles has been developed. Gold nanoparticles have been used for coupling to proteins,1 DNA,2–4 and RNA in various applications like immunoassays,5,6 detection of analytes7–10 and toxic compounds,11 for nuclear targeting,12 as carrier agents,13 and also as enzymes for biosensors.14–18
The chemisorption of thiol or disulfide-containing compounds to gold lattices is well known and was described, eg, by Nuzzo et al19 for simple organic thiols and disulfides and, in succession, was used for staining proteins (gold stain)20 and also thiol-labeled DNA or RNA strands.21 The high sensitivity of the nanoparticle gold staining method can also be used for application in diverse types of biosensors as, eg, for a concanavalin-A biosensor;22 an open access review is available of Li et al.23
Proteins such as antibodies (Abs) bind very stably to the surfaces of gold particles via intercalation of their sulfur atoms with the gold lattice. A “good” conjugate of gold particle and protein is one where the protein is adsorbed passively to the surface of the gold.24
Abs attach firmly to the gold via the Fc region and leave the Fab region jutting through the double-ionic layer surrounding the gold particle and are, thus, able to bind the analyte. Adsorption of proteins to gold particles occurs within seconds through the following mechanisms:
Charge attraction of the negative gold particle to the positively charged amino acids within the protein (eg, lysine)
Hydrophobic adsorption of the protein to the surface of gold particle through certain amino acid residues including tryptophan
Dative binding between the gold particle and sulfur residues on the protein (from cysteine residues).
These binding interactions were utilized in a cluster-linked immunosorbent assay (CLISA) to quantify Ags, such as, bovine serum albumin (BSA), human serum albumin (HSA), β-lactoglobulin (β-LG), and IgEs, in sera that were dotted onto a NC membrane20 or microtiter plate.
Some proteins such as pc-antiperoxidase Abs led to the precipitation of gold colloid clusters (GCCs) observable by its color turning from cherry red to dark violet or black, which indicates the formation of larger gold aggregates. To avoid this problem, either protein G was used to stabilize the GCC solution or the concentration of Ab diminished. Another possibility is the use of an inverse setup, where Abs are dotted onto the NC membrane and the Ag is tagged to GCC.20 Protein G binds to the surface of gold particles and protects them from precipitation after addition of proteins. In this case, the additional proteins (Abs) bind to protein G (Figure 1).
Figure 1.
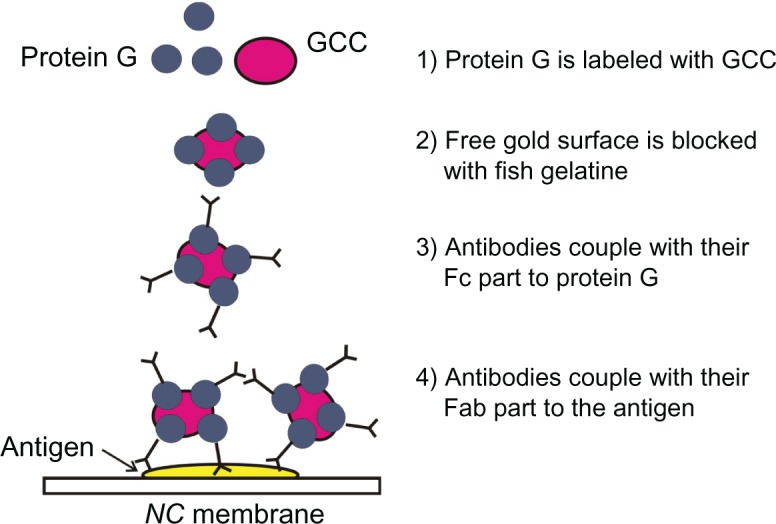
Scheme of stabilization of GCC using protein G.
Abbreviations: GCC, gold colloid cluster; NC, nitrocellulose.
We could show that the sensitivity of the method is comparable with enzyme-linked immunosorbent assay (ELISA). Furthermore, this GCC-labeling method was also applied for the detection of allergens in patients’ sera.
The preparation of GCC solutions was carried out according to the method of Frens.25 This method generates monodisperse colloidal gold with a size ranging from 14 to 50 nm. The size of the clusters can be adjusted by the amount of reducing agent (trisodium citrate) used as a larger number of nucleation sites for a given amount of gold chloride in solution, resulting in smaller final size of each gold particle. GCCs with an average diameter of 17 nm were prepared by the reduction of gold tetrachloric acid (HAuCl4) with trisodium citrate.
Materials and methods
Synthesis of 17 nm GCCs according to Frens
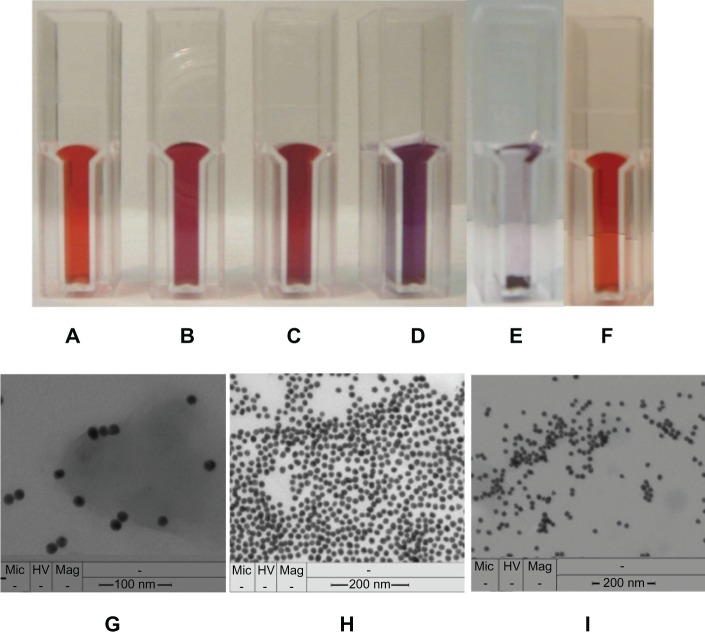
According to Frens,25 it is possible to produce monodispersed colloidal gold with a size range from 14 to 50 nm. A detailed protocol for the preparation of 17 nm GCCs used in this work is given in previous research.20 The distribution of generated GCCs was determinated by dynamic light scattering (Zetasizer Nano; Malvern Instruments Ltd, Worcestershire, UK) in triplicate at 20°C giving a polydispersity index of 0.151. The average size of GCCs was characterized using transmission electron microscopy (TEM, Figure 2G).
Figure 2.
Color of GCC solution. A) after the addition of 1 μL. B) after the addition of 10 μL. C) pc-anti-BSA Ab to 10 mL of GCC solution, GCC flocculates after the addition of 1 μL of pc-antiperoxidase Ab to 10 mL of GCC solution, and its red color turns to violet and then blue. D) black color by total precipitation of GCC solution. E) GCC solution after the addition of fish gelatin. F) TEM image of GCC in A. G) TEM image of C. H) TEM image of D (I).
Preparation of GCC-labeled Abs
For a specific test either on the NC membranes20 or on the microtiter plate, either the Ag or the Ab has to be bound or adsorbed to the GCCs, and the aggregate then stabilized with fish gelatin. Fish gelatin stabilizes gold clusters during the following incubation steps and blocks free binding sites of GCCs, avoiding their direct binding to the detected Ag on the NC membrane or microtiter plate.26,27
Stock solution of GCCs (10 mL) was mixed with mc-anti-HSA Ab (7.8 mg/mL; Sigma-Aldrich®, St Louis, MO) under gentle stirring for 30 minutes at room temperature to generate the GCC–Ab conjugate. 1 mL of GCC-blocking solution (1% [w/v] fish gelatin [Sigma-Aldrich] dissolved in ddH2O including 0.1% [v/v] Tween 20 [Sigma-Aldrich]) was added under further stirring for 30 minutes. Fish gelatin solution in ddH2O (10 μL, 1% [v/v]) stabilizes 100 μL of 17 nm GCCs.20,26,27
As a negative control, 10 mL stock solution of GCC was mixed with 1 mL GCC-blocking solution under stirring for 30 minutes. The GCC-labeled Ab solution and the negative control solution (GCC with fish gelatin and Tween 20) were checked by microscopy (Olympus, Wendenstrasse, Hamburg, Germany) concerning their aggregation status.
Qualitative determination of Ags on NC membranes
For Ag sample preparation, a dilution series in Tris or HCl buffer (50 mM Tris/HCl, pH 7.3) was made in a concentration range between 10 mg/mL and 1 μ/mL β-LG (Sigma-Adrich) and 100 mg/mL peroxides’ sample (502 U/mg; Fluka, Milwaukee, WI). A test sample (1 μL Ag) was dotted onto the surface of an NC membrane (pore size 0.45 μm Protran® BA 85; Schleicher and Schuell, Keene, NH). The dotted sample was dried for 1 hour at room temperature. The membrane was then immersed in blocking solution containing 2% (w/v) fish gelatin and 0.1% (v/v) Tween 20 and Tris-HCl buffer for 20 minutes at room temperature under gentle shaking. Fish gelatin was used for eliminating unspecific background staining as it is free of reactive –SH groups that would form a tight chemical bond with the gold cluster. Therefore, blocking with –SH group-containing reagents, such as BSA or milk, is not recommended. Blocking is further enhanced by addition of the nonionic detergent Tween 20. The membrane was treated 2 times for 5 minutes in excess washing solution (Tris-HCl buffer containing 0.5% [v/v] Tween 20) and 3 times rinsed in ddH2O to remove residual NaCl, which otherwise causes flocculation of GCCs. Flocculation occurs immediately if the gold sol is unstable, and the red color of gold clusters turns into violet and later blue. The washed membrane was then incubated for 2 hours in the GCC-labeled Ab solution at room temperature under gentle shaking. For preparation of GCC-labeled Ab solution, GCC stock solution was mixed with protein G (1 mg/mL [Fluka] dissolved in 50 mM Tris/HCl buffer, pH 7.3) under gentle stirring for 20 minutes at room temperature. Then, Ab solution (either pc-anti-β-LG [1 mg/mL, Bethyl] or pc-antiperoxidase [41 mg/mL; Sigma-Aldrich]) was added and further stirred for 30 minutes. Finally, GCC-blocking solution was added under further stirring for 30 minutes. Table 1 shows quantities and concentrations of the solutions used.
Table 1.
The solutions used to produce labeled-GCC-Ab including protein G
| GCC solution | Protein G (1 mg/mL) | Antibody | GCC-blocking solution |
|---|---|---|---|
| 5 mL | 5 μL | 5 μL pc-anti-β-LG (1 mg/mL) |
0.5 mL |
| 20 mL | 10 μL | 1 μL pc-antiperoxidase (41 mg/mL) |
2 mL |
The NC membrane was washed 3 times in the washing solution for 10 minutes under gentle shaking at room temperature. The detection of a positive signal (red color of gold clusters) is observable with the naked eye, and hence, does not require any instruments. The used amounts resemble the optimum conditions to get sure that the entire surface of gold clusters is covered and no unspecific binding will occur.
Detection of HSA Ag using GCC-labeling (CLISA)
Dynatech Microlite™ 2 plates (Dynatech Laboratories Inc, Chantilly, VA), which are flat-bottomed, white color, and free of electrostatic charge, were used. For Ag sample preparation, a dilution series in Tris or HCl buffer (50 mM Tris/HCl, pH 7.3) was made in a concentration range between 200 mg/mL and 1 μg/mL HSA (Sigma-Aldrich). The wells of the microtiter plate (Microlite 2; Dynatech) were filled with the sample (60 μL per well of HSA dilutions in 50 mM Tris/HCl, pH 7.3) and kept for an hour at room temperature. The microtiter plate was emptied by shaking off. The blocking solution was added to the wells for 20 minutes and then washed 3 times for 5 minutes in washing solution under shaking, followed by rinsing two times with ddH2O. The samples in the microtiter plate were incubated in 350 μL GCC-labeled Ab solution per well for 2 hours at room temperature under shaking. The GCC-labeled Ab solutions were prepared by addition of 1 μL Ab (mc-anti-HSA Ab [7.8 mg/mL; Sigma-Aldrich], pc-anti-BSA Ab [3.6 mg/mL; Sigma-Aldrich], or mc-anti-β-galactosidase Ab [2.5 mg/mL; Promega, Fitchburg, WI] separately) to 10 mL GCC stock solution under stirring for 30 minutes at room temperature, followed by the addition of 1 mL GCC-blocking solution under continued stirring for additional 30 minutes. The samples were washed 3 times in washing solution and then rinsed 3 times with dd. H2O. The detection of a positive signal (red color of gold clusters) was observed with the naked eye. The incubation of coated HSA samples in GCC labeled with pc-anti-BSA Ab served for testing of Ab specificity, and the incubation in GCC labeled with mc-β-galactosidase served as a negative control. Further negative controls were as follows: the incubation of blocked and washed wells (without coated HSA samples) in GCC labeled with mc-anti-HSA Ab; incubation of wells with coated HSA samples in GCC including only GCC-blocking solution; and finally, to show the importance of incubation of GCC-blocking solution with only GCCs.
Detection of HSA Ag using ELISA
The wells of the microtiter plate (Maxisorp™; Nunc, Roskilde, Denmark) were coated with 60 μL of samples per well overnight at 4°C (wells of negative control were coated only with Tris/HCl buffer). The samples were blocked and washed as described before. The dotted samples were incubated in 100 μL Ab solution per well (diluted 1:5000 in 0.01 M phosphate-buffered saline [PBS], pH 7.4) for 2 hours at room temperature under shaking. The samples were washed 3 times with washing solution for 5 minutes. The microtiter plate was incubated for 1 hour with 100 μL per well of the conjugated horseradish peroxidase secondary Ab (antimouse IgG; Sigma-Aldrich) solution (diluted 1:10.000 in 0.01 M PBS, pH 7.4) at room temperature under shaking. The plate was washed 3 times with washing solution for 5 minutes. 100 μL of tetramethylbenzidine substrate (Sciotec, Vienna, Austria) was added per well and incubated for 10 minutes at room temperature. The color of tetramethylbenzidine substrate changes to blue in the presence of Ag. The color intensity is proportional to the amount of detected protein. The reaction was stopped using 50 μL of 1M H2SO4. The color intensity of individual wells was quantified at 450 nm with a microplate reader (Model 450; Bio-Rad, Hercules, CA). The coated wells were also incubated with pc-anti-BSA Ab solution (prepared in analogy to mc-anti-HSA Ab solution described above) for testing of Ab specificity.
Detection of allergens in patients’ sera using gold cluster labeling
GCC solutions were labeled with several allergens (allergy-causing substance) from prick or scratch testing solutions. The prepared GCC-labeled allergen solutions were incubated in the dotted allergic patients’ sera on the microtiter plate. The specific interaction of the IgE in the allergic patients’ serum and the suspected allergen, labeled with GCCs, shows a positive color signal on the surface of microtiter plate.
2 μL serum of each allergic patient was dotted onto 1 row (A–C, see Figure 7) of a microtiter plate (Microlite 2, Dynatech) from wells 1–9 and incubated overnight at 4°C. Wells of row D on the microtiter plate were blocked washed and used as negative control. The patients were found to be allergic to the following:
Figure 7.
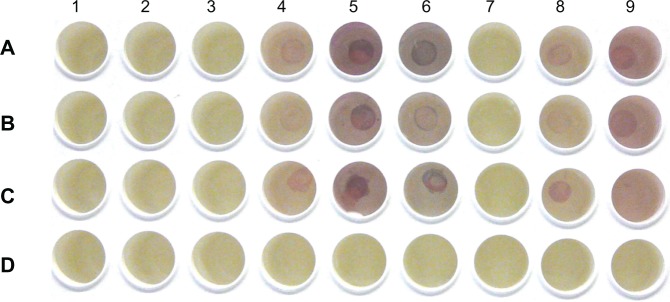
Detection of IgE in patients’ sera shows cherry red color signals. Columns: allergens: 1, 044 fungi I (380 μg protein/mL); 2, 106 mugwort (standardized concerning biological activity); 3, 154 short ragweed (320 μg/mL); 4, 015 grasses/cereals (630 μg/mL); 5, 708 Dermatophagoides farinae (130 μg/mL; dust mite, Ed.); 6, 725 Dermatophagoides pteronyssinus (standardized concerning biological activity; house dust mite, Ed.); 7, 108 birch tree (standardized for biological activity); 8, 116 ash tree (320 μg/mL); 9, GCC solution including GCC-blocking solution as a negative control – see section Results and Discussion. Wells of rows A, B, and C were incubated with patients’serum. Wells of row D were blocked, washed, and used as negative control.
Patient A: grasses, rye, birch tree, ash tree, and ragweed
Patient B: grasses and trees
Patient C: pollen mix, foodstuff, and animal mix
All wells of the microtiter plate were blocked and washed with washing solution (Tris-HCl buffer containing 0.5% [v/v] Tween 20; 100 μL per well) for 5 minutes under shaking. Each washed well (1–8 of rows A–D) was incubated in 350 μL of GCC-labeled allergen solutions, each row including one of the following allergens (1–8 see below), overnight at 4°C. Well 9 was incubated in GCC solution, including GCC-blocking solution as a negative control. Eight different GCC-labeled allergen solutions were separately prepared by stirring 10 mL GCC stock solution with 500 μL of the following allergen solutions (fungi I 300 μL) for 30 minutes at room temperature. 1 mL GCC-blocking solution was added to each GCC-labeled allergen solution under stirring for 30 minutes. The GCC solution, including GCC-blocking solution as negative control, was a prepared analog to GCC-labeled solution omitting allergen.
Allergens:
044 fungi I (380 μg protein/mL)
106 mugwort (standardized for biological activity)
154 short ragweed (320 μg/mL)
105 grasses or cereals (630 μg/mL)
708 Dermatophagoides farinae (130 μg/mL; dust mite, Ed.)
725 Dermatophagoides pteronyssinus (standardized concerning biological activity) (house dust mite, Ed.)
108 birch tree (standardized concerning biological activity)
116 ash tree (320 μg/mL)
The wells were rinsed with ddH2O, and positive signals were visible with the naked eye. For testing the stability of the GCC-labeled Ag solutions, the same experiment was repeated after 21 days using the same solutions of GCC-labeled allergens (stored at 4°C).
Results and discussion
In order to achieve specific binding of the GCC conjugates to the respective samples on the NC membrane, either the Ag or the Ab is coupled to the GCCs. Abs bind with their disulfide groups on the Fc domain or free –SH groups, in case of split Abs, to the GCCs20 and with the Fab domain to the Ag situated either on an NC membrane or in the microtiter plate well.
Imaging of GCC particles
The color of the synthesized GCCs is cherry red (Figure 2A), and the intensity of the color increases after addition of protein (Figure 2B and C). However, the concentration of added protein is critical due to the tendency of GCCs to flocculate immediately above a certain protein concentration (Figure 2D and E). The amount of addition of GCC-blocking solution to GCC solution was optimized and thus, no color change of GCC solution was observed after the addition of GCC-blocking solution (Figure 2F). The size of GCCs in solution, aggregation, and flocculation was determined using TEM (Figure 2G, H, and I), respectively.
Transmitted light microscopy images in Figure 3 show that individual GCC particles bind to Abs and form dark cherry red aggregates. The color and size of the aggregates depend on the concentration of added Abs (Figure 3A and C). The change in color intensity of GCC-labeled Ab solutions was shown with either 10 μL or 1 μL pc-anti-HSA Ab by detection of 1 μL HSA sample (200 mg/mL) dotted onto the NC membrane (Figure 3B and D). This phenomenon illustrates that the detection of protein depends on the color, size, and type of aggregate. However, the GCCs tolerate only a certain amount of protein added which needs to be tested for each protein separately. Adding too much of a protein turns the red color of GCCs into violet, blue, and finally, black due to the flocculation of GCC (Figure 2D and E).
Figure 3.
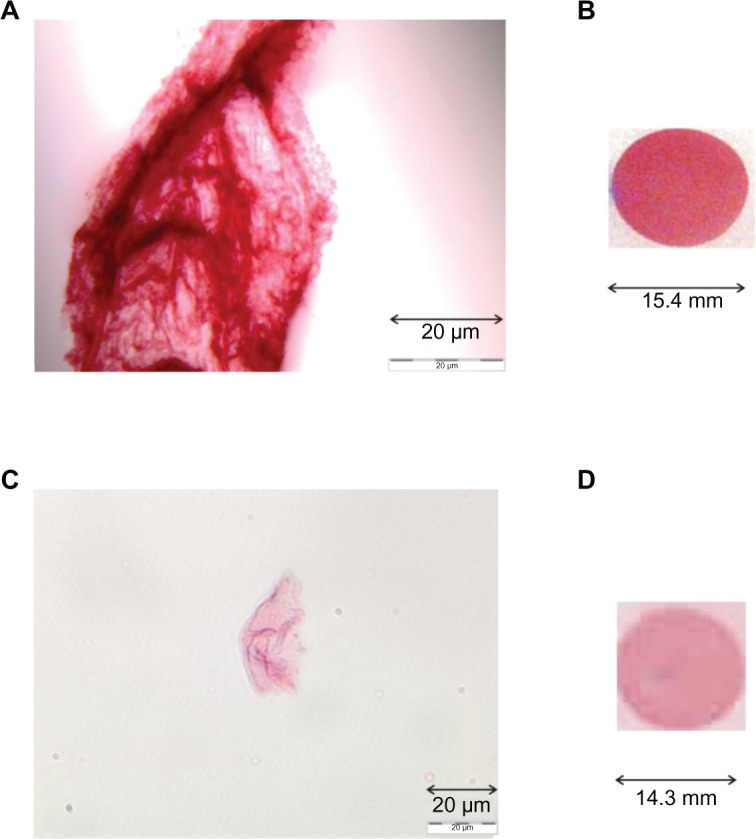
Detection of 1 μL of HSA (200 mg/mL) dotted onto NC membrane after incubation in GCC (10 mL), including different amounts (B, 10 μL; D, 1 μL) of labeled mc-anti-HSA Ab. Images of these GCC-labeled Ab solutions (A, 10 μL; C, 1 μL) were checked by microscopy (Olympus).
Detection of Ag using GCC-labeled Abs and ELISA – a comparison
The sensitivity of CLISA was shown in a previous work.20 The specificity of CLISA was chosen to adjust ELISA to CLISA by labeling the read-out Ab with GCCs by development of an optical biosensor based on the resonance-enhanced absorption effect using the milk allergen β–LG.28
For evaluation of the presented GCC labeling method, the method was compared with ELISA for HSA, as standard ELISA test is available for the protein.
Detection of HSA Ags by means of GCC labeling was carried out in a microtiter plate format and compared with detection by ELISA.
The GCC-labeling method provides the following advantages:
The GCC detection method is sensitive and, therefore, requires only very low amounts of Abs.
There is no need of enzyme-conjugated secondary Abs and their substrates, which make this test cost effective, as the amount of gold used for production of clusters is quite small.
The signal detection of the cherry red color of GCC can be observed by the naked eye, and hence, does not require any instruments.
The procedure is not time consuming (the whole procedure takes 4 hours) and is economic.
It is useful also for analytical field studies.
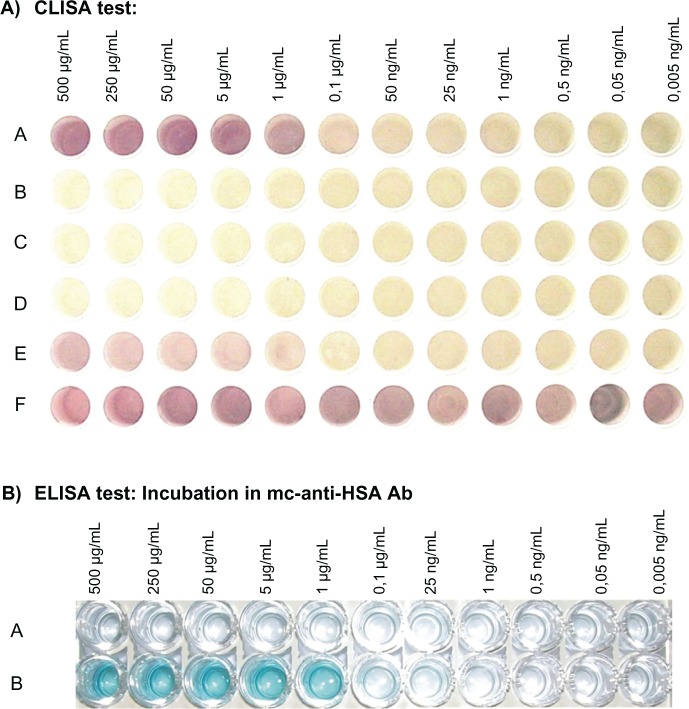
The detection of HSA samples using CLISA (GCC labeled with mc-anti-HSA Ab) was similar to ELISA, and the lower limit of detection was observed at 25 ng/mL (Figure 4A, row A). This result can be easily detected from the original plates. After incubation of the coated HSA samples in the microtiter plate with GCC including only blocking solution, no signal was observed (Figure 4A, row B [negative control]). No color reaction was observed for the negative controls after incubation of coated samples in GCCs labeled with nonbinding Ab (mc-anti-β-galactosidase Ab; Figure 4A, row C) and after incubation of GCC-labeled Ab in blocked and washed wells without coated HSA samples (Figure 4A, row D). After incubation of the coated HSA samples in GCCs labeled with pc-anti-BSA Ab, a certain degree of cross-reactivity was observed due to some degree of homology between HSA and BSA as can be seen in Figure 4A, row E. This cross-reactivity was also detected with ELISA (data not shown). The importance of blocking GCC particles using fish gelatin is illustrated in Figure 4A, row F. A positive unspecific signal was observed after the incubation of coated HSA samples (see Materials and Methods) in GCC solution due to the high affinity of GCC to bind albumins. The impact of the amount of fish gelatin on the detection can be deduced from the salt flocculation test.26,27
Figure 4.
A) CLISA test: wells of microtiter plates were coated with HSA samples and incubated in the following:
A: GCC labeled with mc-anti-HSA Ab including GCC-blocking solution
B: GCC solution including GCC-blocking solution (negative control)
C: GCC labeled with mc-anti-β-galactosidase Ab including GCC-blocking solution (negative control)
D: GCC labeled with mc-anti-HSA Ab, including GCC-blocking solution, incubated in blocked, washed, and without coated HSA-sample wells (negative control)
E: GCC labeled with pc-anti-BSA Ab including GCC-blocking solution (for testing the specificity of Ab)
F: incubation of GCC solution (without GCC-blocking solution) in wells coated with samples (to show the importance of blocking GCC particles using GCC-blocking solution)
Figure 4 B) ELISA test, incubated in:
A: wells of microtiter plates were coated with tris or HCl buffer and incubated in mc-anti-HSA Ab solution (negative control)
B: wells of microtiter plates were coated with HSA samples and incubated in mc-anti-HSA Ab solution.
Abbreviations: CLISA, cluster-linked immunosorbent assay; ELISA, enzyme-linked immunosorbent assay; HSA, human serum albumin.
Avoiding GCC flocculation during reaction with certain proteins
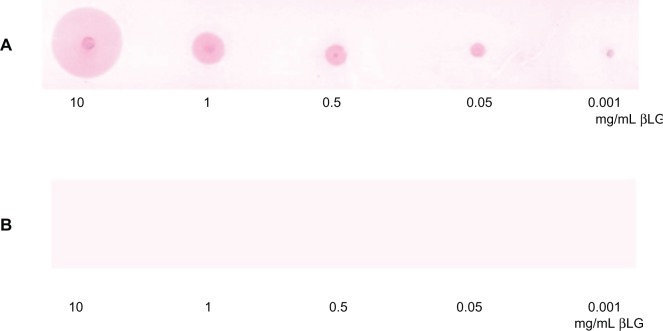
GCCs tend to flocculate after addition of certain proteins. To avoid the GCC flocculation appearing immediately after the addition of some proteins such as pc-antiperoxidase Ab, either protein G is added to GCC solution or the GCC amount is increased. Figure 5A and B illustrate the positive signal observed after the incubation of GCC-labeled Ab(pc-antiperoxidase Ab) with dotted peroxidase with increased amount of GCC solution (20 mL incubated in 1 μL peroxidase Ab) or additional use of protein G, and no signal was observed with dotted β-LG protein (negative control). When incubated with GCC solution, protein G binds to the surface of gold particles. This prevents premature precipitation of gold particles after addition of proteins.
Figure 5.
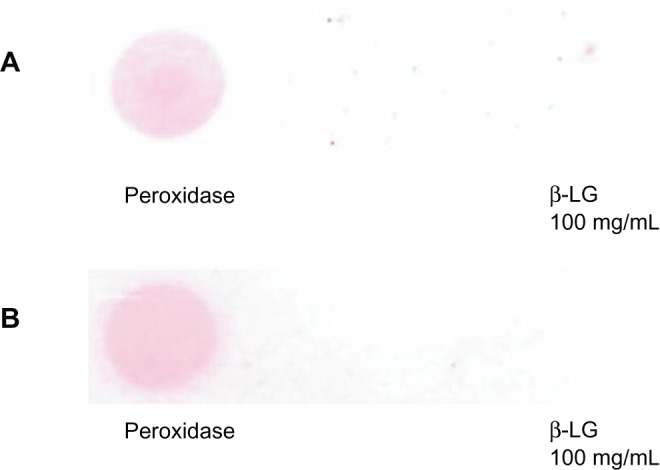
GCC flocculation can be avoided by increasing the amount of GCC solution, which was incubated with pc-antiperoxidase Ab to produce GCC-labeled Ab. A) GCC flocculation can be avoided by addition of protein G to the GCC solution before incubation with the Ab. B) No color reaction was observed by dotted β-LG protein (negative control).
Abbreviation: β-LG, β-lactoglobulin.
This effect is shown in Figure 6 and illustrated again by detection of β-LG, the major allergen in milk. The detected signal of β-LG using GCC labeling is still intense at 0.001 mg/mL (Figure 6A). No signal was observed after the incubation of NC membrane containing dotted β-LG samples in GCC solution including protein G and GCC-blocking solution in the absence of Ab (negative control, Figure 6B).
Figure 6.
Positive signal of detected β-LG using the GCC labeling method on NC membranes.
Application of GCC labeling for detection of allergens in patients’ sera
Wells of microtiter plate containing the dotted sera of allergic patients were incubated with GCC-labeled allergen solutions overnight at 4°C. Positive color reactions were obtained (Figure 7) for specific interactions of specific IgE in allergic patients’ serum to the suspected allergen labeled with GCC. The allergy of patient A was suspected to be against grasses, rye, birch tree, ash tree, and ragweed. The GCC-labeling test indicates specific IgEs against grasses or cereals, D. farinae, D. pteronyssinus, and ash tree in his serum. The quality of used ragweed allergen from skin prick test solutions seemed to be not sensitive enough as no color signal could be observed for patient A who had been tested allergic against ragweed before.
Patient B was previously tested positive for allergy against grasses and trees. The GCC test procedure showed corresponding positive allergy results for grasses and ash tree. Patient C suffered from allergy against different pollen, foodstuff, and animals. GCC-labeled tests demonstrated a positive color reaction for grasses or cereals, ash tree, D. farinae, and D. pteronyssinus. Allergen cross-reactivity occurs when IgE Abs originally raised against 1 allergen recognizes or binds a similar protein from another source.29–31 A cross-reactivity between D. farinae and D. pteronyssinus allergens to crustaceans and sea food is known.32–34 This could be the reason for higher color intensity of the detected signal in patient C compared with patients A and B. The signal intensity for allergens of grasses and cereals was low in patient B, due to allergy against only grasses; however, patient A had allergy against grasses and rye, and patient C against food and different pollen and, therefore, the signal intensity was higher. These findings show that cross reactivity with other Ags could increase the total signal in 1 sample.
It was remarkable that the sera of patients A and B gave a positive color reaction with D. farinae and D. pteronyssinus allergens although this was not indicated for those patients. It might have derived from nonallergic immune response of patients developed against both allergens, and the positive signal might have derived from the respective IgGs in their serum. To increase the specificity of this test for the detection of allergens, purification of sera is necessary for the isolation of IgEs.
No signal was observed with the other supplied allergens against which the patients’ sera did not contain specific IgEs, such as fungi I and mugwort (Artemisia vulgaris). Moreover, the specificity of the GCC-based test is highly dependent on the allergens’ concentration and purification.
As is shown in Figure 7, a positive color reaction was obtained while incubating the dotted patients’ sera in GCC solution, including GCC-blocking solution. This, on the first view of the unexpected result of an intended negative control, derives from the fact that the concentration of added GCC-blocking solution was too low for blocking in the presence of high concentrations of albumins. HSA is the most abundant protein in human blood plasma, and albumin reacts with GCCs with high affinity. HSA contains free reactive –SH groups that form a tight chemical bond with the GCCs. However, addition of allergens and GCC-blocking solution was enough to block the whole surface of the GCC particles, therefore, not all the used GCCs labeled with allergens show a signal with dotted serum. These aggregates were then able to bind specifically to the IgEs in serum and gave a positive signal on the surface of the microtiter plate and gave no color reaction in the absence of suspected allergens. Wells of row D on the microtiter plate were blocked, washed, and used as negative control. Figure 7 shows no color reaction after incubation with the different prepared GCC-labeled allergens.
Furthermore, the same GCC-based detection was carried out with 21-day-old GCC-labeled allergens, stored at 4°C, to test the stability of labeled allergens after storage. This makes the presented method potentially applicable in a kit format, as long-time storage of components is a prerequisite for kit development. A GCC-based allergy test could promise more simplification and patient comfort compared with the currently available allergy tests such as the skin prick tests.
Acknowledgments
The authors thank the Department of Biochemistry, University of Vienna (Dr Bohr Gasse 9, A-1030 Vienna, Austria) for the financial support and Daniela Gruber (Institute of Cell Imaging and Ultrastructure Research, Althanstrasse 14 A-1091 Vienna, Austria) for TEM images.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Burt JL, Wing CG, Yoshida MM, Yacama MJ. Noble-metal nanoparticles directly conjugated to globular proteins. Langmuir. 2004;20:11778–11783. doi: 10.1021/la048287r. [DOI] [PubMed] [Google Scholar]
- 2.Cai H, Xu C, He P, Fang Y. Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J Electroanal Chem. 2001;510:78–85. [Google Scholar]
- 3.Amirkin C, Letsinger RL, Mucic RC, Sterhoff JJ. A DNA based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 4.Castaneda MT, Alegret S, Merkoci A. Electrochemical sensing of DNA using gold nanoparticles. Electroanalysis. 2007;19:743–753. [Google Scholar]
- 5.Dequaire M, Degrand C, Limoges B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal Chem. 2000;72:5521–5528. doi: 10.1021/ac000781m. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Aaron J, Sokolov K. Directional conjugation of antibodies to nanoparticles for synthesis of multiplexed optical contrast agents with both delivery and targeting moieties. Nat Protoc. 2008;3:314–320. doi: 10.1038/nprot.2008.1. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Chena J, Nie L, Kuang Y, Yao S. A label-free electrochemical immunoassay for carcinoembryonic antigen (CEA) based on gold nanoparticles (AuNPs) and nonconductive polymer film. Biosens Bioelectron. 2007;22:1061–1067. doi: 10.1016/j.bios.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Authier L, Grossiord C, Brossier P. Gold nanoparticle-based quantitative electrochemical detection of amplified human cytomegalovirus DNA using disposable microband electrodes. Anal Chem. 2001;73:4450–4456. doi: 10.1021/ac0103221. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Yuan R, Chai Y, Zhang Z, Zhuo Y, Zhang Y. Amperometric immunosensor based on toluidine blue/nano-Au through electrostatic interaction for determination of carcinoembryonic antigen. J Biotechnol. 2006;123:356–366. doi: 10.1016/j.jbiotec.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Dua D, Liub S, Chena J, Jua H, Liana H, Lia J. Colloidal gold nanoparticle modified carbon paste interface for studies of tumor cell adhesion and viability. Biomaterials. 2005;26:6487–6495. doi: 10.1016/j.biomaterials.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 11.Hua SQ, Xie JW, Xu QH, Rong KT, Shen GL, Yu SQ. A label-free electrochemical immunosensor based on gold nanoparticles for detection of paraoxon. Talanta. 2003;61:769–777. doi: 10.1016/S0039-9140(03)00368-0. [DOI] [PubMed] [Google Scholar]
- 12.Tkachenko AG, Xie H, Coleman D, et al. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Chen Y, Li WY, Liu Y. Synthesis of oligo (ethylenediamino)-â-cyclodextrin modified gold nanoparticle as a DNA concentrator. Mol Pharm. 2007;4:189–198. doi: 10.1021/mp060045s. [DOI] [PubMed] [Google Scholar]
- 14.Yi X, Xian JH, Yuan CH. Direct electrochemistry of horseradish peroxidase immobilized on a colloid/cysteamine-modified gold electrode. Anal Biochem. 2000;278:22–28. doi: 10.1006/abio.1999.4360. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Ju H. Electrocatalysis via direct electrochemistry of myoglobin immobilized on colloidal gold nanoparticles. Electroanalysis. 2003;15:1488–1493. [Google Scholar]
- 16.Ju H, Liu S, Ge B, Lisdat F, Scheller FW. Electrochemistry of cytochrome c immobilized on colloidal gold modified carbon paste electrodes and its electrocatalytic activity. Electroanalysis. 2002;14:141–147. [Google Scholar]
- 17.You CC, de M, Han G, Rotello VN. Tunable inhibition and denaturation of á-chymotrypsin with amino acid-functionalized gold nanoparticles. J Am Chem Soc. 2005;127:12873–12881. doi: 10.1021/ja0512881. [DOI] [PubMed] [Google Scholar]
- 18.Gole A, Dash C, Soman C, Sainkar SR, Rao M, Sastry M. On the preparation, characterization, and enzymatic activity of fungal protease gold colloid bioconjugates. Bioconjug Chem. 2001;12:684–690. doi: 10.1021/bc0001241. [DOI] [PubMed] [Google Scholar]
- 19.Nuzzo RG, Zegarski BR, Dubois LH. Fundamental studies of the chemisorption of organosulfur compounds on gold (111). Implications for molecular self-assembly on gold surfaces. J Am Chem Soc. 1978;109(3):733–740. [Google Scholar]
- 20.Al-Dubai H, Pittner G, Pittner F. A dot-blot test using gold colloid cluster technology as a miniaturizable alternative to ELISA and hapten inhibition tests. Chemical Monthly. 2008;139:1531–1536. [Google Scholar]
- 21.Mengsu Y, Hellas CMY, Hing LC. Adsorption kinetics and ligand-binding properties of thiol-modified double-stranded DNA on a gold surface. Langmuir. 1998;14(21):6121–6129. [Google Scholar]
- 22.Guo C, Boullanger P, Jiang L, Liu T. Highly sensitive gold nanoparticles. Biosens Bioelectron. 2007;22(8):1830–1834. doi: 10.1016/j.bios.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Schluesener HJ, Xu S. Gold nanoparticle-based Biosensors. Gold Bull. 2010;43(1):29–41. [Google Scholar]
- 24.Chandler J, Gurmin T, Robinson N. The place of gold in rapid tests: superior stability, sensitivity, and precision and reproducibility of manufacture make gold suitable for use in membrane-based tests. IVD Technology; 2000. http://www.devicelink.com/ivat/archive/00/03/004.html [Google Scholar]
- 25.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature (London), Phys Sci. 1973;241:20–22. [Google Scholar]
- 26.Walter H, Bauer G. Optical visualization of polymer-polymer interactions. J Nanosci Nanotechnol. 2004;4:121–124. doi: 10.1166/jnn.2004.227. [DOI] [PubMed] [Google Scholar]
- 27.Prodinger V. Studies to develop new nanotechnological biosensor chips for assaying of allergenicity [diploma thesis] 2006. p. p 41.
- 28.Hohensinner V, Maier I, Pittner F. A “gold cluster-linked immunosorbent assay”: optical near-field biosensor chip fort the detection of allergenic-lactoglobulin in processed milk matrices. J Biotechnol. 2007;130(4):385–388. doi: 10.1016/j.jbiotec.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–238. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 30.Aalberse RC, Akkerdaas J, van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy. 2001;56:478–490. doi: 10.1034/j.1398-9995.2001.056006478.x. [DOI] [PubMed] [Google Scholar]
- 31.Weber RW. Patterns of pollen crossallergenicity. J Allergy Clin Immunol. 2003;112(2):229–239. doi: 10.1067/mai.2003.1683. [DOI] [PubMed] [Google Scholar]
- 32.Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: IgE-binding crossreactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 33.Sidenius KE, Hallas TE, Poulsen LK, Mosbech H. Allergen crossre-activity between house-dust mites and other invertebrates. Allergy. 2001;56:723–733. doi: 10.1034/j.1398-9995.2001.056008723.x. [DOI] [PubMed] [Google Scholar]
- 34.Jeong KY, Hong CS, Yong TS. Allergenic tropomyosins and their cross-reactivities. Protein Pept Lett. 2006;13:835–845. doi: 10.2174/092986606777841244. [DOI] [PubMed] [Google Scholar]