Abstract
Nanobodies are the smallest fragments of naturally occurring single-domain antibodies that have evolved to be fully functional in the absence of a light chain. Conventional antibodies are glycoproteins comprising two heavy and two light chains. Surprisingly, all members of the Camelidae family possess a fraction of antibodies devoid of both light chains and the first constant domain. These types of antibodies are known as heavy-chain antibody (HcAb) nanobodies. There are three subclasses of IgG in dromedaries, namely IgG1, IgG2, and IgG3 of which IgG2 and IgG3 are of the HcAb type. These heavy chain antibodies constitute approximately 50% of the IgG in llama serum and as much as 75% of the IgG in camel serum. In the present work, the different IgG subclasses from an immunized camel (Camelus dromedarius) with divalent diphtheria-tetanus vaccine were purified using their different affinity for protein A and protein G and their absorbance measured at 280 nm. Purity control and characterization by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis of IgG subclasses was done under reducing conditions. Protein bands were visualized after staining with Coomassie Blue, showing two bands at 50 kDa and 30 kDa for IgG1, while IgG2 and IgG3 produced only one band at 46 kDa and 43 kDa, respectively. An enzyme-linked immunosorbent assay test using diphtheria toxin and purified IgG subclasses from the immunized camel were performed to evaluate their efficiency. Compared with conventional IgG1, heavy chain antibodies (nanobodies) were shown to be more efficient in binding to diphtheria toxin antigen. This study revealed the possibility of using IgG2 and IgG3 nanobodies as an effective antitoxin for the treatment of diphtheria in humans.
Keywords: camel, heavy chain antibody, HcAb, nanobodies, immunoglobulin, IgG subclasses, diphtheria toxin
Introduction
Antibodies belong to a class of proteins called immunoglobulins that are produced by the immune system in response to foreign antigens. Numerous immunoassays take advantage of the high affinity and selectivity of in vivo mature antibodies derived from animals. The main class of antibody used for diagnostic applications is IgG. In most mammals IgG is a large 160 kDa protein made up of two pairs of different polypeptide chains, ie, two heavy and two light chains connected by disulfide bonds. Each chain is composed of one variable domain plus either one constant domain for the light chain or three constant domains for the heavy chains. A variable domain from a light chain combines with a variable domain from a heavy chain to form one of two antigen-binding sites, which upon binding help to neutralize and eliminate pathogens and their toxins.1
The members of the Camelidae family (camels and llamas) have IgG subclasses that possess a unique structural arrangement (Figure 1). The IgG2 and IgG3 subclasses consist of only two heavy chains. Their antigen-binding site is reduced to a single VHH domain. Because the variable domain of the heavy chain antibodies is the smallest fully functional antigen-binding fragment with a molecular mass of only 15 kDa, we refer to this entity as a “nanobody”. The small size of nanobodies makes them particularly suitable for targeting antigens in anatomic locations in at which tissue penetration is difficult but critical.2 Also, the structure of this domain is altered by the replacement of selected surface amino acids to increase its hydrophilicity in compensation for the lack of a light chain.3,4
Figure 1.
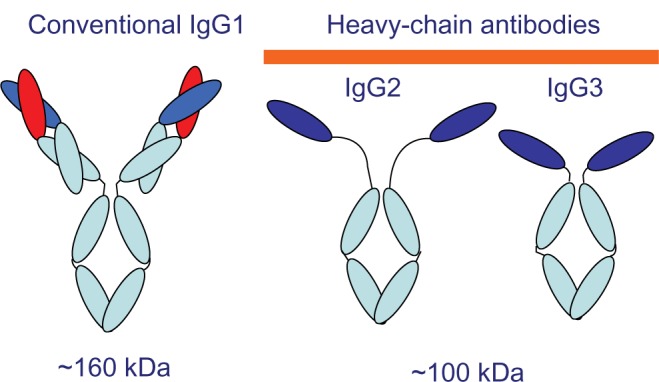
Schematic of camel IgG subclass structures. IgG1, conventional antibody, contains both heavy and light chains. IgG2 and IgG3 consist of only heavy chains and have a long- or short-hinge region, respectively.
Abbreviation: IgG, immunoglobulin G.
Previous studies have shown that HcAbs can act as effective high-affinity binding ligands for foreign target molecules.5,6 To ascertain whether this is the case for a camel immunized against diphtheria toxin and to better evaluate HcAb capabilities found in the natural immune repertoire, we purified and tested IgG subclasses obtained from a camel that had been immunized for diphtheria toxoid.
Diphtheria is an acute bacterial disease caused by toxigenic strains of Corynebacterium diphtheriae. The disease causes considerable morbidity and mortality in developing countries where childhood vaccination coverage is low.7 Travelers to countries with endemic diphtheria are at a higher risk of disease following exposure to toxigenic C. diphtheriae if they are inadequately immunized or not up-to-date with diphtheria booster immunizations.8 The salvage measure in established diphtheria cases is administration of antitoxin. However, the current antitoxin is not approved by the Food and Drug Administration (FDA), causes hypersensitivity reactions, is expensive to manufacture, and requires special storage. An alternative to using conventional immunoglobulins is the use of monovalent fragments derived from heavy chain antibodies found in Camelidae.9 These antibodies (nanobodies) are devoid of light chains and have been shown to have broad antigen-binding properties.10 According to World Health Organization (WHO) recommendations, an antidiphtheria IgG antibody concentration ≥0.1 IU/mL is considered protective, a concentration between 0.01 and 0.09 IU/mL provides basic immunity (but the subject is still susceptible), and below 0.01 IU/mL the individual is not protected.11
Conventional antibodies have many important features, such as high affinity and selectivity for a target, ease of discovery, and low inherent toxicity. In addition, nanobodies have characteristics that make them superior to conventional antibodies. Due to their small size (one-tenth that of a conventional antibody), nanobodies penetrate tissues more effectively than conventional antibodies, and are easily engineered into multivalent and multifunctional formats. Moreover, they are stable to heat, pH, proteases, and other protein-denaturing agents. They are very soluble, tend not to aggregate, and engage with epitopes and targets that cannot be addressed by conventional antibodies such as enzyme-active sites. The VHH domain can interact with the active site of certain enzymes, acting as effective enzyme inhibitors.12,13 Furthermore, nanobodies are relatively rapidly eliminated from the circulation, making them ideal for neutralizing and eliminating toxins.2,14,15
In this study, the aim was to evaluate the avidity of camel IgG2 and IgG3 for ligand recognition of diphtheria toxin. The camel IgG was fractionated into its various subclasses, and the conventional antibody (IgG1) and HcAbs (IgG2 and IgG3) were evaluated for diphtheria target specificity using the enzyme-linked immunosorbent assay technique.
Material and methods
Chemicals
The chemicals used in this study were analytical quality products from different companies. Whatman microgranular diethylaminoethyl-cellulose was obtained from H. Reeve Angel and Co., Inc. (Clifton, NJ). Sephadex G-100 and Sepharose 4B were from Pharmacia Fine Chemicals, Inc. (Piscataway, NJ). Trypsin, soybean trypsin inhibitor, and bovine serum albumin were from Sigma Chemical Co. (St Louis, MO). All other chemicals were reagent grade and were purchased commercially. The following buffer solutions were used: buffer 1–0.01 M sodium phosphate (pH 7.7); buffer 2–0.05 M sodium phosphate (pH 7.0), containing 0.02% (wt/vol) NaN3; buffer 3–0.05 M glycine-chloride (pH 3.5); buffer 4–0.05 M glycine-chloride (pH 3.0); buffer 5–0.082 M NaCl, 0.043 M Na2HPO4−2H2O, 0.0107 M KH2PO4 (pH 7.4); buffer 6–0.077 M NaCl, 0.024 M Na2HPO4 2H2O, 0.0508 M KH2PO4 (pH 6.4).
Animal and microorganism
A two-year-old male camel (C. dromedarius) reared at the breeding farm of the Ministry of Agriculture, Kingdom of Saudi Arabia, was used for induction of antibodies and nanobody response against divalent diphtheria–tetanus vaccine (Glaxo-SmithKline, Riyadh, Kingdom of Saudi Arabia). The camel showed no clinical signs of any infection. A standard strain of toxigenic C. diphtheriae (ATCC 13812) was obtained from Watin-Biolife Company (Riyadh, Kingdom of Saudi Arabia).
Immunization procedure
The camel was inoculated intramuscularly with divalent diphtheria–tetanus toxoid vaccine three times at four-week intervals to reach a high immune status.16 Blood was collected aseptically in sterile containers containing heparin as an anticoagulant at the end of the vaccination period.
Antibody purification and IgG subclass fractionation
IgG subclasses were fractionated by a combination of affinity chromatography on PG and PA columns as described elsewhere.9 Briefly, camel serum diluted fivefold with phosphate-buffered saline (PBS) was first loaded onto a 5 mL PG-Sepharose column (Sigma Chemical Co). After washing to background with PBS, IgG3 was eluted with 150 mM NaCl-0.58% acetic acid (pH 3.5), then the IgG1 fraction was eluted with 100 mM glycine-HCl (pH 2.7). The fraction not adsorbed on PG was loaded onto a 5 mL PA-Sepharose column (Sigma Chemical Co). After washing with PBS, the IgG2 fraction was eluted using 150 mM NaCl-0.58% acetic acid (pH 4.5). All collected IgG fractions were immediately neutralized using 0.1 M Tris-Cl (pH 9.0) and transferred to dialysis tubing to dialyze with three changes of PBS. The final protein concentration of the isolated serum fractions was determined by the Bradford method (Bio-Rad Hercules, CA) and protein purity was assayed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing condition.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was performed according to a procedure described elsewhere.17 Cobalt-irradiated 96-well polystyrene microtiter plates (M129 B; Dynatech, Plochingen, Germany) were coated with 100 μL of the purified diphtheria toxin (1 μg/mL PBS pH 7.2) and left overnight at 4°C. After washing four times with PBS, the 1:100 diluted IgG samples were added and incubated at 37°C for one hour and washed three times with the same washing buffer. Then 100 μL of 1:3000 sheep anticamel horseradish peroxidase conjugate (Kirkegaard and Perry Laboratories, UK) was added and left to react at 37°C for 30 minutes (the optimum concentrations of the antigen and the conjugate were determined by checkerboard titration). The plates were washed again three times and finally 100 μL (0.2 mg/mL) of the substrate o-phenylenediamine dihydrochloride (Sigma Chemical Co) with 0.001% H2O2. After 20 minutes of incubation at 37°C the reaction was stopped using 25 μL of 2 M H2SO4 This basic procedure was repeated to investigate numerous variables. For example, PBS was used as a control to measure nonspecific binding of anticamel IgG to the precoated wells. Absorbance was measured at 490 nm using an automatic ELISA reader (Dynatech, Tarpon Springs, FL). Antigen, IgG samples, conjugates, and control substrates were set up as appropriate controls, and the endpoint was calculated as OD (absorbance).
Production of diphtheria toxin
Bacteria C. diphtheriae strain C7 was constructed by lysogenization of strain C7 with the recombinant corynebacteriophage tox 3 h′ strains C7 (f345) and C7 (f830) prepared by lysogenization of strain C7 with the nontoxinogenic mutant phages f345 and/830.18 C7 strains were cultivated in potato extract glucose thymine (PGT) medium with 2% maltose supplement. In tests for production of diphtheria toxin by lysogenic C7 strains, 25 mL samples of PGT medium containing 2% maltose supplement and 0.075 μg of iron per mL in 125 mL acid-cleaned Erlenmeyer flasks were inoculated with 0.25 mL samples from overnight low iron cultures and were incubated for 17 hours at 34°C with rotary shaking at 240 rpm. Cells were removed by centrifugation for 10 minutes at 12,000 × g and the cell-free culture supernatants were assayed for diphtheria toxin.
Purification of diphtheria toxin
Diphtheria toxin was produced in 250 mL cultures of C. diphtheriae strain PW8r (P)′tX+ as described.19 All subsequent procedures were carried out at 4°C. Ammonium sulfate was added to 2000 mL of pooled culture supernatants containing 7.4 × 104 flocculating units (Lf) of toxin, and the material that precipitated between 40% and 70% of saturation was dialyzed against buffer 1 and applied onto a diethylaminoethyl-cellulose column (3.1 cm × 37 cm). Elution was performed with a 0 to 0.15 M gradient of NaCl in buffer 1, and the toxin peak, recovered at approximately 0.08 M NaCl, was subjected to gel filtration on a Sephadex G-100 column (4.9 cm × 53 cm) in buffer 1. Diphtheria toxin eluted from the Sephadex G-100 column as a single peak, and the fractions containing toxin were combined and stored frozen at −70°C. This sample contained about 72 mg of protein. Analysis of 20 μg samples of toxin by discontinuous polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate revealed a single band of protein with a molecular weight of 62,000. The same result was obtained in the presence or absence of 2-mercaptoethanol, indicating that the purified toxin was “intact toxin” and contained no detectable “nicked toxin”.20 Procedures for production and purification of diphtheria toxin were performed in the Animal Reproduction Research Institute, Egypt.
Statistical analysis
A computer SPSS program was used and the results were expressed as mean ± standard deviation (SD). A P value < 0.05 was considered to be significant.
Results
Purification and separation of camel IgG subclasses
The IgG was successfully fractionated into its various subclasses using PG and PA affinity chromatography as shown in Figure 2. PG affinity chromatography allowed the isolation of one HcAb subclass (fraction, camel IgG3) and the conventional antibody subclass IgG1 fraction. PA chromatography results revealed the presence of another HcAb subclass fraction corresponding to camel IgG2.
Figure 2.
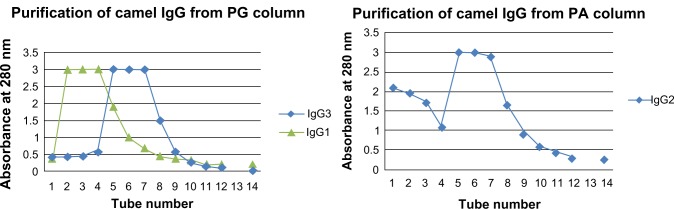
Purification of IgG subclasses by affinity chromatography.
Abbreviations: IgG, immunoglobulin G; PA, protein A; PG, protein G.
Characterization of IgG subclasses
The eluted fractions from PA and PG chromatography were analyzed by 12% SDS-PAGE with reducing agent.21 Protein bands were visualized after staining with 0.1% Coomassie Blue R250 as shown in Figure 3. Fraction IgG1, upon reduction, yielded two bands, ie, 50 kDa heavy chains and 30 kDa light chains (lane b). The two other immunoglobulin fractions, upon reduction, yielded only heavy chains of 46 kDa (IgG2 fraction binding only to PA, lane c) and 43 kDa (IgG3 fraction binding to PG, lane a), respectively. These IgG2 and IgG3 subclasses appeared to lack the light chain completely.
Figure 3.
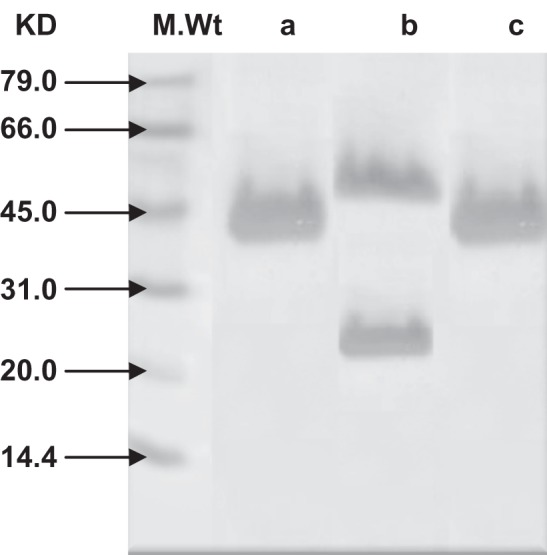
Analysis of purified camel IgG subclasses by 12% SDS-PAGE under reducing condition followed by Coomassie Blue staining reveals a, b IgG3 and IgG1 eluted from PG column and c, IgG2 eluted from PA column.
Abbreviations: IgG, immunoglobulin G; M. Wt, molecular weight; PA, protein A; PG, protein G; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Direct binding immune assays
Screening for affinity of the different IgG subclasses for different concentrations of diphtheria toxin in the range 0.01–10 μg/mL was performed as shown in Figure 4. This graph reveals a parallel and positive relationship between serial concentrations of antigen (diphtheria toxin) and recorded OD in the presence of different IgG fractions. Moreover, IgG2 and IgG3 displayed remarkably greater (more sensitive and potent) increases of OD than IgG1 in the presence of different concentrations of diphtheria antigen.
Figure 4.
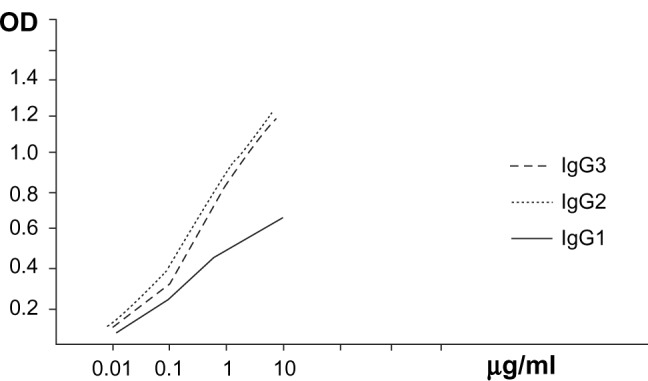
The relationship between the antigen (toxin with different concentrations in μg/mL) and different IgG fractions against the resulting absorbance.
Abbreviations: IgG, immunoglobulin G; OD, absorbance.
The ability of camel IgG antibodies to bind to diphtheria toxin was verified by ELISA (Figure 5). Application of IgG2 and IgG3 produced significant increases in OD compared with IgG1. This shows that application of IgG2 and IgG3 was more sensitive and potent than IgG1 during ELISA. Each column corresponds to the mean value of five measures, and the error bars represent the SD.
Figure 5.
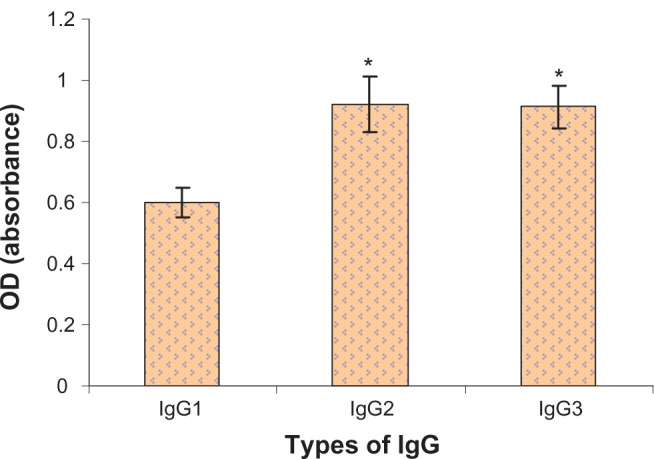
The resulting OD due to application of different IgG fractions with diphtheria toxin using ELISA technique.
Note: *Significance at P < 0.05.
Abbreviations: IgG, immunoglobulin G; OD, absorbance; ELISA, enzyme-linked immunosorbent assay.
Discussion
Diphtheria remains a serious disease throughout much of the world. Most life-threatening cases occurred in inadequately immunized persons. Travelers to disease-endemic areas are at increased risk of exposure to toxigenic strains of C. diphtheriae. Because the disease process depends on the action of bacterial toxins, the life-saving measure is treatment with antitoxins (immunoglobulins, IgG). However, antibody preparations are not without problems because they can lead to development of hypersensitivity, their production is limited by high cost, and they need to be stored under specific conditions to maintain efficiency.
Diphtheria outbreaks in many European countries indicate that immunity to the disease is decreasing among adults.22 The increased risk of diphtheria of outbreaks could have been foreseen because immunity after childhood vaccination is temporary.23 Diphtheria can be treated with diphtheria antitoxin (DAT). However, use of these IgG antibody preparations in developing countries is limited, mainly by high cost. DAT is not licensed by the FDA for use in the US. Clearly, an antitoxin that has better properties in terms of stronger affinity, rapid elimination, easy production, stability upon storage, and tolerance to pH and temperature is still needed. Therefore, in the future we plan to investigate the possibility of successfully producing diphtheria-specific nanobody fragments from the immunized camel and undertake a trial to produce a possibly more effective antitoxin for treatment of diphtheria in humans.
Antibodies, often described as “magic bullets”, are large, complicated, and expensive. Nanobodies, being smaller, may be able to infiltrate a wider range of diseases at lower cost. The binding site formed from a single domain that is small, highly stable, and able to refold properly after denaturation, makes the HcAb variable domain a valuable source of alternative recombinant binding ligand for many applications.24–26
The aim of this research was to evaluate the diphtheria toxin-binding capability of antibodies obtained from the immunized camel. Earlier work had shown that camel HcAb was active towards pathogens, and the ability to create single-domain antibodies from immunized libraries attests to their antigen recognition capability. However, camel HcAbs have not previously been evaluated for their applicability in immunodiagnostics.
Figure 2 demonstrates that a combination of affinity chromatography on PG and PA columns was effective in separating low molecular weight nanobodies from immunized camel serum. It could be easily seen that IgG was fractionated into its constituent subclasses (IgG1, IgG2, and IgG3). The successful separation of the targeted nanobodies was ascertained by SDS-PAGE electrophoresis (Figure 3). The obtained fractionated profile of IgG could be supported by considering the work of Daley et al3 who used the same columns to purify llama IgG isotypes.
Initial studies performed to evaluate the efficiency of purified IgG subclasses were done using microplates coated with different concentrations of diphtheria toxin, and direct binding of each IgG subclass was tested to estimate the relative affinities and specificities of camel HcAbs in comparison with conventional antibodies (Figure 4). The results show a parallel and positive relationship between serial concentrations of antigen (diphtheria toxin) and recorded OD in the presence of different IgG fractions. Most interesting was the fact that the IgG2 and IgG3 nanobodies appeared to display remarkable increases in OD, more than IgG1 in presence of different concentrations (0.01–10 μg/mL) of the used antigen.
In addition, camel HcAbs were shown to be effective as both capture and recognition molecules using ELISA (Figure 5). Statistical analysis of the results show that IgG2 has the highest potency as a capture molecule than the other camel IgGs (P < 0.05). This indicates that application of IgG2 and IgG3 is more sensitive and potent than IgG1 during ELISA. Our results are in good agreement with Emilio et al27 who used ELISA for the measurement of IgM and IgG subclasses in sera from llamas against different antigens. In addition, other researchers have concluded that antigen-specific fragments of the heavy chain IgGs are readily accessible from the llama,28 providing highly valuable binding molecules for a variety of applications. Previous studies have shown that HcAbs can act as effective high-affinity binding ligands for foreign target molecules.5,6,9 However, other studies have found no added benefit from these unusual molecules; one group failed to detect any specific HcAb binding following immunization,29 while another found HcAb to be ineffective as an enzyme inhibitor.30
More investigations are needed to understand the role of these nanobodies and finding the reasons for their existence in nature, because there is some work suggesting that the emergence of HcAbs would correspond to an evolutionary process favoring the development of protective responses against pathogens.31,32
Acknowledgments
The author would like to thank the research center of the female section, King Saudi University, for supporting the present work. The efforts of Dr Suleman O Alobaied in performing immunization of the camel is highly appreciated.
Footnotes
Disclosure
The author report no conflicts of interest in this work.
References
- 1.Brockmann EC, Cooper M, Stromsten N, Vehniainen M, Saviranta P. Selecting for antibody scFv fragments with improved stability using phage display with denaturation under reducing conditions. J Immunol Methods. 2005;296:159–170. doi: 10.1016/j.jim.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Dumoulin M, Conrath K, Van Meirhaeghe A, et al. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;1:500–515. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daley LP, Gagliardo LF, Duffy MS, Smith MC, Appleton JA. Application of monoclonal antibodies in functional and comparative investigations of heavy-chain immunoglobulins in new world camelids. Clin Diagn Lab Immunol. 2005;12:380–386. doi: 10.1128/CDLI.12.3.380-386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muylderman S, Atarhouch T, Saldanha J, Barbosa JA, Hamers R. Sequence and structure of VH domain from naturally-occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994;7:1129–1135. doi: 10.1093/protein/7.9.1129. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Reekmans G, Saerens D, et al. Prostate-specific antigen immunosensing based on mixed self-assembled monolayers, camel antibodies and colloidal gold enhanced sandwich assays. Biosens Bioelectron. 2005;21:483–490. doi: 10.1016/j.bios.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Maass DR, Sepulveda J, Pernthaner A, Shoemaker CB. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs) J Immunol Methods. 2007;324:13–25. doi: 10.1016/j.jim.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO World Health Organization expanded program on immunization. Feasibility of elimination of vaccine preventable disease. Weekly Epidemiol Rec. 1984;59:143–145. [Google Scholar]
- 8.CDC General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) MMWR Morb Mortal Wkly Rep. 2002;51:1–35. [PubMed] [Google Scholar]
- 9.Hamers-Casterman C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 10.Van der Vaart JM, Pant N, Wolvers D, et al. Reduction in morbidity of rotavirus induced diarrhea in mice by yeast produced monovalent llama-derived antibody fragments. Vaccine. 2006;24:4130–4137. doi: 10.1016/j.vaccine.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 11.Ditmann S, Wharton M, Vitek C, Ciotti M, Galazka A, Guichard S. Successful control of epidemic diphtheria in the states of the former Union of Soviet Socialist Republics: Lessons learned. J Infect Dis. 2000;181:S10–S22. doi: 10.1086/315534. [DOI] [PubMed] [Google Scholar]
- 12.Conrath K, Lauwereys M, Galleni M, et al. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in Camelidae. Antimicrob Agents Chemother. 2001;45:2807–2812. doi: 10.1128/AAC.45.10.2807-2812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decanniere K, Desmyter A, Lauwereys M, Ghahroudi MA, Muyldermans S, Wyns LA. Single-domain antibody fragment in complex with Rnase A: Non-canonical loop structures and nanomolar affinity using two CDR loops. Structure. 1999;7:361–370. doi: 10.1016/s0969-2126(99)80049-5. [DOI] [PubMed] [Google Scholar]
- 14.Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem. 2001;276:7346–7350. doi: 10.1074/jbc.M007734200. [DOI] [PubMed] [Google Scholar]
- 15.Ghahroudi MA, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragment from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–526. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 16.Frenken LG, van der Linden RH, Hermans PW, et al. Isolation of antigen specific llama VHH anti-body fragments and their high level secretion by Saccharomyces cerevisiae. J Biotechnol. 2000;78:11–21. doi: 10.1016/s0168-1656(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Jayprakasan V. Use of different antigenic preparations of Pasteurella multocida in enzyme immunoassay. Indian Vet J. 2001;78:379–381. [Google Scholar]
- 18.Barksdale L. Corynebacterium diphtheriae and its relatives. Bacterial Rev. 1970;34:378–422. doi: 10.1128/br.34.4.378-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lampidis T, Barksdale L. Park-Williams number 8 strain of Corynebacterium diphtheriae. J Bacteriol. 1971;105:77–85. doi: 10.1128/jb.105.1.77-85.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill DM, Dinius LL. Observations on the structure of diphtheria toxin. J Biol Chem. 1971;246:1485–1491. [PubMed] [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Kjeldsen K, Simonson O, Heron I. Immunity against diphtheria and tetanus in the age group 30–70 years. Scand J Infect Dis. 1988;20:177–185. doi: 10.3109/00365548809032435. [DOI] [PubMed] [Google Scholar]
- 23.Simonson O, Klaerke M, Klaerke A, Bloch A, Hansen B, Hald N. Revaccination of adults against diphtheria. II. Combined diphtheria and tetanus revaccination with different doses of diphtheria toxoid 20 years after primary vaccination. Acta Pathol Microbiol Immunol Scand. 1986;94:219–225. doi: 10.1111/j.1699-0463.1986.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldman ER, Anderson GP, Liu JL, et al. Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semi synthetic llama library. Anal Chem. 2006;78:8245–8255. doi: 10.1021/ac0610053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladenson RC, Crimmins DL, Landt Y, Ladenson JH. Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Anal Chem. 2006;78:4501–4508. doi: 10.1021/ac058044j. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood LJ, Osborn LE, Carrion R, Jr, Patterson JL, Hayhurst A. Rapid assembly of sensitive antigen-capture assays for Marburg virus, using in vitro selection of llama single-domain antibodies, at biosafety level 4. J Infect Dis. 2007;196:S213–S219. doi: 10.1086/520586. [DOI] [PubMed] [Google Scholar]
- 27.Emilio A, De S, Natalia S, Alejandro F, Juliana L. Development of ELISAs for the measurement of IgM and IgG subclasses in sera from llamas (Lama glama) and assessment of the humoral immune response against different antigens. Vet Immunol Immunopathol. 2008;126:64–73. doi: 10.1016/j.vetimm.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 28.van der Linden R, de Geus B, Stok W, et al. Induction of immune responses and molecular cloning of the heavy chain antibody repertoire of Lama glama. J Immunol Methods. 2000;240:185–195. doi: 10.1016/s0022-1759(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 29.Lange IG, Daxenberger A, Meyer HH. Studies on the antibody response of Lama glama – evaluation of the binding capacity of different IgG subtypes in ELISAs for clenbuterol and BSA. Vet Immunol Immunopathol. 2001;83:1–9. doi: 10.1016/s0165-2427(01)00376-2. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari A, Rodríguez MM, Power P, et al. Immunobiological role of llama heavy-chain antibodies against a bacterial beta-lactamase. Vet Immunol Immunopathol. 2007;117:173–182. doi: 10.1016/j.vetimm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Conrath KE, Wernery U, Muyldermans S, Nguyen VK. Emergence and evolution of functional heavy-chain antibodies in Camelidae. Dev Comp Immunol. 2003;27:87–103. doi: 10.1016/s0145-305x(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 32.Stijlemans B, Conrath K, Cortez-Retamozo V, et al. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. J Biol Chem. 2004;279:1256–1261. doi: 10.1074/jbc.M307341200. [DOI] [PubMed] [Google Scholar]


