Abstract
Alcohol-induced osteonecrosis of the femoral head (ONFH) is observed in alcohol abusers and patients with alcoholic fatty liver disease. It has been reported that Toll-like receptor 4 (TLR4) signalling plays a crucial role in the pathogenesis of alcoholic fatty liver disease. We previously reported a corticosteroid-induced ONFH rat model, and suggested that TLR4 signalling contributes to the pathogenesis of ONFH. Thus, it is thought that the pathogenesis of alcohol-induced ONFH is probably similar to that of corticosteroid-induced ONFH. The aim of this study was to develop a new animal model for alcohol-induced ONFH and to evaluate the relationship between the pro-inflammatory response via TLRs and the development of ONFH in rats. Male Wistar rats were fed a Lieber–DeCarli liquid diet containing 5% ethanol (experimental group) or dextran (control group) for 1–24 weeks. Histopathological and biochemical analyses were performed. Feeding the ethanol-containing liquid diet resulted in the development of ONFH with hepatic steatosis, hepatic dysfunction and hyperlipidaemia, whereas feeding the dextran-containing diet did not cause ONFH. However, we could not recognize any relationship between the pro-inflammatory response via TLR4 and the development of alcohol-induced ONFH. Thus in this study we have developed a new rat model for alcohol-induced ONFH based on the feeding of an ethanol liquid diet. ONFH was observed within seven days from the start of feeding with 5% ethanol-containing liquid diet. Although this was linked to hepatic steatosis, a TLR4 association was not a feature of this model.
Keywords: alcohol, animal model, femoral head osteonecrosis, toll-like receptor
Non-traumatic osteonecrosis of the femoral head (ONFH) has been regarded as a complication of corticosteroid therapy with inflammatory disease or alcohol abuse (Abeles et al. 1978; Mont et al. 2006). However, the pathogenesis of alcohol-induced ONFH has not been clarified apart from epidemiological investigations referring to ONFH. The risk of alcohol-induced ONFH was increased by an alcohol intake dose and drinking period (Hirota et al. 1993). Furthermore, most drinkers who develop ONFH also contract alcoholic fatty liver disease (McClain et al. 2004; Lucey et al. 2009). The pathogenesis of alcoholic fatty liver disease has been studied using experimental rat and mouse models fed on an alcohol-containing liquid diet, especially the Lieber–DeCarli liquid diet (Lieber et al. 1989). Further, it has been reported that the Toll-like receptor 4 (TLR4) signalling pathway plays a crucial role in the pathogenesis of alcoholic fatty liver disease (McClain et al. 2004; Hritz et al. 2008). On the other hand, Ichiseki et al. 2011 reported oxidative stress-induced ONFH in a rat model and demonstrated that glutathione level acts as an index of oxidative stress-induced changes in the liver. Further, we previously reported that the disturbance of liver function was observed in patients with corticosteroid-induced ONFH (Okazaki et al. 2013) and that corticosteroid treatment after an injection of lipopolysaccharides (LPS), a ligand for TLR4, induced ONFH with fatty liver in rats, suggesting that TLR4 signalling contributes to the pathogenesis of corticosteroid-induced ONFH in rat (Okazaki et al. 2009). These reports indicate that any liver damage (fatty liver, liver function, oxidative stress) may contribute to the pathogenesis of non-traumatic ONFH, as Solomon reported that the pathogenesis of alcohol-induced osteonecrosis is probably similar to that of corticosteroid-induced osteonecrosis (Solomon 1985). Therefore, we focused on alcoholic fatty liver disease to develop an alcohol-induced ONFH animal model. We hypothesized that the feeding of this alcohol-containing liquid diet would cause ONFH in rats and that TLR4 signalling may play any role in the development of alcohol-induced ONFH in rats. In this study, to clarify the above hypothesis, we evaluated the relationship between the pro-inflammatory response via TLR4 signalling and the development of ONFH in rats fed with an ethanol-containing liquid diet.
Materials and methods
Animals and ethanol feeding
Male Wistar rats (6 weeks of age) were obtained from the Sankyo Labo Service Co., Ltd. (Sapporo, Japan) and housed individually in a temperature- and humidity-controlled room with a 12-h light/dark cycle. Ninety-four rats were divided into two groups. The alcohol group received the Lieber–DeCarli liquid diet (Lieber et al. 1989) (ORIENTAL YEAST Co., Ltd., Tokyo, Japan) containing 5.0% (weight/volume) ethanol (35% ethanol-derived calories) for 1, 2, 3, 4, 6 and 24 weeks. Control rats were pair-fed the same liquid diet without ethanol (the ethanol was replaced with dextran–maltose isocalorically) for the same periods as the alcohol group. All rats were fed the control liquid diet ad libitum for 1 week prior to the start of experiments. To accustom the alcohol group rats to the alcohol-containing liquid diet, they were fed with the alcohol-containing liquid diet (1.0–4.0% ethanol) ad libitum for 1 week prior to the start of experiments. The rats were weighed each day. The rats in the alcohol group were allowed unrestricted access to the alcohol-containing liquid diet. The intake of the pair-fed control rats was strictly limited to the amount ingested on the previous day by the pair-matched ethanol-fed rats to ensure that the calorie intakes of the two groups were the same. After pair feeding, the rats were sacrificed. Blood was collected from the inferior vena cava at the time of sacrifice and immediately centrifuged. The supernatant was stored as platelet-rich plasma (PRP) at −84 °C until analysis. The femur and liver were harvested and fixed with a 10% formalin–0.1 M phosphate buffer (pH 7.4).
Ethical approval
All experiments were performed in accordance with the guidelines of the Ministry of Sports, Culture, Science and Technology, Japan. They also followed protocols that were approved by the Animal Care and Use Committee, Sapporo Medical University School of Medicine (Approval Number: 09-008).
Histopathology
Bone specimens of the femur were decalcified with Kalkitox™ (Wako Pure Chemical Industries, Ltd. Osaka, Japan) and then neutralized with sodium sulphate buffer. A small section of the liver or femur was dissected and fixed with a 10% formalin–0.1 M phosphate buffer (pH 7.4). The tissues were then processed for routine haematoxylin and eosin staining. Osteonecrosis was defined as the presence of diffuse empty lacunae or pyknotic osteocytic nuclei in the bone trabeculae accompanied by and surrounding bone marrow cell necrosis, as described previously (Yamamoto et al. 1997; Ichiseki 2006; Okazaki et al. 2009; Tateda et al. 2012).
Biochemical assay
The PRP concentrations of aspartate aminotransferase (AST), alanine transaminase (ALT), triglyceride (TG), total cholesterol (TC) and high-density lipoprotein (HDL) were measured using a SPOTCHEM® D system (ARKRAY, Inc., Kyoto, Japan) in accordance with the manufacturer's instructions.
Electrophoretic mobility shift assay
NF-κB, Interferon (IFN) regulatory factor 3 (IRF3) and IRF7 are signal transcription factors related to the pro-inflammatory response via TLR4 signalling. The activation of transcription factors in the liver was assessed by Electrophoretic mobility shift assay (EMSA) as described previously (Matsumoto et al. 2002). Briefly, equal amounts of liver nuclear extracts (2 mg of protein) were incubated for 1 h at room temperature with 32P-labelled NF-κB, IRF3 and IRF7 double-strand consensus oligonucleotide probes (NF-κB: 5′-AGTTGAGGGGACTTTCCCAGGC-3′; IRF3: 5′-GAAAGCGAAACTGAAACTGACT-3′; IRF7: 5′-ACTGATCGGAACCGAACGATCTATG-3′). The probe labelled with 32P- [ATP] [adenosine triphosphate (ATP)] and nuclear protein were mixed in the binding buffer (10 mM HEPES [pH 7.9] [4-(2-hydroxyethyl)- 1- piperazineethanesulfonic acid (HEPES)], 50 mM KCl, 0.2 mM ethylenediaminetetraacetic acid, 2.5 mM dithiothreitol and 10% glycerol, 0.05% NP-40). The DNA–protein complexes were separated on 7% non-denaturing polyacrylamide gels at a constant voltage of 100 V at room temperature. The gels were then exposed to an Image Plate (Fuji Film, Co., Tokyo, Japan) at room temperature. The radioactivity of individual bands on the gel was analysed using an FLA3000 Image Analyzer (Fuji Film) and Image Quant Software (Molecular Dynamics, Sunnyvale, CA, USA).
[ATP] [adenosine triphosphate (ATP)] and nuclear protein were mixed in the binding buffer (10 mM HEPES [pH 7.9] [4-(2-hydroxyethyl)- 1- piperazineethanesulfonic acid (HEPES)], 50 mM KCl, 0.2 mM ethylenediaminetetraacetic acid, 2.5 mM dithiothreitol and 10% glycerol, 0.05% NP-40). The DNA–protein complexes were separated on 7% non-denaturing polyacrylamide gels at a constant voltage of 100 V at room temperature. The gels were then exposed to an Image Plate (Fuji Film, Co., Tokyo, Japan) at room temperature. The radioactivity of individual bands on the gel was analysed using an FLA3000 Image Analyzer (Fuji Film) and Image Quant Software (Molecular Dynamics, Sunnyvale, CA, USA).
Blood cytokine analysis
The blood pro-inflammatory cytokine concentration–related TLR4 signalling was determined as follows. The blood concentrations of IL-1α, IL-1β, IL-17, IFNγ and adiponectin were determined using a Milliplex®MAP Rat Cytokine/Chemokine/Adipocyte Immunoassay kit (Millipore Co. Billerica, MA, USA) or Luminex 100 KT01 (Luminex Co. Austin, TX, USA) in accordance with the manufacturers' instructions. Blood IFNα concentration was determined using an enzyme-linked immunosorbent assay kit (Uscn Life Science Inc. Wuhan, China).
Glutathione peroxidase assay
The activity of glutathione peroxidase (GPx), as an index of oxidative stress, in the liver was evaluated using the spectrophotometric method. A BIOTECH® GPx-340™ assay kit (Oxis Research ™ Burlingame, CA, USA) was used in accordance with the manufacturer's instructions.
Statistical analysis
Data are expressed as means ± SEM. Data were compared between the control and alcohol groups using the Fisher's exact test or the unpaired t-test with Welch's correction. A P-value of <0.05 was considered statistically significant. All statistical analysis was performed using graphpad prism 5.0c software for Mac OS X (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Rats were fed with a liquid diet for 3–26 weeks. The animals were sacrificed after 1, 2, 3, 4, 6 and 24 weeks of administration of a 5% alcohol-containing liquid or a pair-fed control diet. The body weights of the control and alcohol rats increased in parallel, indicating the absence of any starvation or malnutrition in the control and alcohol-fed rats (Figure 1). The mean liquid diet ingestion was 79.8 ± 2.4 ml/day in the alcohol-fed rats for an ethanol intake of 12.94 g/kg/day. The incidences of ONFH in the control and the alcohol groups are shown in Table 1. No ONFH was observed in any rats fed with the control diet. In the control group, there was no change in the trabeculae or in the numbers of haematopoietic and fat cells. On the other hand, ONFH was observed in three of eight, four of eight and six of 12 rats, at 1, 2, 3 and 4 weeks respectively, after alcohol feeding. The incidence of ONFH in the 4-week alcohol rats was significantly higher than that in the 4-week control rats (P = 0.0137; Fisher's exact test). Figures 2 and 3 show the histopathological appearance of the femoral head after haematoxylin and eosin staining in typical experiments. Empty lacunae within the necrotic bone trabeculae, bone marrow cells including adipocytic necrosis and an accumulation of cell debris in the medullary space in most areas of the femoral head were observed at 1–3 weeks in the alcohol group. Feeding with the alcohol liquid diet for 4 weeks resulted in the formation of appositional bone around the necrotic bone trabeculae and, as part of the repair process, the deposition of fibrous and granulation tissue in the medullary space (Figures 2 and 3). Moreover, the repair process was more advanced in the alcohol group at 6 and 24 weeks, with the necrotic bone trabeculae observed to be surrounded by appositional bone. Additionally, partial appositional bone thickening of the bone trabeculae and normal haematopoietic and fat cells were observed in the alcohol group. These histopathological findings were observed in two of six and two of five rats in the alcohol group at 6 and 24 weeks respectively.
Figure 1.

Changes in body weight and ingestion in the control and alcohol liquid diet–fed rats. The body weights of the control and alcohol rats increased in parallel.
Table 1.
Incidence of osteonecrosis of the femoral head (ONFH) in the control and alcohol groups
| Feeding interval (week) | |||||
|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 6 |
| Control | 0/8 | 0/8 | 0/8 | 0/12 | 0/6 |
| Alcohol | 3/8 | 3/8 | 4/8 | 6/12* | 2/6 |
P = 0.0137 vs. control by Fisher's exact test.
Figure 2.

Histological appearance (low magnification) of the femoral head after haematoxylin and eosin staining in typical experiments. The alcohol group rats show the presence of diffuse empty lacunae in the bone trabeculae and bone marrow cell necrosis in most areas of the femoral head. The control group shows normal trabeculae and normal hematopoietic and fat cells over time. Scale bar represents 100 μm.
Figure 3.

Histological appearance (high magnification) of the femoral head after haematoxylin and eosin staining. The alcohol group rats show the formation of appositional bone around necrotic bone trabeculae, which represents part of the repair process, at 4, 6 and 24 weeks (black arrow). Scale bar presents 100 μm.
In the control rats, the liver had the expected regular chord-like architecture, whereas the liver in the alcohol-fed rats showed mild disorganization of the hepatic chord architecture and hepatocellular microsteatosis without necrosis, which progressed with time (Figure 4).
Figure 4.
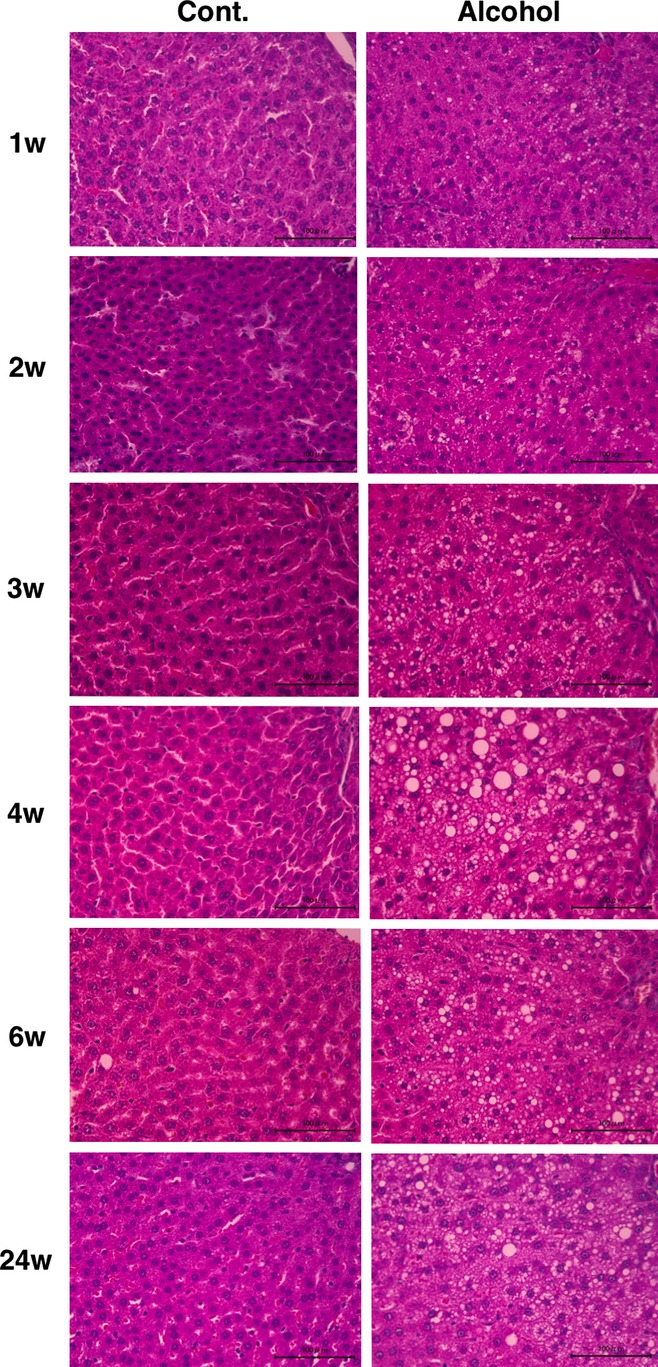
Histological appearance of the liver in the control and alcohol-fed rats. Control group rats show the expected regular chord-like architecture over time. The alcohol group rats show mild and moderate hepatocellular microsteatosis without necrosis. Scale bar represents 100 μm.
Figure 5 shows the blood concentrations of AST, ALT, TG, TC and HDL. All the parameters except TG were significantly higher in the alcohol group than in the control group from 1 week to 24 weeks. No significant changes were observed in blood IFNα levels at 1–6 weeks (Figure 5). Adiponectin was significantly lower at 1–4 weeks in the alcohol group than in the control group (Figure 5). On the other hand, no cytokines regulated by TLR signalling, such as IL-1α, IL-1β, IL-17 and IFNγ, were detected in either group (Data not shown). NF-κB activity did not significantly change at 1, 3 and 24 weeks, but was significantly decreased at 2 weeks in the alcohol group and significantly increased at 4 and 6 weeks in the alcohol group. On the other hand, the activity of IRF3 significantly changed only after 3 weeks of alcohol consumption. IRF7 activity also showed a significant change at 4 weeks in the alcohol-fed rats. Glutathione peroxidase activity in the liver was significantly increased at 2 and 4 weeks in the alcohol group, but was significantly decreased at 6 weeks in the alcohol group (Figure 6).
Figure 5.

Increases in blood concentrations of AST, ALT, TG, TC and HDL and changes in blood IFNα and adiponectin levels in rats. AST, ALT, TC and HDL were significantly increased in the alcohol group over time. IFNα was not significantly changed in the alcohol group at 1–6 weeks, while the adiponectin level was significantly decreased in the alcohol group at 1–4 weeks. *P < 0.05 as control, **P < 0.01 as control.
Figure 6.

Activity of transcription factors NF-κB, IRF3 and IRF7, and GPx activity in the liver. NF-κB activity was significantly decreased at 2 weeks and was significantly increased at 4 and 6 weeks. IRF3 activity was significantly decreased at 3 weeks, and IRF7 activity was significantly increased at 4 weeks. GPx activity tended to be higher in the alcohol group than in the control group at 1–4 weeks. *P < 0.05 as control, **P < 0.01 as control.
Discussion
In the present study, we found that feeding an alcohol liquid diet induced ONFH in rats. There have been a few reports on alcohol-induced ONFH in animal models; however, those models take 4–6 months to develop osteonecrosis through the administration of alcohol containing >45% ethanol intragastrically daily (Wang et al. 2003, 2008). In contrast, the present study showed that ONFH developed in rats fed with the 5% ethanol-containing liquid diet for 1 week, suggesting that ONFH can be initiated in a period as short as 7 days after feeding the animals with a 5% ethanol-containing liquid diet. Clinically, ONFH is observed in patients with a long-term history of drinking (Hirota et al. 1993). However, the precise time of onset of alcohol-induced ONFH in long-term drinkers remains unclear. The reasons for this include the fact that the collapse of the femoral head in humans may occur several months to years after the initial development of ONFH and may not show any symptoms until collapse (Mont et al. 2010).
The Lieber–DeCarli diet used in the present study is known to induce experimental alcoholic fatty liver disease (Lieber & DeCarli 1982; Lieber et al. 1989). The advantage of using this diet in animal experiments is that it allows the efficient consumption of ethanol (Lieber & DeCarli 1982; Lieber et al. 1989). In the present study, ONFH was observed within 7 days from the start of feeding with the 5% ethanol-containing liquid diet. This finding suggests that the alcohol-induced ONFH in humans may develop very early in drinkers with excessive alcohol consumption.
There have been some reports of changes in the histopathological findings of the femur induced by alcohol administration in animals. Solomon and Ikemura et al. reported that alcohol administration induced fatty bone marrow, but not osteonecrosis (Solomon 1985; Ikemura et al. 2011). Wang et al. reported that alcohol administration also induced fatty bone marrow and increased the percentage of empty lacunae as an index of osteonecrosis (Wang et al. 2008). However, we could not find any experimental reports on appositional bone formation as part of the repair process of bone necrosis after alcohol administration. Clinically, the detection of a band pattern on a magnetic resonance image is an important early finding for the diagnosis of non-traumatic ONFH and histopathologically corresponds to the repair tissue formed in cases of osteonecrosis (Bullough & DiCarlo 1990; Arlet et al. 1993; Sugano et al. 1999). The histopathological findings in the present study were very similar to those in patients with ONFH (Bullough & DiCarlo 1990; Arlet et al. 1993). We observed empty lacunae within the necrotic bone trabeculae and bone marrow cells including adipocytic necrosis in most areas of the femoral head in the alcohol group; results were similar to those observed in corticosteroid-induced ONFH model treated with corticosteroids after the administration of a TLR4 ligand, LPS (Okazaki et al. 2012). In particular, histological examination of the epiphysis showed osteocytic death and necrotic bone marrow with or without appositional bone. On the other hand, we did not observe any collapse of the femoral head in the rats fed an alcohol diet over 24 weeks. It was a difference between human and rat that the femoral head did not collapse. However, we reported that the initial development of ONFH and the collapse formation in the rat become independent and may not influence to elucidate the pathogenesis of ONFH (Okazaki et al. 2012). Therefore, our results suggest that this rat model could be used to unravel the underlying mechanisms of human ONFH. However, it should be noted that the structure of the femoral head differs between rats and humans. The epiphyseal line in the rat femur remains throughout adulthood (Okazaki et al. 2009). Focal ischaemia or thrombosis could not be observed in the proximal femur of the ONFH rats in the present study.
Enomoto et al. 1999 reported that chronic alcohol administration induces an increase in gut permeability resulting in elevated portal endotoxin levels. Endotoxin, a TLR4 ligand, induces a pro-inflammatory response via the TLR4 signalling pathway (Akira & Takeda 2004). We confirmed that NF-κB, IRF3 and IRF7, which are downstream transcriptional factors of the TLR4 signalling pathway, were activated in the liver during chronic alcohol consumption (Figure 6). However, we could not observe any activation of NF-κB, IRF3 or IRF7 in the liver at one week, i.e. the time at which the OFNH was first observed. And we could not observe any activation of pro-inflammatory cytokines regulated by TLR4 signalling. Therefore, whether the TLR4 signalling is associated with the initiation of alcohol-induced ONFH remains unclear. On the other hand, hypercholesterolaemia and hepatic steatosis were observed in the rats fed the alcohol liquid diet in the present study. We previously reported that hypercholesterolaemia and hepatic steatosis were also observed in corticosteroid-induced ONFH rat model after corticosteroid treatment (Okazaki et al. 2009). Further, previous clinical studies have reported an association between hyperlipidaemia and ONFH (Moskal et al. 1997; Kabata 2005). Therefore, hypercholesterolaemia may be related to the development of ONFH. Ichiseki et al. reported that administration of an oxidative stressor induced ONFH in rats and demonstrated that glutathione level acts as an index of oxidative stress-induced changes in the liver (Ichiseki et al. 2011). However, we could not observe any significant activation of GPx activity in the liver at 1 week, but we could observe any increase in transaminase levels in the fatty liver of rats fed the alcohol liquid diet. These findings suggest that any liver damage may contribute to the development of ONFH.
In the present study, adiponectin was significantly decreased in the alcohol group at 1, 2, 3 and 4 weeks, which is consistent with the findings of previous reports (You et al. 2005; Breitkopf et al. 2009). Hypoadiponectinaemia is associated with mortality following ischaemic stroke (Nishimura et al. 2007), and it has been suggested that it could be used as a biomarker for ischaemic stroke (Urbonaviciene et al. 2010). Therefore, the low adiponectin levels observed in the present study suggest the hypothesis that ONFH results from ischaemia of the femoral head.
In conclusion, we have developed a new rat model of alcohol-induced ONFH based on the feeding of an ethanol liquid diet, whereas it remains unclear whether the pro-inflammatory response via TLR4 signalling contributes to the development of alcohol-induced ONFH in rats fed with an ethanol liquid diet. This rat model will help us to unravel the underlying mechanisms of human ONFH.
Funding source
This work was supported in part by Grants-in-Aid for Young Scientists (B) (S.O., 22791390) and for Scientific Research (B) (H.M., 20390196) of the Japanese Society for the Promotion of Science.
Conflict of interest
The authors have no conflict of interests to declare.
References
- Abeles M, Urman JD, Rothfield NF. Aseptic necrosis of bone in systemic lupus erythematosus. Relationship to corticosteroid therapy. Arch. Intern. Med. 1978;138:750–754. [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Arlet J, Laroche M, Soler R, Thiechart M, Pieraggi MT, Mazieres B. Histopathology of the vessels of the femoral heads in specimens of osteonecrosis, osteoarthritis and algodystrophy. Clin. Rheumatol. 1993;12:162–165. doi: 10.1007/BF02231520. [DOI] [PubMed] [Google Scholar]
- Breitkopf K, Nagy LE, Beier JI, Mueller S, Weng H, Dooley S. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2009;33:1647–1655. doi: 10.1111/j.1530-0277.2009.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough PG, DiCarlo EF. Subchondral avascular necrosis: a common cause of arthritis. Ann. Rheum. Dis. 1990;49:412–420. doi: 10.1136/ard.49.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto N, Yamashina S, Kono H, et al. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology. 1999;29:1680–1689. doi: 10.1002/hep.510290633. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Hirohata T, Fukuda K, et al. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am. J. Epidemiol. 1993;137:530–538. doi: 10.1093/oxfordjournals.aje.a116706. [DOI] [PubMed] [Google Scholar]
- Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiseki T. Oxidative stress by glutathione depletion induces osteonecrosis in rats. Rheumatology. 2006;45:287–290. doi: 10.1093/rheumatology/kei149. [DOI] [PubMed] [Google Scholar]
- Ichiseki T, Kaneuji A, Ueda Y, et al. Osteonecrosis development in a novel rat model characterized by a single application of oxidative stress. Arthritis Rheum. 2011;63:2138–2141. doi: 10.1002/art.30365. [DOI] [PubMed] [Google Scholar]
- Ikemura S, Yamamoto T, Motomura G, et al. Lipid metabolism abnormalities in alcohol-treated rabbits: a morphometric and haematologic study comparing high and low alcohol doses. Int. J. Exp. Pathol. 2011;92:290–295. doi: 10.1111/j.1365-2613.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabata T. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology. 2005;44:1233–1237. doi: 10.1093/rheumatology/keh721. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol. Clin. Exp. Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N. Engl. J. Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Sato Y, Azumi J, Kato J, Niitsu Y, Tamaki K. Role of endotoxin in NF-kappaB activation by ethanol in rat hepatocytes. Alcohol. Clin. Exp. Res. 2002;26:6S–10S. doi: 10.1097/01.ALC.0000026827.79925.C9. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I. Recent advances in alcoholic liver disease. IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G497–502. doi: 10.1152/ajpgi.00171.2004. [DOI] [PubMed] [Google Scholar]
- Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J. Bone Joint Surg. Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J. Bone Joint Surg. Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- Moskal JT, Topping RE, Franklin LL. Hypercholesterolemia: an association with osteonecrosis of the femoral head. Am. J. Orthop. (Belle Mead NJ) 1997;26:609–612. [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase-dependent mechanisms. Circulation. 2007;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Nishitani Y, Nagoya S, Kaya M, Yamashita T, Matsumoto H. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology. 2009;48:227–232. doi: 10.1093/rheumatology/ken462. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Nagoya S, Tateda K, et al. Weight bearing dose not contribute to the development of osteonecrosis of the femoral head. Int. J. Exp. Pathol. 2012;93:458–462. doi: 10.1111/j.1365-2613.2012.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki S, Nagoya S, Yamamoto M, et al. High risk of osteonecrosis of the femoral head in autoimmune disease patients showing no immediate increase in hepatic enzyme under steroid therapy. Rheumatol. Int. 2013;33:51–55. doi: 10.1007/s00296-011-2295-y. [DOI] [PubMed] [Google Scholar]
- Solomon L. Mechanisms of idiopathic osteonecrosis. Orthop. Clin. North Am. 1985;16:655–667. [PubMed] [Google Scholar]
- Sugano N, Kubo T, Takaoka K, et al. Diagnostic criteria for non-traumatic osteonecrosis of the femoral head. A multicentre study. J. Bone Joint Surg. Br. 1999;81:590–595. doi: 10.1302/0301-620x.81b4.9393. [DOI] [PubMed] [Google Scholar]
- Tateda K, Okazaki S, Nagoya S, et al. The suppression of TRIM21 and the accumulation of IFN-alpha play crucial roles in the pathogenesis of osteonecrosis of the femoral head. Lab. Invest. 2012;92:1318–1329. doi: 10.1038/labinvest.2012.89. [DOI] [PubMed] [Google Scholar]
- Urbonaviciene G, Frystyk J, Flyvbjerg A, Henneberg EW, Lindholt JS. Association of serum adiponectin with risk for cardiovascular events in patients with peripheral arterial disease. Atherosclerosis. 2010;210:619–624. doi: 10.1016/j.atherosclerosis.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin. Orthop. Relat. Res. 2003;410:213–224. doi: 10.1097/01.blo.0000063602.67412.83. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yin L, Li Y, Liu P, Cui Q. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin. Orthop. Relat. Res. 2008;466:1059–1067. doi: 10.1007/s11999-008-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40:2055–2064. doi: 10.1002/art.1780401119. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]


