Abstract
Stem cell transplantation for spinal cord injury (SCI) along with new pharmacotherapy research offers the potential to restore function and ease the associated social and economic burden in the years ahead. Various sources of stem cells have been used in the treatment of SCI, but the most convincing results have been obtained with neural progenitor cells in preclinical models. Although the use of cell-based transplantation strategies for the repair of chronic SCI remains the long sought after holy grail, these approaches have been to date the most successful when applied in the subacute phase of injury. Application of cell-based strategies for the repair and regeneration of the chronically injured spinal cord will require a combinational strategy that may need to include approaches to overcome the effects of the glial scar, inhibitory molecules, and use of tissue engineering strategies to bridge the lesion. Nonetheless, cell transplantation strategies are promising, and it is anticipated that the Phase I clinical trials of some form of neural stem cell-based approach in SCI will commence very soon.
Keywords: stem cell therapy, regeneration, spinal cord injury, cell dosing, cell tracking
Introduction
Spinal cord injury (SCI) is a major cause of disability and, at present, there is no universally accepted treatment. The cervical and lumbar regions of the spine are the most commonly affected areas in SCI. The functional decline following SCI is contributed to by both direct mechanical injury and secondary pathophysiological mechanisms that are induced by the initial trauma. These mechanisms initially involve widespread hemorrhage at the site of injury and necrosis of cellular components of the central nervous system (CNS). At later stages of injury, the cord is observed to display reactive gliosis. The actions of astrocytes, as well as numerous other cells in this response, create an environment that is not conducive to axonal regrowth, and the immune system worsens this further.1
The discovery of the potential utility of stem cells in neurological repair and regeneration is an exciting development in neuroscience. Despite advances in medical and surgical care, the current clinical therapies for SCI are largely ineffective. During the last two decades, the search for new therapies has been revolutionized with the discovery of stem cells, which has inspired scientists and clinicians to search for a stem cell-based reparative approach to treat SCI. Stem cells, in the niches around the ependymal layer, proliferate after neuronal loss. This proliferation is triggered by various messenger cascades provoked by the injury. Gliosis in most situations impairs the attempts of axons to regrow and re-establish communications. Cell replacement approaches in the setting of SCI can be used to achieve two broad goals, ie, regeneration, which seeks to replace lost or damaged neurons and induce axonal regeneration or plasticity, and repair, which seeks to replace supportive cells such as oligodendrocytes in order to induce remyelination and prevent progressive myelin loss.2 In the setting of SCI, stem cell therapy could potentially be used to stimulate the endogenous stem cell population to proliferate along neuronal lines or to supplement stem cells in the repair process.3,4 The mature CNS harbors endogenous stem cells which develop into neurons constantly in at least a few areas of the CNS, ie, the subventricular zone, which includes the linings of the lateral ventricles, the subgranular zone of the dentate gyrus, and the central canal of the spinal cord. These cells normally differentiate only into astrocytes and oligodendrocytes in vivo.5,6 There are some factors in the CNS which normally limit or prevent endogenous stem cells from becoming mature neurons.4
After injury, with resultant loss of both nerve cells and cells that provide the myelin for appropriate conduction properties, the obvious solution would be to provide cells that can replace the lost function. A variety of tissues and cells have been used to encourage restoration of function.7 These include stem cells, olfactory ensheathing cells (cells that form the myelin on olfactory nerves), Schwann cells (cells that form the myelin on peripheral nerves), dorsal root ganglia, adrenal tissue, hybridomas, peripheral nerves, or transplanted conduits of Schwann cells, which would serve as a source for chemical and mechanical guidance. It is postulated that these tissues would rescue, replace, or provide a regenerative pathway for injured adult neurons, which would then integrate or promote regeneration of the spinal cord circuitry and restore function after injury.8
The route through which stem cells can be instilled is a major question in cell therapy. Direct injection into the injury site at the time of surgery, delivery through the vascular route by highly selective angiography, through the central canal by instillation into the fourth ventricle, and intrathecal instillation are the possible and frequently used routes for stem cell transplantation in SCI. Of these routes, intrathecal instillation is technically simple and least likely to provoke iatrogenic damage of regenerative potential.
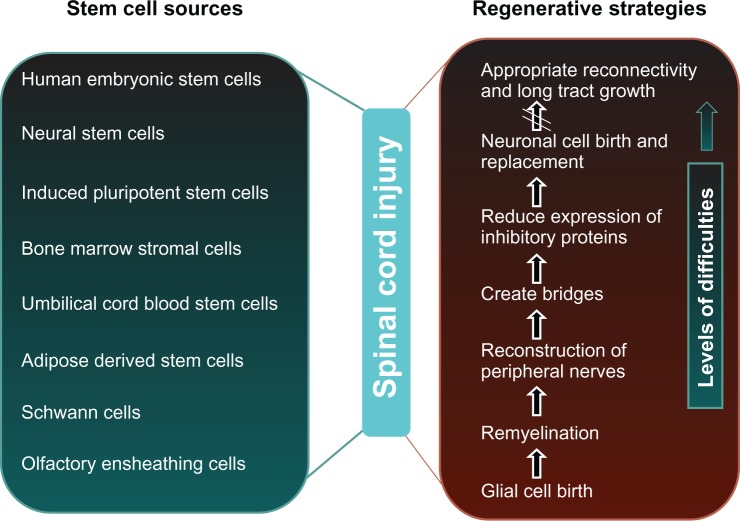
Due to cellular loss, regeneration after SCI is limited, and current approaches to treatment of SCI do not lead to complete cure. Various sources of stem cell transplantation have been shown to replace host neurons, enhance axonal growth, and improve functional recovery successfully in rat models of SCI.9 New approaches in stem cell therapy using neural stem/progenitor cells are widely accepted in the treatment of degenerative diseases for the repair of damaged or lost tissues (Figure 1). There are several cell sources for stem cell therapy, including fetal spinal cord tissue,10 neuronal stem cells from adult brains, and mesenchymal stem cells from bone marrow11 and other organs, in addition to embryonic stem cells. Human embryonic and adult stem cells both have potential use as cell-based regenerative therapies. Of course they differ in many respects, eg, in the number and differentiated cell types that they can become.
Figure 1.
Major stem cell sources, regenerative strategies, and levels of difficulties in the treatment of spinal cord injury.
Pathophysiology of SCI
To understand the rationale of the recent advances, it is first necessary to review the pathophysiology of SCI. There are four general types of SCI: cord maceration, in which the morphology of the cord is severely distorted; cord lacerations (gun shot or knife wounds); contusion injury, which leads to a central hematomyelia that may evolve into a syringomyelia; and solid cord injury, in which there is no central focus of necrosis as in contusion injury. There are three phases of SCI response that occur after injury, ie, acute, secondary, and chronic injury processes.12–14 Experimental models and clinical observations of acute SCI support the concepts of primary and secondary injury, in which the initial mechanical insult is succeeded by a series of deleterious events that promote progressive tissue damage and ischemia. Whereas the primary injury is fated by the circumstances of the trauma, the outcome of the secondary injury may be amenable to therapeutic modulation.
Acute SCI
In the acute phase, which encompasses the moment of injury and extends for the first few days, a variety of parallel pathophysiological processes begins, which can be summarized in two complex phases.15
Primary injury mechanism
The primary injury phase is due to compression or contusion. Primary injury mechanisms include impact plus persistent compression; impact alone with transient compression; distraction; and laceration/transection.13 The first mechanism in SCI involves cord compression, fracture-dislocations, and acute disc ruptures. The second mechanism involves impact alone with only transient compression and distraction. Forcible stretching of the spinal column in the axial plane provides a third mechanism and becomes apparent when distractional forces resulting from flexion, extension, rotation, or dislocation produce shearing or stretching of the spinal cord and/or its blood supply. This type of injury may underlie SCI without radiological abnormality, especially in children where cartilaginous vertebral bodies, underdeveloped musculature, and ligament laxity are predisposing factors.16
The initial mechanical insult tends to damage primarily the central gray matter, with relative sparing of the white matter, especially peripherally, which can be the result of its softer consistency and greater vascularity.17 Further nerve transmission may be disrupted due to microhemorrhages or edema near the injury site.18–20 Damage of gray matter occurs within the first hour after injury, whereas the white matter is irreversibly damaged within 72 hours after injury.
Secondary injury mechanism
Following an initial impact after SCI, there is a cascade of downstream events termed “secondary injury”, which culminates in progressive degenerative events in the spinal cord. These secondary injury mechanisms include, but are not limited to, ischemia, inflammation, free radical-induced cell death, glutamate excitotoxicity, cytoskeletal degradation, and induction of extrinsic and intrinsic apoptotic pathways. After SCI, several factors, including glutamate release, lipid peroxidation, and ionic imbalance, contribute to the events, leading to acute local ischemia and secondary degeneration.21 There is emerging evidence that glutamate excitotoxicity plays a key role not only in neuronal cell death but also in delayed post-traumatic spinal cord white matter degeneration. Importantly however, the differences in cellular composition and expression of specific types of glutamate receptors in grey versus white matter require a compartmentalized approach to understand the mechanisms of secondary injury after SCI.
Chronic SCI
In chronic SCI, the wave of secondary cell death, which mainly affects neurons and oligodendrocytes, spreads rostrally and caudally from the site of impact, leading to structural and functional disturbance of the spinal cord.22 Later, secondary mechanisms cause the chronic phase of the SCI, when they produce vasculature and ischemia, glutamatergic excitotoxicity, oxidative cell stress, lipid peroxidation, and inflammation, all of which alone or in concert can trigger apoptosis.21 Mortality rates are highest in the first year after SCI. For patients surviving at least 1 year after traumatic SCI, life expectancy is approximately 90% of normal.23,24 Higher neurological level, severity of injury, and older age at the time of SCI negatively impact survival.
Current treatments and clinical trials for SCI
Pharmacological agents must be given within a narrow window of opportunity to be effective. Although many therapeutic agents show potential promise in animal models, only methylprednisolone has been shown in large, randomized, double-blind human studies to enhance the functional recovery of neural elements after acute SCI.25 Several studies have investigated chronic SCI models using whole tissue grafts and cells of the peripheral nervous system. Transplantation of fetal spinal tissue, fetal brain cortex, olfactory ensheathing cells, peripheral nerve grafts, and Schwann cells after SCI have all been shown to improve locomotor recovery,26–30 suggesting that the chronic post-injury period may be a feasible target for repair (Table 1).
Table 1.
Recent clinical trials of stem cell treatment for SCI listed on http://www.clinicaltrials.gov
| Status | Study | NCT number | Cells | Phase | Sponsors/investigators | Duration | Number enrolled | Country | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|
| Completed | Cell transplant in SCI patients, condition: chronic SCI, Procedure: physical therapy | NCT00816803 | Autologous bone marrow stem cell | I/II | Cairo University, Cancer Institute of New Jersey | May 2005 to December 2008 | 80 | Egypt | Safety of autologous bone marrow transplant measured by absence of neuronal changes, infections, or increased intracranial tension, and monitoring for any abnormal growth or tumor formation by MRI; Efficacy of bone marrow cell transplant in improving neurological functions in patients with chronic SCI. Improvement in motor, sensory and sphincteric functions, and quality of life using ASIA scores and MRI |
| Completed | Safety and efficacy of autologous bone marrow stem cells in treating spinal cord injury | NCT01186679 | Autologous bone marrow stem cells | I/II | International Stemcell Services Ltd | January 2008 to August 2010 | 12 | India | Significant clinical improvement in ASIA impairment scale and general condition; changes in MRI, neurological improvement (cranial/spinal reflexes) and evoked potential study |
| Completed | Autologous adipose-derived MSCs transplantation in patient with spinal cord injury | NCT01274975 | Autologous adipose-derived MSCs | I | RNL Bio Company Ltd | July 2009 to February 2010 | 8 | Korea | Safety evaluation |
| Active, not recruiting | Autologous bone marrow stem cell transplantation in patients with spinal cord injury | NCT01325103 | Autologous bone marrow stem cell | I | Hospital Sao Rafael; Oswaldo Cruz Foundation; Irep Sociedade de Ensino Superior Médio e Fundamental Limitada; Hospital Espanol | July 2010 to January 2013 | 20 | Brazil | Feasibility and safety of bone marrow stem cell transplantation in patients with SCI; functional improvement in muscle strength; improvement of sphincter control |
| Active, not recruiting | Transfer of bone marrow-derived stem cells for the treatment of spinal cord injury | NCT01162915 | Autologous mesenchymal stem cells | I | TCA cellular therapy | July 2010 to June 2012 | 10 | US | Safety |
| Recruiting | Autologous stem cells for SCI in children; primary SCI to minimize secondary SCI | NCT01328860 | Autologous BMSCs |
I | Memorial Herman Healthcare System, James E Baumgarter |
April 2011 to October 2014 | 10 | US | American Spinal Injury Association, Standard Neurological Classification of Spinal Cord Injury, Standard Neuropathic Pain Rating Scale |
| Recruiting | Study of human CNS stem cells in patients with thoracic SCI | NCT01321333 | Human CNS stem cells | I/II | Stem Cells Inc | March 2011 to March 2016 | 12 | Switzerland | Types and frequencies of adverse events and serious adverse events |
| Recruiting | Study of the safety and efficacy of autologous bone marrow stem cells in patients with SCI | NCT01490242 | Autologous bone marrow derived stem cells | I/II | Totipotent RX Cell Therapy Pvt Ltd; Fortis Healthcare | October 2011 to October 2013 | 15 | India | Number of participants with adverse events as a measure of safety and tolerability; significant improvement in the ASIA scores by the assessment of motor, sensory, and sphincteric function |
| Recruiting | Autologous stem cells for SCI in children | NCT01328860 | Autologous BMPCs | I | Memorial Hermann Healthcare System; The Institute for Rehabilitation and Research Foundation; The University of Texas Health Science Center, Houston, TX | April 2011 to October 2014 | 10 | US | Standard Neurological Classification of Spinal Cord Injury; Standard Neuropathic Pain Rating Scale |
| Recruiting | Difference between rehabilitation therapy and stem cells transplantation in patients with spinal cord injury in China | NCT01393977 | Stem cells | II | General Hospital of Chinese Armed Police Forces | January 2011 to May 2012 | 60 | China | Electromyogram and electroneurophysiology |
| Recruiting | Safety and feasibility of umbilical cord blood cell transplant into injured spinal cord | NCT01046786 | Umbilical cord blood mononuclear cells | I/II | China Spinal Cord Injury Network | January 2010 to June 2012 | 20 | China | Number of participants with adverse events as a measure of safety and tolerability |
Abbreviations: BMPCs, bone marrow progenitor cells; BMSCs, bone marrow stromal cells; MSCs, mesenchymal stem cells; SCI, spinal cord injury; MRI, magnetic resonance imaging.
Regeneration and replacement of neurons and glia that undergo cell death soon after injury are the main goals of all stem cell-based therapies for SCI. Successful development of stem cell-based therapies for SCI requires more intense work to obtain a better understanding of stem cell differentiation pathways and their survival upon transplantation.25 All stem cell replacement strategies should address these two important problems, and this may be accomplished by improved differentiation protocols for stem cells, transplantation of neural progenitor cells, or by activation of endogenous sources of neural progenitors. However, the ideal source of stem cells for efficient and safe cell replacement has remained a challenging issue that requires more investigation (Table 2).
Table 2.
Comparison of different stem cell types used in clinical trials for the treatment of spinal cord injury
| BMSCs | UCBSCs | ESCs | iPSCs | fNPCs | aNPCs | OECs | SCs | Adip MSCs | |
|---|---|---|---|---|---|---|---|---|---|
| Isolation | Challenging | Challenging | Challenging | Challenging | Challenging | Challenging | Challenging | Challenging | Easy |
| Ethical issues | Considerable | − | Significant | None | Significant | Considerable | Few | Considerable | Few |
| Differentiation potential into bone, fat, and cartilage | √ | √ | Pluripotent | Pluripotent | Neural lineages | Neural lineages | − | − | √ |
| Pre-isolation storage | X | √ | X | X | X | X | X | X | ? |
| Post-isolation storage | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Tumorigenicity | X | X | √ | √ | X | X | X | X | X |
| Transfection | √ | √ | √ | √ | √ | √ | √ | √ | ? |
| Autologous | √ | Potential | X | Potential | X | X | √ | √ | √ |
| Safety/risk | √ | √ | √ | ? | √ | √ | √ | √ | √ |
Abbreviations: BMSCs, bone marrow stromal cells; UCBSCs, umbilical cord blood-derived stem cells; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; fNPCs, fetal neural progenitor cells; aNPCs, adult neural progenitor cells; OECs, olfactory ensheathing cells; SCs, Schwann cells; AdipMSCs, adipose tissue-derived mesenchymal stem cells.
An autologous transplantation approach for the treatment of SCI eliminates concerns regarding immune rejection and avoids the controversy over embryonic or neonatal sources. However, an autologous approach necessitates sacrificing a peripheral nerve, and despite improvements in amplification techniques, more time is needed before enough cells can be generated for transplantation. Alternative sources of stem cells from postnatal skin or adult bone marrow have recently been pursued and tested after thoracic transection31 or contusion32 injuries to obviate the need for harvesting a peripheral nerve from a patient. Different sources of stem cells are being exploited for the treatment of spinal cord injury as well as other neurological disorders.
Human embryonic stem cells
Embryonic stem cells are derived from the inner cell mass of mammalian blastocysts and have the ability to proliferate by maintaining both their pluripotency and the ability to differentiate into nearly all cell types, including neuronal and glial fate cells.26,33 Human embryonic stem cells were first derived from human blastocysts by Thomson et al in 1998.27 These cells have an ability to proliferate in long-term cultures while maintaining their pluripotent nature. Therefore, embryonic stem cells are a promising source of differentiated oligodendrocytes and motor neurons for the treatment of SCI. Several reports34–36 have demonstrated the in vitro capacity of human embryonic stem cells to generate neural progenitor cells, including regionally specific neuronal subtypes. Compared with other sources of cell therapy, human embryonic stem cells are one of the most attractive cell sources for spinal cord therapy and this strategy has been validated recently34 by the finding that human embryonic stem cell-derived motor neurons can survive and integrate into the spinal cord. In a recent study by Erceg et al,37 transplantation of human embryonic stem cell-derived oligodendrocyte progenitor cells and/or motor neuron progenitors was done in an adult rat with SCI. The study demonstrated the survival, migration, and differentiation of transplanted cells into appropriate cell types without forming teratomas, and improved locomotor function.
Limitations with regard to this source of cells include the lengthy and complex differentiation protocols needed for obtaining neural progenitors from human embryonic stem cells, application of undesired cell types and undefined factors, and also issues regarding the safety of transplantation of human embryonic stem cells in humans, including formation of teratomas following human embryonic stem cell-derived neural cell engraftment.38
Neural stem cells
Neural stem cells can be isolated from various parts of the embryonic and adult CNS, such as the subventricular zone lining the lateral ventricles39–41 and the dentate gyrus of the hippocampus,42,43 although the multipotentiality of the latter remains a contentious issue. Neural stem cells are multipotent and can differentiate into three lineages, ie, neurons, oligodendrocytes, and astrocytes, and can be efficiently propagated in vitro.44,45 The alternative strategy for treatment of SCI is recruitment of endogenous neural stem cells or transplantation of neural stem cells. There are very few reports46–49 that describe the mechanism of integration of neural stem cells and a mechanism to promote functional recovery after SCI. Recently, Hooshmand et al46 have shown that no change occurs in the host microenvironment (lesion size, tissue sparing, glial scar, and expression of proteins such as fibronectin, NG2, versican, GFAP, and PECAM1) after analysis of neural stem cell-engrafted models of SCI. Sources of transplanted neural stem cells, methods of isolation, and preparation of cells prior to implantation seem to be critical in cell survival and integration after implantation.46,50
Neural stem cells are more preferable to human embryonic stem cells for clinical applications because they are safer for cell therapy, given that neural stem cells have less potential to form tumors compared with embryonic stem cells.51 Isolation of pure populations of differentiated cells, inefficient tracking systems, and moderate cell survival after transplantation45,52 are critical challenges that remain when using neural stem cells. Axonal regeneration and extension by cell replacement using either endogenous or exogenous stem cells suffer from various limitations, including formation of glial scars, a lack of neurotrophic factors, inhibitory myelin-associated molecules, and decreased levels of cAMP.53,54
Induced pluripotent stem cells
Induced pluripotent stem cells represent a breakthrough in the field of stem cell biology and provide a promising cell resource for customized patient-specific cell therapies. Induced pluripotent stem cells are very similar to embryonic stem cells in terms of gene expression, chromatin methylation, and embryoid body and viable chimera formation.55 They are capable of differentiating into all cell types, including neurons, glia, neural progenitor cells, and motor neurons.56,57 Furthermore, the derivation of induced pluripotent stem cells using nonviral methods58 or by chemicals and small molecules,59 such as transcription factors,60 Oct4, Klf4, Sox2, and c-Myc,61 makes this cell replacement strategy very attractive. Nevertheless, this cell type shares disadvantages similar to those of other cell sources. Teratoma formation, aberrant reprogramming, and the presence of transgenes in induced pluripotent stem cell populations are the most concerning obstacles, which should be addressed before their clinical application.62
Bone marrow stromal cells
Bone marrow stromal cells are isolated from the stromal compartment of the bone marrow and are nonteratogenic, with anti-inflammatory and immunomodulatory effects.63,64 These cells also secrete neurotrophic factors and cytokines which make them an attractive source for trophic support in autologous transplantation of endogenous and co-implanted cells at the time of CNS injury. Several studies in rodents have demonstrated that the intraspinal, intrathecal, and systemic (intravenous) routes of delivery have been successful for axonal regrowth and sprouting in SCI repair.65
However, the use of bone marrow stromal cells in SCI is limited because of certain issues, such as bone marrow stromal cell migration beyond the injection site. Variability in clinical outcome of bone marrow stromal cells across the lesion site in SCI repair makes it difficult due to intervariability in efficacy and immunomodulatory potency. Also, there is an absence of more appropriate models for SCI using bone marrow stromal cells to distinguish between functional preservation of axons and de novo axonal regrowth.66,67
Umbilical cord blood-derived stem cells
Identification of a suitable source of stem cells for use in regenerative medicine research and its applications has been challenging. For effective clinical application, a readily, more accessible, abundant, and compatible source of stem cells is required. Umbilical cord blood provides an answer to these problems because it is an abundant source of nonembryonic stem cells.68 Compared with the collection of bone marrow, umbilical cord blood collection is more feasible because it is noninvasive with no side effects for either the baby or the mother.69,70 Moreover, umbilical cord blood stem cells have a higher proliferating potential, with telomeres that are longer than those of other somatic stem cells.71,72 Further, umbilical cord blood can be stored and cryopreserved in cord blood banks for later use in transplant applications.
It has been shown that the mononuclear fraction of umbilical cord blood contains pluripotent stem cells with the potential to become neural cells.73 The use of these cells in neural clinical applications is still in the initial phase, but the results obtained so far are very promising. Kang et al74 purified mesenchymal stem cells from umbilical cord blood and transplanted them to the injury site in a 37-year-old woman with SCI. The results showed regeneration of the spinal cord at the injury site and the patient showed improved sensory perception and mobility.74
Preclinical trials based on umbilical cord blood stem cells in animal models have shown promising results in the treatment of neural diseases and injuries.75 Many researchers have demonstrated that umbilical cord blood stem cells are amenable to neurological application, including preclinical animal models of disease, and more recently clinical trials.76 Therefore, umbilical cord blood stem cells are unique in their suitability for use in stem cell transplantation for the treatment of neurological disease.77
Adipose-derived mesenchymal stem cells
Studies have been conducted with mesenchymal stem cells for the treatment of various diseases in the field of regenerative medicine. For many years, bone marrow stromal cells have been the primary source of stem cells for tissue engineering applications.78–80 However, studies have shown that subcutaneous adipose tissue has an advantage over other stem cell sources, ie, the ease of isolating stem cells from harvested tissue.81 Adipose mesenchymal stem cells show stable growth and proliferation kinetics, and can differentiate into adipogenic, osteogenic, chondrogenic, myogenic, or neurogenic lineages in vitro.82–84
Adipose mesenchymal stem cells express CD90, CD105, CD106, CD117, and STRO-1 markers, and are negative for hematopoietic lineage markers (eg, CD45 and CD14) as well as for endothelial cell markers (CD31, CD144) and von Willebrand factor.82,84 Adipose mesenchymal stem cells are fibroblast-like cells in their morphology, and preserve their shape after expansion in vitro.82,85,86
Adipose mesenchymal stem cells can be used in the treatment of many CNS injuries.87–91 Ryu et al91 used this type of stem cell for the treatment of acute spinal injury in a canine model, and reported significant improvement in neurological function. In another study, adipose mesenchymal stem cells were used to treat SCI in rats after in vitro differentiation into Schwann cells.92 In this study, the authors stated that functional improvement was not observed despite the significant histological improvement achieved at the primary injury site by the transplantation of Schwann cells. Therefore, it was speculated that complete recovery of spinal cord function requires a much more complex treatment strategy combining different modalities. Adipose mesenchymal stem cell therapies appear very promising based on the results of in vitro and in vivo research. Currently, a number of clinical trials for the regeneration of soft tissue, craniofacial tissue, cardiovascular, and CNS tissues are underway.93
Schwann cells
Schwann cells are typical gliocytes that are involved in axon regeneration in the peripheral nervous system and form the myelin sheaths around axons.94 Several neurotrophic factors, extracellular matrix, and cell adhesion molecules expressed by Schwann cells have been used to promote axon regeneration and improve the glial environment after SCI.95,96
It is believed that astroglial scars block the growing axons and prevent migration of Schwann cells into the astroglial domain, so they are unable to provide a bridge for newly born axons to grow through the astroglial scar.95 Therefore, more research is needed to maintain the biological activity of Schwann cells and increase their migration after being transplanted to the SCI site. Woodhoo et al97 implanted Schwann cell precursors into the injured spinal cord and found that these cells have greater ability than normal Schwann cells to migrate and survive in the astroglial environment.
In another study, Papastefanaki et al98 transplanted Schwann cells with altered adhesion properties into an in vivo mouse model of SCI and found that these cells promoted faster and significantly greater functional recovery compared with normal Schwann cells or no cells at all. The ability of Schwann cells to repair SCI can be enhanced by combined therapeutic measures, such as increasing the secretion of neurotrophic factors by genetic engineering, filling the gap, and providing channels through the injury site.
Olfactory ensheathing cells
Olfactory ensheathing cells are present along the full length of olfactory nerves crossing the peripheral nervous system and CNS junction.99,100 Unlike most of the adult CNS, the olfactory bulb retains its regenerative capacity. Compared with other cells, olfactory ensheathing cells have a greater ability to migrate to areas distal from the transplantation site, so they can bridge the gap between the lesion site and normal spinal cord to grow the axons.101 These cells can be harvested and then transplanted back into the original human donor, thereby eliminating rejection-related problems and being ideal for translational research.102
Olfactory ensheathing cell transplantation studies in animals suggest that delaying transplantation after SCI may be beneficial to the survival of ensheathing cells.98 There are many published reports about autologous transplantation as a treatment for SCI.106 Olfactory ensheathing cells have more potential advantages than Schwann cells, but more basic research is needed to identify the mechanisms by which they promote axonal regeneration. Recent findings suggest that the beneficial effect of these cells can be enhanced by genetic engineering approaches or transplanting them with other cell types.103 When compared with Schwann cells and other sources of stem cells, problems of rejection, overgrowth, disease transmission, and ethical issues can be avoided because a subject’s own olfactory mucosa can be used for in vitro studies and clinical trials.106
Challenges in developing cellular therapies for SCI
Cell sourcing
A number of cell sourcing issues need to be considered when developing cell-based therapies for the treatment of SCI and other neurological disorders. To develop such a strategy, a suitable source and quantity of cells for transplantation need to be considered first. If the cells are obtained from an animal source for preclinical studies, there should be a mechanism for translating them into the clinical setting. A second consideration is how to characterize the cells obtained to determine which specific cell phenotype should be implanted for a particular disease. Another issue arises when the stem cells are continuously cultured, including reproducibility of cell lines and the number of times a cell line can be passaged due to variation in passage numbers, feeder layers, and composition of the medium.
Transplantation issues
The first transplantation issue concerns the route of delivery, which should preserve cell integrity and viability. For progenitor cell transplantation, maximum care should be taken to eliminate unwanted differentiation or proliferation, which can lead to formation of teratomas. A second issue is related to the immune response of patients at the time of transplantation, which can lead to graft rejection.
Confirming an experimental therapy before transfer into humans
To obtain the necessary experimental data to begin clinical studies, compelling evidence of benefit must be demonstrated in reproducible animal models of SCI, although no single experimental model exactly mimics clinical conditions in humans. The rodent models that are currently being used include compression, contusion, and transection methods leading to reproducible patterns of structural damage in specific gray and white matter structures. Injury severity varies with each model, so that a spectrum of histopathological and behavioral deficits is reproduced. Also, the SCI patient population is a very heterogeneous group, so no one SCI patient is exactly the same as another. With this in mind, the American Society of Neural Transplantation and Repair comments that the exact type of animal model required will depend on the target condition being considered.105
Cell fate and tracking in vivo
Determination of cell migration and fate in real time and in the long term is of major interest as it relates to dosing, efficacy, optimization, and safety concerns in translational approaches for cell therapy. For both research and clinical purposes, tracking of stem cells after their administration is crucial in vivo and in vitro for determination of their distribution, location, quantity, viability, and final differentiation. There are several strategies to track the cells in vivo and in vitro. Noninvasive strategies include: direct labeling of target cells with paramagnetic contrast agents and tracking them with functional magnetic resonance imaging, using either supermagnetic iron oxide particles,106,107 gadolinium,108,109 19 F isotopes,110 direct labeling with quantum dots using cadmium nanocrystals,111,112 or traditional fluorochromes like PKH26,113 or internal labeling using transfected enhanced green fluorescence protein and firefly luciferase reporter genes via the bioluminescence mechanism.114,115 Each of these cell tracking methods suffers from many drawbacks, including: limited uptake of paramagnetic contrast and weak magnetic resonance imaging signals (except when using the supermagnetic iron oxide particles); traditional fluorochromes being prone to bleaching; cadmium in quantum dot crystals being toxic to cells; and bioluminescence imaging being limited by low tissue penetrance.116
Cell dosing and delivery
In terms of the upper limit and frequency of administration, stem cell dosing remains controversial in the indication of SCI. One would expect an incremental response with dose escalation, but the available data are limited. Recently, Usvald et al117 demonstrated an optimum dosing regimen for intraparenchymal injection of human spinal cord-derived stem cells into the minipig spinal cord for neuronal repopulation, although some studies have suggested that intrathecal and intravenous delivery of stem cells is less efficacious than direct injection into spinal cord tissue.118,119 Vaquero et al118 also showed that intralesional injection of stem cells into rats with SCI produced a better outcome than intravenous administration. A recent study by Wu et al120 proposed fibrin glue as a vehicle for delivering mesenchymal stem cells to injured neural tissues, with good functional recovery, although it is still a challenge to target the stem cells within cellular precision.
Conclusion and future directions
In the field of translational biology, we are rapidly moving toward translation into clinical practice, which provides advanced cell culture techniques and plasticity with regard to choosing different stem cell sources to test the ability of these cells in the treatment of SCI and other neurological disorders. Research using in vitro and in vivo regenerative medicine models has demonstrated the potential use of stem cells in the treatment of various diseases, but uncertainty regarding a suitable source have raised controversial problems concerning their identification and development of new cell-based therapies.
Although various factors are involved in causing different types of SCI, destruction of the signal pathway is the same. Reier et al121 performed the first human fetal stem cell transplantation for SCI and the procedure explicitly recognized the advantage of using stem cells, with a positive outcome in regards to both safety and efficacy. More studies are needed to enhance graft cell viability and application of controlled differentiated cells in SCI. Several other studies have also demonstrated that histological changes in axonal regeneration and sprouting are crucial for behavioral recovery, therefore promoting axonal elongation and sprouting is now a well accepted strategy for treatment of SCI. These cellular-based therapies combined with additional strategies such as neurotrophins, neuroprotective agents, and engineering of neurobiocompatible materials to bridge the cord injury increase the hope of improved treatment of SCI.
Because of the complexity of the issues involved, different viewpoints, and variations between protocols at different institutions for graft replacement transplantation studies, it is difficult to develop a common protocol. Questions regarding relevant animal modeling in both large and small animals also require continued discussion, and controlled preclinical data in Phase I studies are essential to evaluate the safety of cell therapy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Webb AA, Ngan S, Fowler JD. Spinal cord injury I: A synopsis of the basic science. Can Vet J. 2010;51:485–492. [PMC free article] [PubMed] [Google Scholar]
- 2.Ulzheimer JC, Peles E, Levinson SR, Martini R. Altered expression of ion channel isoforms at the node of Ranvier in P0-deficient myelin mutants. Mol Cell Neurosci. 2004;25:83–94. doi: 10.1016/j.mcn.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- 4.Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- 5.Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vroemen M, Aigner L, Winkler J, Weidner N. Adult neural progenitor cell grafts survive after acute spinal cord injury and integrate along axonal pathways. Eur J Neurosci. 2003;18:743–751. doi: 10.1046/j.1460-9568.2003.02804.x. [DOI] [PubMed] [Google Scholar]
- 7.Horner P, Power A, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zompa EA, Cain LD, Everhart AW, Moyer MP, Hulsebosch CE. Transplant therapy: recovery of function after spinal cord injury. J Neurotrauma. 1997;14:479–506. doi: 10.1089/neu.1997.14.479. [DOI] [PubMed] [Google Scholar]
- 9.Di Giovanni S. Regeneration following spinal cord injury, from experimental models to humans: where are we? Expert Opin Ther Targets. 2006;10:363–376. doi: 10.1517/14728222.10.3.363. [DOI] [PubMed] [Google Scholar]
- 10.Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 12.Tator CH. Biology of neurological recovery and functional restoration after spinal cord injury. Neurosurgery. 1998;42:696–708. doi: 10.1097/00006123-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Tator CH. Experimental and clinical studies of the pathophysiology and management of acute spinal cord injury. J Spinal Cord Med. 1996;19:206–214. doi: 10.1080/10790268.1996.11719436. [DOI] [PubMed] [Google Scholar]
- 14.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 15.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 16.Pang D, Wilberger JE., Jr Spinal cord injury without radiographic abnormalities in children. J Neurosurg. 1982;57:114–129. doi: 10.3171/jns.1982.57.1.0114. [DOI] [PubMed] [Google Scholar]
- 17.Wolman L. The disturbances of circulation in traumatic paraplegia in acute and late stages: a pathological study. Paraplegia. 1965;2:213–226. doi: 10.1038/sc.1964.39. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DK, Hall ED. Pathophysiology of spinal cord trauma. Ann Emerg Med. 1989;22:987–992. doi: 10.1016/s0196-0644(05)82739-8. [DOI] [PubMed] [Google Scholar]
- 19.Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal cord injury – a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324:1829–1838. doi: 10.1056/NEJM199106273242601. [DOI] [PubMed] [Google Scholar]
- 20.Lapchak PA, Araujo DM, Song D, Zivin JA. Neuroprotection by the selective cyclooxygenase-2 inhibitor SC-236 results in improvements in behavioral deficits induced by reversible spinal cord ischemia. Stroke. 2001;32:1220–1225. doi: 10.1161/01.str.32.5.1220. [DOI] [PubMed] [Google Scholar]
- 21.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 22.Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 23.Frankel HL, Coll JR, Charlifue SW, et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- 24.Hagen EM, Lie SA, Rekand T, Gilhus NE, Gronning M. Mortality after traumatic spinal cord injury: 50 years of follow-up. J Neurol Neurosurg Psychiatry. 2010;81:368. doi: 10.1136/jnnp.2009.178798. [DOI] [PubMed] [Google Scholar]
- 25.Bracken M, Shepard M, Holford T, et al. Results of the third acute spinal cord injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 26.Barakat DJ, Gaglani SM, Neravetla SR, et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PM, Mous GW, Cummings JL, Toga AW, et al. Cortical Variability and Asymmetry in Normal Aging and Alzheimer’s Disease. Cereb Cort. 1998;8(6):492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- 28.Lu J, Feron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- 29.Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J Neurosci. 2003;23:28–34. doi: 10.1523/JNEUROSCI.23-28-09428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci Lett. 2006;398:129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 31.Kamada T, Koda M, Dezawa M, et al. Transplantation of bone marrow stromal cell-derived Schwann cells promotes axonal regeneration and functional recovery after complete transection of adult rat spinal cord. J Neuropathol Exp Neurol. 2005;64:37–45. doi: 10.1093/jnen/64.1.37. [DOI] [PubMed] [Google Scholar]
- 32.Biernaskie J, Sparling JS, Liu J, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erceg S, Ronaghi M, Stojkovic M. Human embryonic stem cell differentiation toward regional specific neural precursors. Stem Cells. 2009;27:78–87. doi: 10.1634/stemcells.2008-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee H, Shamy GA, Elkabetz Y, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 35.Erceg S, Lainez S, Ronaghi M, et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PloS One. 2008;3:e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee G, Kim H, Elkabetz Y, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 37.Erceg S, Ronaghi M, Oria M, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transaction. Stem Cells. 2010;28:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 40.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 41.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seaberg RM, Van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmer TD, Ray J, Gage FH. FGF-2 responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 44.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 45.Hsu YC, Lee DC, Chiu IM. Neural stem cells, neural progenitors, and neurotrophic factors. Cell Transplant. 2007;16:133–150. [PubMed] [Google Scholar]
- 46.Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PloS One. 2009;4:e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PloS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babu H, Cheung G, Kettenmann H, Palmer TD, Kempermann G. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PloS One. 2007;2:e388. doi: 10.1371/journal.pone.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim BG, Hwang DH, Lee SI, Kim EJ, Kim SU. Stem cell-based cell therapy for spinal cord injury. Cell Transplant. 2007;16:355–364. doi: 10.3727/000000007783464885. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J, Wu X, Zhang HL. Adult neural stem cell therapy: expansion in vitro, tracking in vivo and clinical transplantation. Curr Drug Targets. 2005;6:97–110. doi: 10.2174/1389450053345055. [DOI] [PubMed] [Google Scholar]
- 53.Coutts M, Keirstead HS. Stem cells for the treatment of spinal cord injury. Exp Neurol. 2008;209:368–377. doi: 10.1016/j.expneurol.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 57.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 60.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 63.Atkins H, Freedman M. Immune ablation followed by autologous hematopoietic stem cell transplantation for the treatment of poor prognosis multiple sclerosis. Methods Mol Biol. 2009;549:231–236. doi: 10.1007/978-1-60327-931-4_16. [DOI] [PubMed] [Google Scholar]
- 64.Freedman MS, Bar-Or A, Atkins HL, et al. International MSCT Study Group The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis. Mult Scler. 2010;16:503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 65.Okada S, Ishii K, Yamane J, et al. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005;19:1839–1841. doi: 10.1096/fj.05-4082fje. [DOI] [PubMed] [Google Scholar]
- 66.Neuhube B, Timothy HB, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 67.Koda M, Kamada T, Hashimoto M, et al. Adenovirus vector-mediated ex vivo gene transfer of brain-derived neurotrophic factor to bome marrow stromal cells promotes axonal regeneration after transplantation in completely transected adult rat spinal cord. Eur Spine J. 2007;16:2206–2214. doi: 10.1007/s00586-007-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGuckin C, Basford C, Hanger K, Habibollah S, Forraz N. Cord blood revelations: the importance of being a first born girl, big, on time and to a young mother! Early Hum Dev. 2007;83:733–741. doi: 10.1016/j.earlhumdev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Watt S, Contreras M. Stem cell medicine: umbilical cord blood and its stem cell potential. Semin Fetal Neonatal Med. 2005;10:209–220. doi: 10.1016/j.siny.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Ballen K, Barker N, Stewart K, Greene F, Lane A. Collection and preservation of cord blood for personal use. Biol Blood Marrow Transplant. 2008;14:356–363. doi: 10.1016/j.bbmt.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Pipes B, Tsang T, Peng S, Fiederlein R, Graham M, Harris D. Telomere length changes after umbilical cord blood transplant. Transfusion. 2006;46:1038–1043. doi: 10.1111/j.1537-2995.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 72.Slatter M, Gennery A. Umbilical cord stem cell transplantation for primary immunodeficiencies. Expert Opin Biol Ther. 2006;6:555–565. doi: 10.1517/14712598.6.6.555. [DOI] [PubMed] [Google Scholar]
- 73.Lim J, Park S, Oh J, et al. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res. 2008;86:2168–2178. doi: 10.1002/jnr.21669. [DOI] [PubMed] [Google Scholar]
- 74.Kang K, Kim S, Oh Y, et al. A 37-year-old spinal cord-injured female patient transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005;7:368–373. doi: 10.1080/14653240500238160. [DOI] [PubMed] [Google Scholar]
- 75.Dasari VR, Spomar DG, Li L, Gujrati M, Rao JS, Dinh DH. Umbilical cord blood stem cell mediated downregulation of fas improves functional recovery of rats after spinal cord injury. Neurochem Res. 2008;33:134–149. doi: 10.1007/s11064-007-9426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walczak P, Chen N, Eve D, et al. Long-term cultured human umbilical cord neural-like cells transplanted into the striatum of NOD SCID mice. Brain Res Bull. 2007;74:155–163. doi: 10.1016/j.brainresbull.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho SR, Yang MS, Yim SH, et al. Neurally induced umbilical cord blood cells modestly repair injured spinal cords. Neuroreport. 2008;19:1259–1263. doi: 10.1097/WNR.0b013e3283089234. [DOI] [PubMed] [Google Scholar]
- 78.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 79.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 80.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 81.Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 82.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romanov YA, Darevskaya AN, Merzlikina NV, Buravkova LB. Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med. 2005;140:138–143. doi: 10.1007/s10517-005-0430-z. [DOI] [PubMed] [Google Scholar]
- 85.Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res. 2009;338(3):401–411. doi: 10.1007/s00441-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 86.Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 87.Chi GF, Kim MR, Kim DW, Jiang MH, Son Y. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol. 2010;222:304–317. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Zhang HT, Cheng HY, Cai YQ, et al. Comparison of adult neurospheres derived from different origins for treatment of rat spinal cord injury. Neurosci Lett. 2009;458:116–121. doi: 10.1016/j.neulet.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 89.Ohta Y, Takenaga M, Tokura Y, et al. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877–886. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 90.Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15:583–594. doi: 10.1089/scd.2006.15.583. [DOI] [PubMed] [Google Scholar]
- 91.Ryu HH, Lim JH, Byeon YE, et al. Functional recovery and neural differentiation after transplantation of allogeneic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10:273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohta Y, Takenaga M, Tokura Y, et al. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877–886. doi: 10.3727/096368908786576516. [DOI] [PubMed] [Google Scholar]
- 93.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 94.Gilson JM, Blakemore WF. Schwann cell remyelination is not replaced by oligodendrocyte remyelination following ethidium bromide induced demyelination. Neuroreport. 2002;13:1205–1208. doi: 10.1097/00001756-200207020-00027. [DOI] [PubMed] [Google Scholar]
- 95.Buss A, Pech K, Kakulas BA, et al. Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury. Brain. 2007;130:940–953. doi: 10.1093/brain/awl374. [DOI] [PubMed] [Google Scholar]
- 96.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 97.Woodhoo A, Sahni V, Gilson J, et al. Schwann cell precursors: a favourable cell for myelin repair in the central nervous system. Brain. 2007;130:2175–2185. doi: 10.1093/brain/awm125. [DOI] [PubMed] [Google Scholar]
- 98.Papastefanaki F, Chen J, Lavdas AA, Thomaidou D, Schachner M, Matsas R. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain. 2007;130:2159–2174. doi: 10.1093/brain/awm155. [DOI] [PubMed] [Google Scholar]
- 99.Graziadei PPC, Levine RR, Montigraziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals: morphological aspects of differentiation of and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 100.Farbman AL. Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci. 1990;13:362–365. doi: 10.1016/0166-2236(90)90017-5. [DOI] [PubMed] [Google Scholar]
- 101.Schwob J. Neuronal regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- 102.Alexander CL, FitzGerald UF, Barnett SC. Identification of growth factors that promote long-term proliferation of olfactory ensheathing cells and modulate their antigenic phenotype. Glia. 2002;37:349–364. [PubMed] [Google Scholar]
- 103.Ruitenberg MJ, Levison DB, Lee SV, Verhaagen J, Harvey AR, Plant GW. NT-3 expression from engineered olfactory ensheathing glia promotes spinal sparing and regeneration. Brain. 2005;128:839–853. doi: 10.1093/brain/awh424. [DOI] [PubMed] [Google Scholar]
- 104.Féron F, Perry C, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 105.Redmond DE, Jr, Freeman T. Commentary: The American Society for Neural Transplantation and Repair Considerations and Guidelines for Studies of Human Subjects. The Practice Committee of the Society. Approved by Council. Cell Transpl. 2001;10:661–664. [PubMed] [Google Scholar]
- 106.Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- 107.Kallur T, Farr TD, Bohm-Sturm P, Kokaia Z, Hoehn M. Spatio-temporal dynamics, differentiation and viability of human neural stem cells after implantation into neonatal rat brain. Eur J Neurosci. 2011;34:382–393. doi: 10.1111/j.1460-9568.2011.07759.x. [DOI] [PubMed] [Google Scholar]
- 108.Rudelius M, Daldrup-Link HE, Heinzmann U, et al. Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. Eur J Nucl Med Mol Imaging. 2003;30:1038–1044. doi: 10.1007/s00259-002-1110-0. [DOI] [PubMed] [Google Scholar]
- 109.Liu Y, He ZJ, Xu B, et al. Evaluation of cell tracking effects for transplanted mesenchymal stem cells with jetPEI/Gd-DTPA complexes in animal models of hemorrhagic spinal cord injury. Brain Res. 2011;1391:24–35. doi: 10.1016/j.brainres.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 110.Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One. 2011;6:e29040. doi: 10.1371/journal.pone.0029040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu S, Xu X, Zhao W, et al. Targeting of embryonic stem cells by peptide-conjugated quantum dots. PLoS One. 2010;5:e12075. doi: 10.1371/journal.pone.0012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shah BS, Mao JJ. Labeling of mesenchymal stem cells with bioconjugated quantum dots. Methods Mol Biol. 2011;680:61–75. doi: 10.1007/978-1-60761-901-7_4. [DOI] [PubMed] [Google Scholar]
- 113.Seyed Jafari SS, Ali Aghaei A, Asadi-Shekaari M, Nematollahi-Mahani SN, Sheibani V. Investigating the effects of adult neural stem cell transplantation by lumbar puncture in transient cerebral ischemia. Neurosci Lett. 2011;495:1–5. doi: 10.1016/j.neulet.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 114.Daadi MM, Li Z, Arac A, et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swijnenburg RJ, Schrepfer S, Cao F, et al. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008;17:1023–1029. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Almeida PE, van Rappard JR, Wu JC. In vivo bioluminescence for tracking cell fate and function. Am J Physiol Heart Circ Physiol. 2011;301:H663–H671. doi: 10.1152/ajpheart.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Usvald D, Vodicka P, Hlucilova J, et al. Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs. Cell Transplant. 2010;19:1103–1122. doi: 10.3727/096368910X503406. [DOI] [PubMed] [Google Scholar]
- 118.Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci Lett. 2006;398:129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 119.Khalatbary AR, Tiraihi T. A comparative study of therapeutic benefits of intraspinal and intravenous bone marrow stromal cell administration to spinal cord injuries. Iran Biomed J. 2009;13:43–48. [PubMed] [Google Scholar]
- 120.Wu X, Ren J, Li J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy. 2012;14:555–562. doi: 10.3109/14653249.2011.638914. [DOI] [PubMed] [Google Scholar]
- 121.Reier PJ. Cellular transplantation strategies for spinal cord injury and translational neurobiology. Neuro Rx. 2004;1:424–451. doi: 10.1602/neurorx.1.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]