Abstract
OBJECTIVES
The following study presents a special independent atrial fibrillation (AF) risk factor—preoperative fluctuation of heart rate variability (HRV), as well as other perioperative AF risk factors in patients qualified for pneumonectomy and undergoing pneumonectomy or lobectomy for lung cancer.
METHODS
The prospective study was performed in patients who had undergone anatomical resection for non-small-cell lung cancer. A total of 117 patients (92 men and 25 women) qualified for statistical research. In order to determine the risk factors, all patients were divided into two groups: Group A—98 patients without AF and Group B—19 patients with AF during the perioperative time. A number of different risk factors of AF have been analysed and further divided into preoperative, operative and postoperative.
RESULTS
Postoperative AF occurred in 19 patients (16%), all of them were male. The patients with higher short-term HRV parameters (SD1, RMSSD), slower mean heart rate and those with a lower fluctuation of HRV-related parameters (HRV Afternoon, Night, Day (A/N/D)) before the operation, were more prone to AF. Postoperative risk of AF was higher in patients with a higher number of ventricular ectopic beats before the operation, a higher number of supraventricular and ventricular ectopic beats and a higher maximal heart rate after the operation. Statistical analysis revealed that male gender and the extent of pulmonary resection, particularly left pneumonectomy, constituted significant risk factors. AF was more often observed in patients who had ASA physical status score of III, in comparison with ASAI and ASAII patients.
CONCLUSIONS
Along with other concomitant AF risk factors presented in this work, the evaluation of the fluctuation tendencies of HRV parameters should be taken into consideration before any major lung resection. The balance disturbance between the sympathetic and parasympathetic nervous systems is responsible for AF.
Keywords: Heart rate variability, Pulmonary resection, Atrial fibrillation risk factors
INTRODUCTION
Atrial fibrillation (AF) is a common post-thoracic surgery complication affecting >25% of patients undergoing pneumonectomy, some of whom require intensive therapy. Preoperative AF is a peculiar non-psychiatric predictor of delirium after cardiac surgery [1]. AF appears to be a strong predictor of early and long-term mortality after general thoracic surgical procedures for lung cancer [2]. It can trigger dangerous ventricular arrhythmias and is conducive to cardiac and cerebrovascular events associated with greater risk of late stroke [3]. Therefore, AF risk factors have been the subject of study for many years. The aforementioned complications indicate the need for better preventive management of AF. AF after pneumonectomy has severe prognostic implications, with prolonged hospital stays, increased health-care costs and higher postoperative mortality rates among patients, compared with those remaining in normal sinus rhythm [4].
The aim of this study was, first, to find the independent AF risk factors in order to predict possible complications and apply proper preventive therapy in the future, and secondly, to answer the question whether preoperative assessment of HRV parameters allows for predicting which patients are likely to suffer from postoperative AF.
The following dissertation presents an evaluation of independent AF risk factors during the perioperative period among patients qualified for and undergoing pneumonectomy and lobectomy for lung cancer.
MATERIALS AND METHODS
The study protocol and research methods were approved by the Bioethical Committee of the Poznań University of Medical Sciences (chairperson: Zygmunt Przybylski, decision number: 1146/02). Each patient signed an informed consent form prior to being included in the study. It is a prospective observational clinical study.
The study group comprised 121 patients treated at the Department of Thoracic Surgery (Poznań University of Medical Sciences in Poznań, Poland), who underwent anatomical resection for non-small-cell lung cancer.
The excluded criteria were as follows: asthma, heart failure, any previous thoracic surgery, cerebral stroke, atrial dysrhythmias or thyroid dysfunction and contraindication to epidural analgesia. β-mimetic, β-blocking and digitalis agents had not been allowed for at least 3 months before the operation. Four patients were excluded from statistical research: 3 because they required rethoracotomy, and 1 due to early death within a 3-day period of time. Ultimately, 117 patients (92 men and 25 women) were qualified for statistical research. Sixty pneumonectomies, six bilobectomies and 51 lobectomies were performed. Among the 117 patients, postoperative AF occurred in 19 cases.
In order to determine AF risk factors, all patients were divided into two groups: Group A—98 patients without AF and Group B—19 patients who developed AF during the perioperative period (which included 1 day before the operation and 3 days following it). Group A consisted of 73 men and 15 women; the patients’ age ranged from 38 to 77 years, the mean age was 57.46. Forty-nine pneumonectomies, five bilobectomies and 44 lobectomies were performed. All 19 patients in Group B were men, age 50–71, mean age 59.94 years. Eleven pneumonectomies, one bilobectomy and seven lobectomies were performed in this group.
Demographic data, the number and types of pulmonary resections performed on patients from the two groups, as well as their ASA grades (I, II or III) are summarized in Table 1. There were no ASA IV and V patients.
Table 1:
Demographic data, ASA grades and the number and type of pulmonary resection performed in patients from Groups A and B
| Data | Group A (98 patients) | Group B (19 patients) |
|---|---|---|
| Men | 73 | 19 |
| Women | 15 | 0 |
| Age (years) | 38–77 | 50–71 |
| Mean age (years) | 57.4 | 59.9 |
| ASA I | 1 | 0 |
| ASA II | 42 | 3 |
| ASA III | 55 | 16 |
| Pneumonectomy right | 26 | 2 |
| Pneumonectomy left | 23 | 9 |
| Bilobectomy upper | 2 | 0 |
| Bilobectomy lower | 3 | 1 |
| Lobectomy upper right | 12 | 5 |
| Lobectomy middle right | 1 | 0 |
| Lobectomy lower right | 4 | 0 |
| Lobectomy upper left | 21 | 2 |
| Lobectomy lower left | 6 | 0 |
During the preoperative interview, we discovered that many of the patients suffered from chronic illnesses. The most frequent disorders included: chronic obstructive pulmonary disease (COPD)—(33 patients), arterial hypertension (30), gastritis and duodenitis (26), lower extremity varicosis (23), arteriosclerosis obliterans (18), diabetes mellitus (12) and coronary heart disease (10). Ninety-six of the patients were smokers. There were no differences between the study groups in terms of pre-existing conditions. The same surgical and anaesthetic techniques were employed in all cases. In all patients, combined general anaesthesia and thoracic epidural analgesia (TEA) were performed. Induction of anaesthesia was performed with propofol 2 mg/kg with subsequent continuous infusion of propofol 6–8 mg/kg/h. Rocuronium and fentanyl were administered prior to endobronchial intubation with a double-lumen endobronchial tube. TEA was performed using a mixture of 0.125% bupivacaine with fentanyl. The infusion of TEA was provided both during surgery and postoperatively for a 3-day period. Additional non-steroidal anti-inflammatory drugs (ketoprofen), and other analgesics (metamizol, tramadol) were also provided if required.
Numerous different risk factors for AF have been analysed and further divided into preoperative, operative and postoperative. Demographic data (age, gender, BMI index and cigarette smoking), spirometry parameters [vital capacity (VC), forced vital capacity (FVC), forced expiratory volume (FEV) at timed intervals of 1.0 s (FEV1), forced expiratory flow 25–75% (FEF 25–75), maximal voluntary ventilation (MVV) and FEV1/VC ratio] and concomitant diseases were analysed before the operation. We examined blood lost, blood required in the perioperative period, length of operation time, kind of pulmonary resection and pericardial bag opening needed. We used the Swan Ganz catheter to diagnosis pulmonary arterial hypertension (PAH) in the perioperative period. PAH was not observed in our patients. Pulmonary artery catheterization was performed before induction in the operative room. Corodyn (B. Braun, Germany) was introduced into the pulmonary artery via a subclavian vein and connected to a disposable pressure transducer (Exadyn Transducer, B. Braun, Germany). Laboratory and haemodynamic data were measured before, during and after surgery, and included the following: complete blood count (CBC), arterial blood gases (ABGs), serum creatinine and urea levels, saturation level of oxygen in haemoglobin (SaO2), capnometry, arterial blood pressure, body temperature, amount of diuresis, pulmonary arterial pressure (PAP), mean pulmonary arterial pressure (mPAP), pulmonary capillary wedge pressure (PCWP), shunt fraction (Qs/Qt) and arterio-venous oxygen difference [(a-v)O2]. Patients with heart failure were excluded from this study. Twenty-four hour electrocardiogram-ECG recording and analysis were performed using Holter equipment (Aspect702 recorder/Aspel) during the day before surgery and for the next 3 days. Arrhythmia analysis typically included the mean, maximum and minimum HR as well as the presence of any supraventricular and ventricular disturbances. Unfortunately, we did not examine echocardiographic data and ECG waves in details.
Three 5-min ECG strips from the 24-h ECG recording made during the day before the operation (Holter monitor) were analysed to evaluate HRV data parameters. The first strip (A) came from the afternoon of the day before the operation, the second (N) from the following night and the third (D) from the early morning of the day of the operation. Heart rate variability (HRV) parameters from each of the 5-min strips were calculated using HRV Analysis Software 1.1, which was obtained from Pasi A. Karjalainen from the Biomedical Signal Analysis Group (Department of Applied Physics, University of Kuopio, Finland. HRV was analysed in accordance with the guidelines of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [5]. Five-minute ECG strips with normal sinus rhythm RR intervals were selected for analysis. The time and frequency domain analysis was performed. Standard deviation of NN intervals (SDNN) reflects total HRV. The square root of the mean squared difference of successive NNs (RMSSD) and the proportion of NN50 divided by the total number of NNs (pNN50) reflects short-time HRV [6, 7]. The Poincaré plot graph was analysed, and both the short-term (SD1) and long-term (SD2) HRV as well as the SD2/SD1 ratio were determined. SD1 measures the dispersion of points perpendicular to the line of identity whereas SD2 measures the dispersion along the line of identity. The SD2/SD1 ratio describes the symphathovagal balance.
Furthermore, we performed a special analysis of related parameters of HRV A/N/D (Afternoon, Night, Day). Each ECG strip presented a set of several parameters; the same type of parameters included on strips from different time periods were then grouped together (e.g. mean HRA—mean heart rate from the strip (A) came from the afternoon of the day before the operation, mean HRN—mean heart rate from the strip (N) came from the night of the day before the operation, mean HRD—mean heart rate from the strip (D) came from the early morning of the day before the operation etc.) and their fluctuation was assessed in time. If the P-value was <0.05, the fluctuation of HRV A/N/D-related parameters was considered statistically significant in the studied patient group. The frequency domain of results, as well as both short- and long-term HRV were examined each time.
A postoperative chest radiogram was performed on all patients and fibrescopic bronchoscopy was obtained when needed. Respiratory and circulatory complications were monitored and necessary postoperative treatment was provided to all patients. Patients suffering from AF were administered amiodarone intravenously to restore sinus rhythm (300 mg/h as a bolus dose and subsequently 1200 mg/24 h through infusion pump if necessary). The data of these patients were then included in the analyses on the following days.
Depending on the data distribution pattern, the quantitative variables are presented as arithmetic means for normal distribution, with SD, and 95% confidence intervals (CIs), or as medians for non-parametric distribution. Qualitative variables are presented as absolute values and percentages. The comparison of parameter groups was performed by using appropriate parametric and non-parametric two-sided tests. The statistical analysis for unrelated variables was performed by using the two-sided Student's t-test for two independent samples, after normal distribution was confirmed with the Shapiro–Wilk test or the Levene's test of homogeneity of variance. If there was no variance identity, the Welch's test was employed. If normal distribution was not noted, the Mann–Whitney non-parametric test for unrelated variables was used. The relationship between the measured variables of normal distribution was analysed using linear (Pearson's) correlation analysis. If at least one variable exhibited a distribution other than normal or was an ordinal variable, Spearman's rank correlation analysis was applied. In order to compare the grouped patient parameters, unifactorial variance analysis (ANOVA) was performed for related variables after normal distribution was confirmed with the Shapiro–Wilk test or the Levene's test of homogeneity of variance and if there was no variance identity, the non-parametric Friedman test for related variables was employed. Some of the significant statistical differences that were revealed also required the application of multicomparative analysis such as the Dunn's test, the Newman–Keuls test or the Tukey's test. Nominal variables were analysed with the McNemar's test for paired data. Unpaired data in two or multidimensional contingency tables were analysed using the χ2 test, the Fisher's exact test or the Fisher–Freeman–Halton test. Relative risk with a 95% CI was applied. The results were considered statistically significant if the P-value was <0.05. All analyses were conducted by means of statistical software: STATISTICA 8.0 (from StatSoft), StatXact (from CytelStudio 8) and InStat (from GraphPad 3.0).
RESULTS
There were no significant differences in terms of concomitant diseases between the groups and the only significant demographic difference was gender. Postoperative AF occurred in 19 patients (16%), all of whom were male. The peak incidence of the onset of AF occurred in postoperative days 2–3. Patients suffering from AF were administered amiodarone intravenously to restore sinus rhythm and the treatment was successful. The results are summarized in Tables 2 and 3.
Table 2:
Significant perioperative AF risk factors
| AF risk factors | Group A (without AF) 98 patients |
Group B (with AF) 19 patients |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Med | SD | n | Mean | Med | SD | ||
| Mean RR Afternoon (s) | 97 | 0.71 | 0.69 | 0.10 | 19 | 0.76 | 0.77 | 0.08 | 0.015 |
| Mean HR Afternoon 1/min | 97 | 86.44 | 87.41 | 11.93 | 19 | 79.53 | 78.31 | 14.84 | 0.025 |
| RMSSD Day (ms) | 97 | 21.7 | 17.40 | 16.26 | 19 | 30.19 | 22.30 | 20.27 | 0.037 |
| SD1 Day (ms) | 97 | 15.36 | 12.27 | 11.52 | 19 | 21.38 | 15.80 | 14.37 | 0.036 |
| V 0 | 96 | 74.12 | 3 | 288.07 | 19 | 416.1 | 23 | 1606.35 | 0.001 |
| Supra 1 | 96 | 238.88 | 118 | 504.24 | 19 | 552.73 | 270 | 695.13 | 0.015 |
| V 1 | 96 | 98.85 | 8 | 479.9 | 19 | 79.89 | 43 | 139.78 | 0.027 |
| Max 2 | 89 | 109.16 | 108 | 17.96 | 17 | 147.52 | 150 | 34.51 | 0.0002 |
| Supra 2 | 95 | 265.62 | 27 | 977.94 | 19 | 1449.78 | 622 | 1999.27 | 0.00002 |
| V 2 | 95 | 110.1 | 2 | 576.38 | 19 | 56.52 | 44 | 61.88 | 0.0022 |
| Max 3 | 96 | 108.1 | 109.5 | 19.57 | 19 | 158.42 | 161 | 24.24 | 0.00001 |
| Supra 3 | 96 | 392.32 | 29 | 1151.47 | 19 | 1911 | 770 | 2915.14 | 0.00071 |
| V 3 | 96 | 78.26 | 2 | 353.24 | 19 | 536.31 | 24 | 1774.52 | 0.0027 |
| PCWP0 (mmHg) | 59 | 8.15 | 8 | 4.35 | 8 | 4.75 | 4.5 | 1.98 | 0.014 |
| PCWP1 (mmHg) | 59 | 8.53 | 8 | 3.83 | 8 | 5.88 | 6 | 2.17 | 0.028 |
| Male gender | 73 patients (74%) | 19 patients (100%) | 0.012 | ||||||
| Left pneumonectomy | 23 patients (23%) | 9 patients (47%) | 0.032 | ||||||
| ASAIII score status | 55 patients (56%) | 16 patients (84%) | 0.022 | ||||||
Mean RR Afternoon (s); RMSSD Day (ms); SD1 Day (ms); Afternoon: time period-afternoon a day before operation; Day: means time period-early morning in a day of operation; V: number of ventricular ectopic beats per day; Supra: number of supraventricular ectopic beats per day; Max: maximal hearth rhythm/min; numbers 1, 2, 3 signify: 0: a day before operation; 1: first day (day of the operation); 2: second day; 3: third day; PCWP0: pulmonary capillary wedge pressure before intubation; PCWP1: pulmonary capillary wedge pressure after intubation; ASA: American Society of Anesthesiologists scale.
Table 3:
Statistically significant differences in Groups A and B obtained from the analysis of HRV fluctuation A/N/D (Afternoon, Night, Day)-related parameters data from each different 5 min time periods examined together a day before operation
| HRV fluctuation of variables A/N/D | Group A (97 patients) |
Group B (19 patients) |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Med | SD | P A/N/D | Mean | Med | SD | P A/N/D | |
| Mean RR A (s) | 0.71 | 0.69 | 0.1 | P <0.000001 | 0.76 | 0.77 | 0.08 | 0.13 |
| Mean RR N | 0.84 | 0.84 | 0.13 | 0.87 | 0.8 | 0.21 | ||
| Mean RR D | 0.77 | 0.75 | 0.14 | 0.8 | 0.79 | 0.15 | ||
| SDNN A (ms) | 0.04 | 0.03 | 0.02 | 0.037 | 0.04 | 0.04 | 0.03 | 0.17 |
| SDNN N | 0.03 | 0.03 | 0.02 | 0.04 | 0.04 | 0.02 | ||
| SDNN D | 0.04 | 0.03 | 0.03 | 0.05 | 0.04 | 0.04 | ||
| Mean HR A (1/min) | 86.44 | 87.41 | 11.93 | P <0.000001 | 79.93 | 81.77 | 9.28 | 0.12 |
| Mean HR N | 73.4 | 72.02 | 11.54 | 72.39 | 75.09 | 14.44 | ||
| Mean HR D | 80.34 | 80.52 | 13.85 | 77.24 | 77.32 | 12.41 | ||
| STD A (1/min) | 4.37 | 3.95 | 2.14 | P <0.000001 | 4.54 | 4.14 | 2.41 | 0.073 |
| STD N | 2.95 | 2.56 | 1.67 | 2.92 | 2.97 | 1.64 | ||
| STD D | 4.33 | 3.32 | 2.77 | 4.45 | 3.69 | 2.76 | ||
| RMSSD A (ms) | 17.43 | 14.9 | 11.11 | 0.0023 | 21.77 | 17.4 | 14.69 | 0.15 |
| RMSSD N | 23.8 | 16.9 | 20.46 | 26.87 | 18.3 | 22.29 | ||
| RMSSD D | 21.7 | 17.4 | 16.26 | 30.19 | 22.3 | 20.28 | ||
| NN50 A | 11.27 | 4 | 19.27 | 0.39 | 16.13 | 4 | 20.67 | 0.40 |
| NN50 N | 23.92 | 3 | 49.95 | 20 | 3 | 36.05 | ||
| NN50 D | 19.56 | 3 | 31.47 | 28.53 | 8 | 35.87 | ||
| PNN50 A | 2.71 | 0.9 | 4.52 | 0.20 | 4.15 | 1.2 | 5.38 | 0.27 |
| PNN50 N | 7.38 | 0.8 | 14.34 | 7.25 | 0.8 | 13.12 | ||
| PNN50 D | 5.49 | 0.6 | 9.34 | 8.36 | 2.1 | 11.25 | ||
| VLF A (ms²) | 179 451.34 | 167 194 | 54 556.77 | P <0.000001 | 198 917.42 | 189 693 | 67 312.37 | 0.031 |
| VLF N | 255 509.44 | 242 323 | 86 048.62 | 286 984.82 | 228 393 | 138 516.47 | ||
| VLF D | 21 6279.88 | 195 958 | 82 345.08 | 235 150.13 | 230 719 | 86 430.96 | ||
| LF A (ms²) | 1201.52 | 1097 | 379.67 | P <0.000001 | 1418.2 | 1289 | 452.67 | 0.13 |
| LF N | 1637.68 | 1537.5 | 632.74 | 1996.27 | 1634 | 1011.17 | ||
| LF D | 1465.07 | 1309 | 642.97 | 1770.93 | 1347 | 1181.97 | ||
| HF A (ms²) | 275.77 | 254 | 86.69 | P <0.000001 | 309.95 | 316 | 108.52 | 0.038 |
| HF N | 441.08 | 380.5 | 227.6 | 510.71 | 421 | 276.34 | ||
| HF D | 369.49 | 309 | 234.1 | 419.67 | 344 | 193.75 | ||
| LF/HF A | 4.39 | 4.29 | 0.66 | 0.00009 | 4.26 | 4.09 | 0.55 | 0.63 |
| LF/HF N | 3.9 | 4.06 | 0.6 | 3.83 | 4.09 | 0.33 | ||
| LF/HF D | 4.17 | 4.18 | 0.62 | 3.65 | 4.02 | 0.83 | ||
| SD1 A (ms) | 12.34 | 11 | 7.79 | 0.0019 | 15.41 | 12.3 | 10.42 | 0.15 |
| SD1 N | 16.85 | 12 | 14.5 | 19.03 | 13 | 15.8 | ||
| SD1 D | 15.36 | 12.3 | 11.52 | 21.38 | 15.8 | 14.37 | ||
| SD2 A (ms) | 48.96 | 44.6 | 25.37 | 0.0056 | 58.47 | 50 | 34.65 | 0.27 |
| SD2 N | 44.39 | 38.1 | 27.43 | 47.11 | 51.7 | 31.32 | ||
| SD2 D | 54.37 | 43.9 | 34.19 | 65.28 | 47 | 49.91 | ||
| SD2/SD1 A | 4.65 | 4.2 | 2.43 | 0.00002 | 4.17 | 3.93 | 1.61 | 0.42 |
| SD2/SD1 N | 3.5 | 2.93 | 1.96 | 3.16 | 2.52 | 1.76 | ||
| SD2/SD1 D | 4.34 | 3.83 | 2.7 | 3.24 | 2.61 | 2.19 | ||
A: means period time afternoon a day before operation; N: data from Holter by night; D: day early morning in day of operation, before operation.
The difference in total subjects reported in Table 2 results from the exclusion of some patients, for whom the Holter ECG data was not interpretable due to the presence of artefacts. There was no difference in survival rates between the study groups.
The risk factors were divided into preoperative, operative and postoperative. Statistical analysis revealed that male gender constituted the most significant preoperative risk factor. AF was more frequently observed among patients who had an ASA physical status score of III, in comparison with ASA I or II patients. Patients with lower PCWP before and after induction at the operative time were more prone to postoperative AF too (Table 2).
Examined preoperatively, Holter data from the day before the operation revealed that the patients with a higher number of ventricular ectopic beats were more prone to postoperative AF (as presented in Table 2).
Evaluation of each of the short 5-min periods of HRV parameters revealed that patients from Group B (those with AF) had a slower heart rate (higher mean RR), measured on the afternoon of the day before operation, than patients from Group A (median 0.77 vs 0.69 s), which responds to mean HR of (median 78.31 vs 87.41/min). Furthermore, patients from Group B had a higher values of RMSSD Day (median 22.3 vs 17.4) as well as SD1 Day (median 15.8 vs 12.27) measured in short 5-min time periods of HRV early in the morning before operation, which was reflected in short-time HRV (Table 2).
In order to evaluate the HRV A/N/D-related parameters in terms of their tendency to fluctuate, our special analysis was conducted for related variables. It proved that patients from Group A (without AF) differed significantly in terms of almost all HRV-related parameters (except NN50 and pNN50 A/N/D) in comparison with the patients from Group B (with AF), who differed significantly only in VLF and HF A/N/D-related variables. It means that patients from Group B (in whom AF was observed in the postoperative period) exhibited lower fluctuation of almost all HRV A/N/D-related variables (as given in Table 3). Our investigation of Holter ECG data after the operation revealed that postoperative risk of AF was higher in those patients who exhibited a higher number of supraventricular and ventricular ectopic beats during the first 3-day period after the operation, and in patients with a higher maximal heart rate on the second and third day after the operation (Table 2).
Circulatory and respiratory complications occurred after the operation in both groups but the difference was not significant. Hypotension (systolic blood pressure <90 mmHg) during the first 3 postoperative days occurred in 21.43% of patients in Group A vs 15.79% in Group B. Cough and obstructive atelectasis occurred in 17.35% of patients in Group A vs 26.32% in Group B. More than 55% of patients in Group A and 57% in Group B required forced diuresis after operation (>80 mg of furosemide/day); the difference between the two groups was not significant in this respect. In both groups, ∼15% of patients underwent fibreoptic bronchoscopy due to atelectasis, in order to remove the sputum from the respiratory tract. Laboratory data also did not show significant differences. After operation the use of catecholamines was necessary in 7.14% of patients from Group A and in 5.26% of patients from Group B (difference not significant). Patients from Group A (without AF) required 1 day less of intensive care unit stay, in comparison with patients from Group B (with AF after operation). In this case, the difference was statistically significant (P = 0.00259).
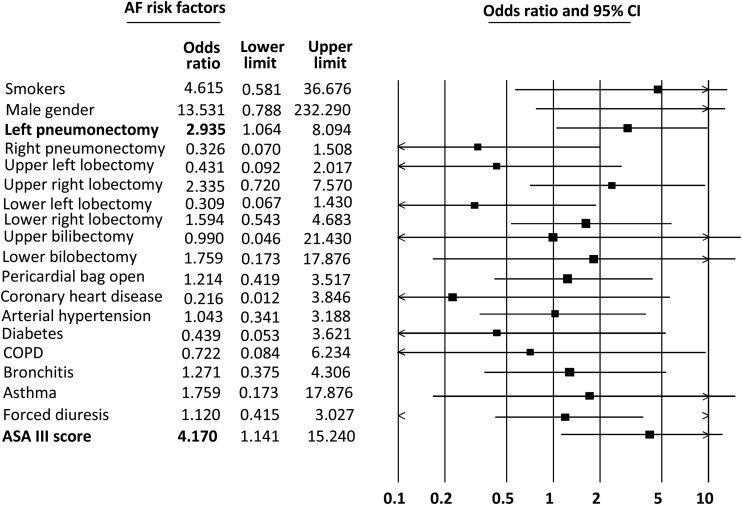
The odds ratio (OR) for the chosen parameters was set; gender, kind of pulmonary resection, pericardial bag opening, concomitant diseases like arterial hypertension, asthma, bronchitis, coronary heart disease, diabetes and COPD, smokers, ASA score III, forced diuresis.
ASA grade III was associated with a >4-fold increased risk for AF after operation (OR = 4.1) and left pneumonectomy with almost 3-fold increased risk (OR = 2.9). Others (OR) can only be an indication for further investigation for the higher number of patients (see Fig. 1).
Figure 1:
Graphical representation of the OR values examined AF risk factors.
DISCUSSION
Most recent series of lung cancer surgery suggest that pneumonectomies currently constitute <10% of all lung cancer surgeries [8]. The very high percentage of pneumonectomy patients in the presently discussed series results from the intent of the study, which was to enrol only patients that qualified for pneumonectomy. Ultimately, many of the patients who initially qualified for pneumonectomy only required a lobectomy. We then included both pneumonectomy and lobectomy patients for further analysis and excluded patients who were deemed inoperable in the operating room.
In the 1970s, the mortality rate after pneumonectomy in elderly patients amounted to 40% [9]. Despite the medical progress, the mortality rate still remains high in this group, as it is ∼10% [10]. It can be attributed to the fact that more elderly patients with serious concomitant diseases are treated with surgery. Postoperative supraventricular tachycardia as a complication of thoracotomy has been observed and described in medical literature for the past 60 years [11]. This is probably best explained by high circulatory catecholamine levels after thoracic operations. TEA attenuates the sympathetic response, but, even with complete pain relief, haemodynamic and endocrine responses are still present. For this reason, additional pharmacological attenuation of response is desired. In order to apply proper treatment and prevent AF, knowledge of all AF risk factors is required.
AF risk factors have been a subject of study for many years. It has been documented that male gender, age of 65 years and above, obesity (BMI >25), coronary diseases, previous myocardial infarction, congestive heart failure (CHF), fibrillatio atriorum paroxysmalis (FAP), arteriosclerosis obliterans (AO), COPD and disturbances of the autonomic nervous system are particular independent perioperative AF risk factors [4, 6, 12, 13]. Our results confirm earlier findings by other authors. All patients with AF were male and most of them had numerous concomitant diseases (they had an ASA physical status score of III). Statistical analysis of the study groups revealed that the extent of pulmonary resection, particularly left pneumonectomy, constituted very significant risk factors.
The aetiology of AF is almost certainly multifactorial, with postoperative hyperadrenergic condition and atrial distention being as the most important factors. In our study, AF occurred more often after left pneumonectomy. It can be attributed to injuries of the pulmonary venous truncus and cardiac plexus associated with lymph node resection and other surgical procedures. Lymphadenectomy was performed routinely in all cases of pneumonectomy and lobectomy. Investigation of other studies confirms this observation [14].
Patients with lower PCWP values before and after anaesthesiology induction at the operative time seem to be more predisposed to AF after operation. It can be caused by dehydratation before operation which can cause later cardiovascular complication but, more prospective studies need to confirm this suggestion.
According to the investigations by other authors, the risk of postoperative AF is higher in patients with a higher number of supraventricular and ventricular ectopic beats [15]. These results indicate the important function of postoperative ECG monitoring, which needs to be provided to all patients after thoracotomy.
It is widely believed that heightened sympathetic nervous system activity plays a fundamental role in the development of perioperative cardiac complications. Electrocardiography, being one of the basic examination tools in the diagnosis and evaluation of conduction disturbances and arrhythmias, allows the assessment of the autonomic nervous system status by means of HRV analysis [16, 17]. Many reports on the prognostic values of HRV in CHF patients have been published to date [16, 18]. The HRV parameters are reduced in the case of enhanced adrenergic activation, as observed in patients exhibiting stress, pain, high body temperature and CHF. Saul et al. and Casolo et al. [19, 20] have demonstrated that HRV decreased in CHF patients. The decrease in HRV variables is also associated with hyper-spasticity of the bronchi, diabetic neuropathy and adverse prognostics for CHF patients, and is considered to be an independent risk factor for sudden heart death [6, 7, 21]. Lower HRV also leads to a higher number of arrhythmic incidents in the future.
The GISSI 2 study (by Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico) included an analysis of temporal HRV parameters among 567 patients, who were observed for 1000 days; it revealed that the relative risk of death among patients after myocardial infarction was 3.5 for NN50 <200/day, 3.0 for SDNN <70 ms and 2.8 for RMSSD <17.5 ms [22].
HRV allows one to indirectly assess the autonomic control of circulatory system activity. SDNN is considered to reflect total HRV, while RMSSD and pNN50 reflect the so-called short-term HRV. RMSSD and pNN50 present a credible picture of the cardiac activity of the parasympathetic nervous system component.
RMSSD = (2 + SD1)1/2, a SD11/2 + SD21/2 = 2 <SDNN1/2. SD1 is considered to be the quantitative measure of short-term HRV, while SD2 is considered to be the quantitative measure of long-term HRV. The norms for HRV parameters are broadly defined. There is currently no normative data for short-term measures of HRV; the values of SDNN, RMSSD and HR decrease with age. The extent of these changes makes it difficult to discuss HRV norms without taking into consideration the age of the studied patients.
Among the patient groups analysed in our study, there were no significant demographic differences. Moreover, patients with cardiac insufficiency and those HRV D strips indicate after myocardial infarction, cerebral stroke, or any previous atrial dysrythmias, in whom significant HRV disturbances could be predicted, were excluded from the study. Can the assessment of preoperative HRV parameters therefore be used for identifying patients with increased risk of postoperative AF after major pulmonary resection?
It is crucial to remember that AF can be associated with increased activity of either the sympathetic or the parasympathetic nerve supply. If AF results from parasympathetic dominance (which happens especially at night and after dinner), β-blocking agents are contraindicated [23, 24]. In such cases, amiodarone appears to be more appropriate.
In our study, patients with higher preoperative short-term HRV were more predisposed to AF after operation.
The lower mean HR in the HRV A ECG strip from the day before operation and the higher values of short-term HRV (SD1, RMMSD) parameters obtained from the HRV D strips indicate an increased activity of the parasympathetic component of the autonomic nervous system in patients from Group B. This suggests balance disturbances in the nervous system activity and constitutes a significant predictor of postoperative AF. Continuous TEA decreases the activity of the sympathetic component of the cardiac and vascular innervation, which may lead to further dysregulation of the sympathetic-parasympathetic balance in favour of the parasympathetic component.
The increased parasympathetic activity of the autonomous nervous system in the patients from Group B suggests that the occurrence of AF in the postoperative period is related to this increase in activity.
The increased participation of the parasympathetic component among Group B patients was reflected in the smaller fluctuation of related variables (HRV A/N/D) at different times of the day. A single 5-min preoperative HRV analysis cannot offer an unambiguous answer to the question whether the patient will suffer from AF after major lung resection, because it is difficult to determine the values of HRV parameters indicating increased risk of postoperative AF occurrence without comparing the results with the control group of healthy patients. There are no widely accepted standard values for HRV that can be used for clinical purposes.
This problem can be solved by comparing the HRV parameters variability (fluctuation) from three 5-min HRV strips from different times of the day (A/N/D) and analysing them in terms of the statistically significant variability of parameters of the same name. All patients from Group B (with AF) exhibited lower fluctuation of almost all related HRV A/N/D variables.
In summary, patients without preoperative fluctuation of HRV A/N/D were more likely to suffer from AF after the operation. Our statistical analysis of related HRV A/N/D variables appears to be a simple method of identifying which thoracic surgery patients are at risk of postoperative AF. HRV analysis could be employed as the standard procedure for all patients qualified for pneumonectomy or lobectomy.
Knowledge of which component of the nervous system activity is dominant helps to indicate adequate antiarrhythmic treatment. Taking into account the aforementioned data, we recommend substituting β-blockers with amiodarone in treating postoperative AF after major lung resection with additional use of TEA. Obviously, all contraindications to amiodarone must be taken into consideration.
One more important question remains: could amiodarone be administered prophylactically to patients in whom the preoperative HRV analysis reveals decreased fluctuation of HRV A/N/D-related variables? In our opinion, if there are no clear contraindications to using amiodarone, such preventive treatment should be employed from the day of the procedure and throughout the perioperative period, but further prospective studies are required to confirm this suggestion. ECG Holter monitoring on the day prior to the surgery and evaluation of changes in HRV parameters should be done before each major pulmonary resection, particularly before each predictable pneumonectomy. The cost of ECG monitoring is lower than the cost of one more day of intensive care unit stay.
CONCLUSIONS
Preoperative lower fluctuation of HRV is an independent AF risk factor in the postoperative period in patients undergoing major pulmonary resection.
The risk of postoperative AF was higher in patients with a higher number of ventricular ectopic beats a day before operation, a higher number of supraventricular and ventricular ectopic beats during the first 3-day period after the operation, and patients with a higher maximal heart rate on the second and third day after operation. Male gender and the extent of pulmonary resection, particularly left pneumonectomy, constituted significant risk factors for AF. AF was more often observed in patients with ASA grade III, in comparison to ASA I and II patients.
The evaluation of changes in HRV parameters should be taken into consideration before each major pulmonary resection. Antiarrhythmic treatment should be recommended individually to each patient, based on the evaluation of the balance between their sympathetic and parasympathetic nervous systems and on their HRV fluctuation during the perioperative period.
Funding
This work was supported by Wielkopolskie Center of Pulmonology and Thoracosurgery E.J. Zeylands Pulmonary Diseases and Thoracic Surgery Center, Szamarzewski street 62, 60-569 Poznan, Poland
Conflict of interest: none declared.
REFERENCES
- 1.Banach M, Kazmierski J, Kowman M, Okonski PK, Sobow T, Kloszewska I, et al. Atrial fibrillation as a nonpsychiatric predictor of delirium after cardiac surgery: a pilot study. Med Sci Monit. 2008;14:286–91. [PubMed] [Google Scholar]
- 2.Amar D, Burt M, Reinsel RA, Leung DH. Relationship of early postoperative dysrhythmias and long-term outcome after resection of non-small cell lung cancer. Chest. 1996;110:437–9. doi: 10.1378/chest.110.2.437. doi:10.1378/chest.110.2.437. [DOI] [PubMed] [Google Scholar]
- 3.Musiał WJ. Udar mozgu i migotanie przedsionkow—niebezpieczny duet. Kard Pol. 2007;65:758–9. [Google Scholar]
- 4.Roselli EE, Murthy SC, Rice TW, Houghtaling PL, Pierce CD, Karchmer DP, et al. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg. 2005;130:438–44. doi: 10.1016/j.jtcvs.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Task Force of The European Society of Cardiology, The North American Society of Pacing, Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–87. doi:10.1093/oxfordjournals.eurheartj.a014868. [PubMed] [Google Scholar]
- 6.Guzik P. Asymetria rytmu serca, krotko- i długotrwałe skladowe zmiennosci rytmu serca u zdrowych osob. Poznan: Uniwersytet Medyczny im. Karola Marcinkowskiego; 2009. [Google Scholar]
- 7.Malik M. Heart rate variability: standards of measurements, physiological interpretation an clinical use. Circulation. 1996;93:1043–65. doi:10.1161/01.CIR.93.5.1043. [PubMed] [Google Scholar]
- 8.Tang SS, Redmond K, Griffiths M, Ladas G, Goldstraw P, Dusmet M. The mortality from acute respiratory distress syndrome after pulmonary resection is reducing: a 10-year single institutional experience. Eur J Cardiothorac Surg. 2008;34:898–902. doi: 10.1016/j.ejcts.2008.06.020. doi:10.1016/j.ejcts.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Kirch MM, Rotman H, Bove E, Agenta L, Cimmino V, Tashian J, et al. Major resection for bronchogenic carcinoma in the elderly. Ann Thorac Surg. 1976;22:369–73. doi: 10.1016/s0003-4975(10)64969-7. doi:10.1016/S0003-4975(10)64969-7. [DOI] [PubMed] [Google Scholar]
- 10.Damhuis RA, Schutte PR. Resection rates and postoperative mortality in 7899 patients with lung cancer. Eur Respir J. 1996;9:7–10. doi: 10.1183/09031936.96.09010007. doi:10.1183/09031936.96.09010007. [DOI] [PubMed] [Google Scholar]
- 11.Bailey CC, Betts RH. Cardiac arrhythmias following pneumonectomy. N Engl J Med. 1943;229:356–9. doi:10.1056/NEJM194308262290902. [Google Scholar]
- 12.Vaporciyan AA, Correa AM, Rice DC, Roth JA, Smythe WR, Swisher SG, Jr, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127:779–86. doi: 10.1016/j.jtcvs.2003.07.011. doi:10.1016/j.jtcvs.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Dyszkiewicz W, Skrzypczak M. Atrial fibrillation after surgery of the lung: clinical analysis of risk factors. Eur J Cardiothorac Surg. 1998;13:625–8. doi: 10.1016/s1010-7940(98)00084-0. doi:10.1016/S1010-7940(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen PS, Chou CC, Tan AY, Zhou S, Fishbein MC, Hwang C, et al. The mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(Suppl. 3):2–7. doi: 10.1111/j.1540-8167.2006.00626.x. doi:10.1111/j.1540-8167.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 15.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–8. doi: 10.1016/s0735-1097(03)00955-0. doi:10.1016/S0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 16.Guzzetti S, La Rovere MT, Pinna GD, Mortara A, Malliani A. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J. 2005;26:357–62. doi: 10.1093/eurheartj/ehi067. doi:10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]
- 17.Musialik-Lydka A, Sredniawa B, Pasyk S. Heart rate variability in heart failure. Kardiol Pol. 2003;58:10–6. [PubMed] [Google Scholar]
- 18.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:514–6. doi: 10.1161/01.cir.0000047275.25795.17. doi:10.1161/01.CIR.0000053944.35059.FA. [DOI] [PubMed] [Google Scholar]
- 19.Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, Cohen RJ. Assessmentestive heart failure by heart rate spectral analysis. Am J Cardiol. 1988;61:1292–9. doi: 10.1016/0002-9149(88)91172-1. doi:10.1016/0002-9149(88)91172-1. [DOI] [PubMed] [Google Scholar]
- 20.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous hart rate variability in congestive heart failure. Am J Cardiol. 1989;64:1162–7. doi: 10.1016/0002-9149(89)90871-0. doi:10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]
- 21.Vinik AI, Erbas T. Cardiovascular autonomic neuropathy: diagnosis and management. Curr Diabetes Rep. 2006;6:424–30. doi: 10.1007/s11892-006-0074-z. doi:10.1007/s11892-006-0074-z. [DOI] [PubMed] [Google Scholar]
- 22.Zuanetti G, Neilson JM, Latini R, Santoro E, Maggioni AP, Ewing DJ. Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era. The GISSI-2 results. Gruppo Italiano per lo Studio della Sopravivenza nell’ Infarto Miocardico. Circulation. 1996;94:432–6. doi: 10.1161/01.cir.94.3.432. doi:10.1161/01.CIR.94.3.432. [DOI] [PubMed] [Google Scholar]
- 23.Shin DG, Yoo CS, Yi SH, Bae JH, Kim YJ, Park JS, et al. Prediction of paroxysmal atrial fibrillation usung nonlinear analysis of the RR interval dynamics before the spontaneous onset of atrial fibrillation. Circ J. 2006;70:94–9. doi: 10.1253/circj.70.94. doi:10.1253/circj.70.94. [DOI] [PubMed] [Google Scholar]
- 24.Maisel WH. Autonomic modulation preceding the onset of atrial fibrillation. J Am Coll Cardiol. 2003;42:1269–70. doi: 10.1016/s0735-1097(03)00959-8. doi:10.1016/S0735-1097(03)00959-8. [DOI] [PubMed] [Google Scholar]