Abstract
OBJECTIVES
Continuous-flow left ventricular assist devices (cf-LVADs) may induce commissural fusion of the aortic valve leaflets. Factors associated with this occurrence of commissural fusion are unknown. The aim of this study was to examine histological characteristics of cf-LVAD-induced commissural fusion in relation to clinical variables.
METHODS
Gross and histopathological examinations were performed on 19 hearts from patients supported by either HeartMate II (n = 17) or HeartWare (n = 2) cf-LVADs and related to clinical characteristics (14 heart transplantation, 5 autopsy).
RESULTS
Eleven of the 19 (58%) aortic valves showed fusion of single or multiple commissures (total fusion length 11 mm [4–20] (median [interquartile range]) per valve), some leading to noticeable nodular displacements or considerable lumen diameter narrowing. Multiple fenestrations were observed in one valve. Histopathological examination confirmed commissural fusion, with varying changes in valve layer structure without evidence of inflammatory infiltration at the site of fusion. Commissural fusion was associated with continuous aortic valve closure during cf-LVAD support (P = 0.03). LVAD-induced aortic valve insufficiency developed in all patients with commissural fusion and in 67% of patients without fusion. Age, duration of cf-LVAD support and aetiology of heart failure (ischaemic vs dilated cardiomyopathy) were not associated with the degree of fusion.
CONCLUSIONS
Aortic valve commissural fusion after support with cf-LVADs is a non-inflammatory process leading to changes in valve layer structure that can be observed in >50% of cf-LVAD patients. This is the first study showing that patients receiving full cf-LVAD support without opening of the valve have a significantly higher risk of developing commissural fusion than patients on partial support.
Keywords: Aortic stenosis, Aortic valve, Rotary blood pump
INTRODUCTION
Many patients with end-stage heart failure are treated with second- and third-generation continuous-flow left ventricular assist devices (cf-LVADs) as bridge to cardiac transplantation, destination therapy or bridge to recovery [1–4]. Compared with the first-generation pulsatile LVADs (P-LVADs), cf-LVADs have demonstrated improved patient survival and mechanical durability enabling prolonged cardiac and circulatory support [5]. However, the use of these devices can induce adverse cardiac structural changes, such as aortic valve regurgitation [6] and commissural fusion of the aortic valve leaflets [7].
Particularly, aortic valve leaflets are affected during LVAD support, as the aortic valve may remain closed depending on the pump speed. This may lead to fusion of these leaflets. This LVAD-related commissural fusion may be the result of a mechanically induced remodelling process of fibrous connective tissue. This process is considered to be different from the degenerative process in senile calcific aortic stenosis [8, 9]. It has been reported in relation to P-LVADs [8, 10–13]. Although partial commissural fusion of the aortic valve is believed to be well known in patients on cf-LVAD support, only 10 patients have been described to date in whom the phenomenon has been studied and it is unknown which clinical patient characteristics are associated with this fusion process [7, 14]. In addition, the histological characterization of aortic valve fusion considering new generation cf-LVADs requires thorough investigation. The aim of this study was to examine gross and histological characteristics of aortic valve fusion after cf-LVAD support in relation to clinical patient variables.
METHODS
Patients
Aortic valves from hearts of 19 patients supported by either a Heartmate II cf-LVAD (n = 17) (HeartMate II, Thoratec Corp, Pleasanton, CA, USA) or a HeartWare cf-LVAD (n = 2) (‘HVAD’, HeartWare, Inc., Miramar, FL, USA) were examined after heart transplantation (n = 14) or autopsy (n = 5). Demographic data and clinical course prior to and during cf-LVAD support were collected retrospectively. Echocardiographic studies were reviewed to assess aortic valve dynamics and dysfunction. Opening (partial support) or continuous closure of the aortic valve (full support) was identified at baseline pump speed throughout the entire support period, while the degree of aortic valve insufficiency (AI) was graded mild, moderate or severe based on colour Doppler echocardiography. After discharge, patients underwent a pump speed change procedure at 3, 6 and 12 months and each year following implantation. During these studies, the pump speed was ramped down to assess the degree of aortic valve (re-) opening in order to assess potential fusion.
Gross examination
The explanted hearts were fixed in 4% formaldehyde and stored until examination. Initially, the aortic valve was macroscopically examined in its anatomical position and its structure and anatomical appearance were analysed. The diameter of the aortic lumen was measured at the level of the sinotubular junction and in cases of severe commissural fusion, the diameter of the rest-lumen (in between the fused valve leaflets) was determined. Subsequently, the right coronary (RC), left coronary (LC) and non-coronary (NC) leaflets were identified and inspected for pathological lesions and commissural fusion. Fusion was identified as a binding of tissue between two leaflets at a commissure, and the length of the fusion was measured. Only fusion of a length greater than 3 mm was considered LVAD-induced fusion, because fusion of ≤3 mm is difficult to discriminate from normal. The location of the nodule of Arantius within the aortic valve was also determined. Finally, the macroscopic morphology of the aortic valves was documented on photographs.
Histology
The aortic valves were dissected from the hearts keeping the entire valve intact. The LC leaflet was marked with ink for later identification. The entire valve was embedded in paraffin as a whole mount specimen and cut into 5 μm-thick whole mount sections. The sections were stained with haematoxylin and eosin and elastic van gieson.
Statistics
Patient clinical data and morphological measurements are presented as mean ± SD or median (interquartile range [IQR]) in case of inhomogeneous variance. Relationship between total fusion length and both age and duration of cf-LVAD support was determined by linear regression. The Mann–Whitney U-test was performed to compare the total length of commissural fusion (total fusion) between two selected groups. Furthermore, the Kruskal–Wallis test was performed to compare the total fusion between the three potential areas of fusion: LC and RC, LC and NC, RC and NC. A P-value of <0.05 was considered to be statistically significant.
RESULTS
Patients
Demographic data and patient characteristics are presented in Table 1. Nineteen explanted hearts were evaluated. Fifteen (79%) patients were male; the average age was 48 ± 14 years. The aetiology of heart failure was dilated cardiomyopathy (DCM) in 12 (63%) patients and ischaemic cardiomyopathy (ICM) in 7 (37%) patients. The duration of support was 718 ± 507 days. Preoperative LVAD echocardiography was available in 18 (95%) patients. Fourteen (78%) of 18 patients had no pre-LVAD aortic insufficiency, whereas 4 (22%) patients had a mild AI prior to cf-LVAD implantation. Postoperatively, echocardiographic evaluation of 16 patients was available and the presence or absence of AI could be assessed in 15 patients as Doppler was absent in 1 patient. Echocardiography review showed that 4 of the 16 patients were on partial support with opening of the aortic valve, while 12 of the 16 patients were on full support without opening of the valve. Eleven of the 15 patients developed an AI after implantation of the cf-LVAD (LVAD-induced AI) and 2 of the 15 patients did not develop AI. One patient with AI prior to cf-LVAD implantation showed consistency of AI after implantation while in another patient, AI was present after cf-LVAD implantation, but due to the absence of preoperative echocardiographic evaluation it could not be determined whether AI was induced by the cf-LVAD. In retrospect, there were no valve-related complications or clinical symptoms reported in the 15 transplanted patients. Causes of death of the five autopsy cases were: perioperative right ventricular failure (n = 1), multiorgan failure and pump obstruction (n = 2), ischaemic stroke (n = 1) and haemorrhagic stroke after thrombolysis due to pump obstruction (n = 1). In the latter case, the aortic valve began to reopen as a result of an acquired pump obstruction after a 5-year period of full support without opening of the valve. Therefore, this case was denoted as full support (echocardiography provided in Supplementary Fig. S1).
Table 1:
Demographic and clinical data from 19 cf-LVAD patients
| Patient (no.) | Age at implant (years) | Gender | Aetiology of HF | Comorbidities | Device | Degree of support based on AV opening | AV function at lowest pump speed | LVAD support duration (days) | Heart | Clinical course |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | Male | ICM | None | HM II | Partial | Good opening | 1393 | HTx | No AI (preoperative + postoperative) |
| 2 | 62 | Male | DCM | None | HM II | Full | Good opening | 506 | HTx | LVAD-induced AI |
| 3 | 37 | Female | ICM | None | HM II | Full | Aortic valve closed | 700 | HTx | LVAD-induced AI |
| 4 | 59 | Male | ICM | None | HM II | n/a | n/a | 530 | HTx | AI (preoperative); postoperative AI data n/a |
| 5 | 44 | Male | DCM | None | HM II | Partial | Good opening | 707 | HTx | LVAD-induced AI |
| 6 | 47 | Male | ICM | None | HM II | Full | Partial opening | 419 | HTx | LVAD-induced AI |
| 7 | 24 | Female | DCM | None | HM II | Full | Partial opening | 758 | HTx | LVAD-induced AI |
| 8 | 38 | Male | DCM | None | HM II | Partial | Good opening | 541 | HTx | No AI (preoperative + postoperative) |
| 9 | 49 | Male | DCM | None | HM II | Full | Good opening | 787 | HTx | LVAD-induced AI |
| 10 | 61 | Female | DCM | Hypertension | HM II | Full | Good opening | 297 | HTx | LVAD-induced AI |
| 11 | 46 | Male | DCM | None | HM II | Full | Good opening | 1529 | HTx | Preoperative AI data n/a, postoperative AI |
| 12 | 23 | Male | DCM | None | HM II | Full | Partial opening | 1035 | HTx | LVAD-induced AI |
| 13 | 65 | Male | DCM | None | HM II | Full | Good opening | 478 | HTx | AI (preoperative and postoperative) |
| 14 | 67 | Male | DCM | None | HM II | Partial | Good opening | 410 | HTx | LVAD-induced AI |
| 15 | 63 | Female | ICM | Diabetes | HM II | n/a | n/a | 1146 | Autopsy | AI (preoperative); postoperative AI data n/a, ischaemic strokea |
| 16 | 63 | Male | ICM | None | HW | Full | n/a | 277 | Autopsy | LVAD-induced AI, MOFa + pump obstruction |
| 17 | 52 | Male | DCM | None | HW | n/a | n/a | 7 | Autopsy | AI (preoperative); postoperative AI data n/a, RVFa |
| 18 | 32 | Male | DCM | None | HM II | Full | n/a | 121 | Autopsy | No AI preoperative; postoperative AI data n/a; MOFa + pump obstruction |
| 19 | 45 | Male | ICM | None | HM II | Full | Good opening | 1995 | Autopsy | LVAD-induced AI; haemorrhagic strokea + pump obstruction |
aCause of death on cf-LVAD.
AV: aortic valve; ICM: ischaemic cardiomyopathy; DCM: dilated cardiomyopathy; HM II LVAD: HeartMate II left ventricular assist device; HW LVAD: HeartWare left ventricular assist device; AI: aortic insufficiency; RVF: right ventricular failure; MOF: multiorgan failure; HTx: heart transplantation; n/a: not available.
Gross examination
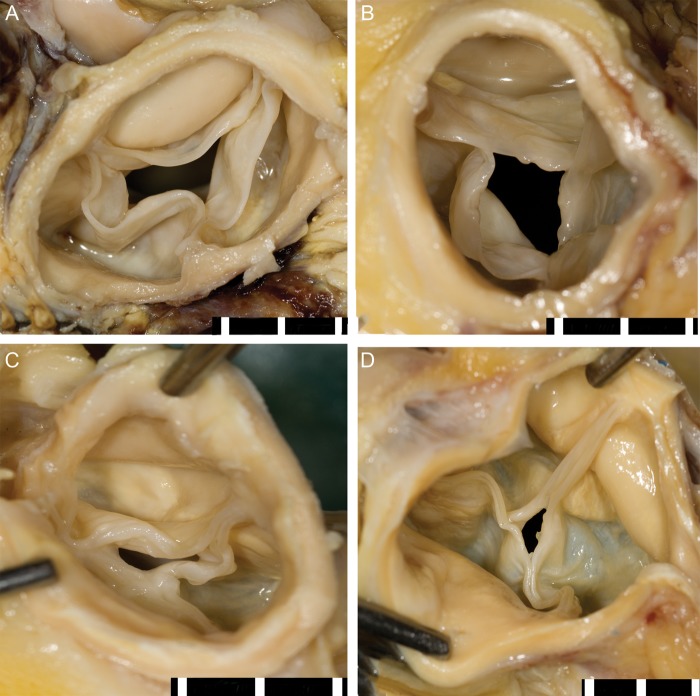
Eleven (58%) of the 19 aortic valves showed evident commissural fusion (>3 mm) of single or multiple commissures with a total fusion length of 11 mm [4–20] (median [IQR]) per valve (Table 2). Examples of multiple degrees of commissural fusion are shown in Fig. 1. In 2 (11%) aortic valves, commissural fusion led to noticeable displacement of the nodule of Arantius to a different anatomical location within the aortic valve. Four (21%) aortic valves showed diffuse commissural fusion of all the three leaflets. The residual-luminal diameters of these valves were 3, 4, 4 and 8 mm. In addition, 13 (68%) aortic valves revealed focal yellow white irregularities appearing as fatty streaks on the base of the valve cusps and in the sinus of Valsalva. Fenestrations were also seen in leaflets of two (11%) aortic valves (Fig. 2). None of the aortic valves showed presence of thrombus on the valve leaflets.
Table 2:
Aortic annulus and fusion data of 19 cf-LVAD patients
| Patient (no.) | AAd (mm) | LC and RC fusion (mm) | LC and NC fusion (mm) | RC and NC fusion (mm) | Max fusion (mm) | Total fusion (mm) | No. of fused commissures (>3 mm) | Residual lumen (mm)a |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 0 | 0 | 2 | 2 | 2 | 0 | |
| 2 | 22 | 0 | 5 | 6 | 6 | 11 | 2 | |
| 3 | 21 | 14 | 0 | 5 | 14 | 19 | 2 | |
| 4 | 25 | 0 | 3 | 0 | 3 | 3 | 0 | |
| 5 | 22 | 0 | 0 | 1 | 1 | 1 | 0 | |
| 6 | 22 | 0 | 0 | 5 | 5 | 5 | 1 | |
| 7 | 15 | 1 | 0 | 10 | 10 | 11 | 1 | |
| 8 | n/a | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | 25 | 4 | 6 | 2 | 6 | 12 | 2 | |
| 10 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 11 | 21 | 0 | 10 | 10 | 10 | 20 | 2 | |
| 12 | 22 | 0 | 9 | 5 | 9 | 14 | 2 | |
| 13 | 28 | 2 | 2 | 2 | 2 | 6 | 0 | |
| 14 | 28 | 1 | 3 | 3 | 3 | 7 | 0 | |
| 15 | 23 | 14 | 15 | 15 | 15 | 44 | 3 | 4 |
| 16 | 24 | 10 | 10 | 14 | 14 | 34 | 3 | 4 |
| 17 | 22 | 2 | 0 | 2 | 2 | 4 | 0 | |
| 18 | 20 | 9 | 9 | 11 | 11 | 29 | 3 | 3 |
| 19 | 22 | 10 | 10 | 11 | 11 | 31 | 3 | 8 |
| 23 ± 3b | 1 [0.7]c | 3 [0.9]c | 5 [2.10]c | 6 [2.11]c | 11 [4.20]c |
aResidual-lumen is computed for valves with all fused commissures.
bMean ± SD.
cMedian [interquartile range].
AAd: aortic annulus diameter; LC: left coronary cusp; RC: right coronary cusp; NC: non-coronary cusp; n/a: not available: disrupted during sample dissection.
Figure 1:
Cranial view of aortic valves after cf-LVAD support. (A) No commissural fusion, (B) one commissure fused with additionally some fatty streaks, (C) two commissures fused leading to nodular displacement and (D) all the three commissures fused resulting in a drastic lumen size reduction. Scale bar in 5 mm.
Figure 2:
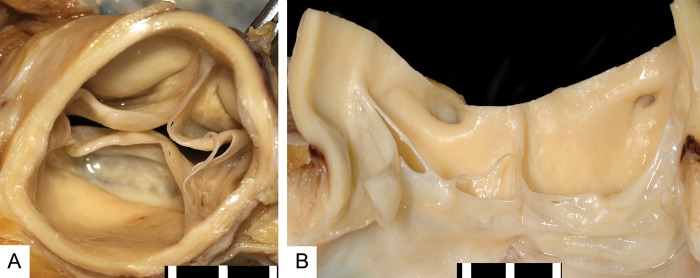
Valve fenestrations. Cranial (A) and frontal view (B) of an aortic valve after cf-LVAD support with two commissures fused with additionally multiple small and large fenestrations on multiple leaflets. Scale bar in 5 mm.
Histology
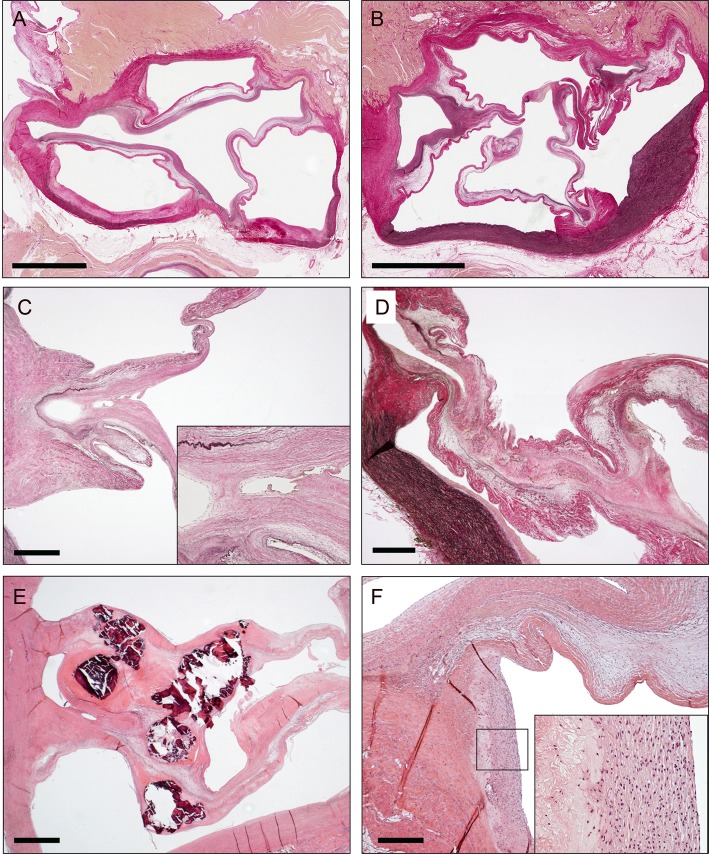
Histological examination confirmed either the presence or absence of commissural fusion in referred cases. Commissural fusion was often identified halfway the commissure, with more or less prominent merger of adjacent leaflets with disappearance of the endothelial layer (Fig. 3). Often, a small lumen was enclosed in between the fused commissures at the place of adherence at the base of the leaflets. Yet, in one aortic valve, this lumen was absent and filled by loose connective tissue. The elastic fibres of the ventricularis layer were disrupted at multiple locations within the fused areas. An increase of extracellular matrix was observed in most cases. In 1 case, severe calcification was observed at the site of fusion. In addition, there was no evidence of inflammatory infiltration seen around these fused regions. Some specimens, however, showed additionally subendothelial areas of calcification, cholesterol clefts, infiltration of macrophages and some lymphocytes. These were located in the sinus of Valsalva in the aortic wall next to the commissures of both fused and non-fused leaflets. These areas corresponded to the areas that were identified as fatty streaks in gross examination.
Figure 3:
Histology of aortic commissural fusion after LVAD support. (A) Whole mount slide of aortic valve without commissural fusion. Elastic van gieson staining. Bar = 5 mm. (B) Whole mount slide of aortic valve with commissural fusion. Bar = 5 mm. (C) Example of commissure with minor fusion of the valve leaflets. Inlay with higher magnification of fused area. Note the disruption of the elastic fibres (in black) in the ventricularis layer of the valve leaflets. Bar = 1 mm. (D) Example of commissure with major commissural fusion. Bar = 1 mm. (E) Commissural fusion with calcifications in the fused area (observed in 1 patient). Haematoxylin and eosin staining. Bar = 1 mm. (F) Example of fatty streak in aortic wall of sinus of Valsalva next to the fused area. Inlay with higher magnification showing macrophages and cholesterol clefts. The fatty streaks also revealed calcification in some patients (not shown). Bar = 1 mm.
Association between clinical characteristics and commissural fusion
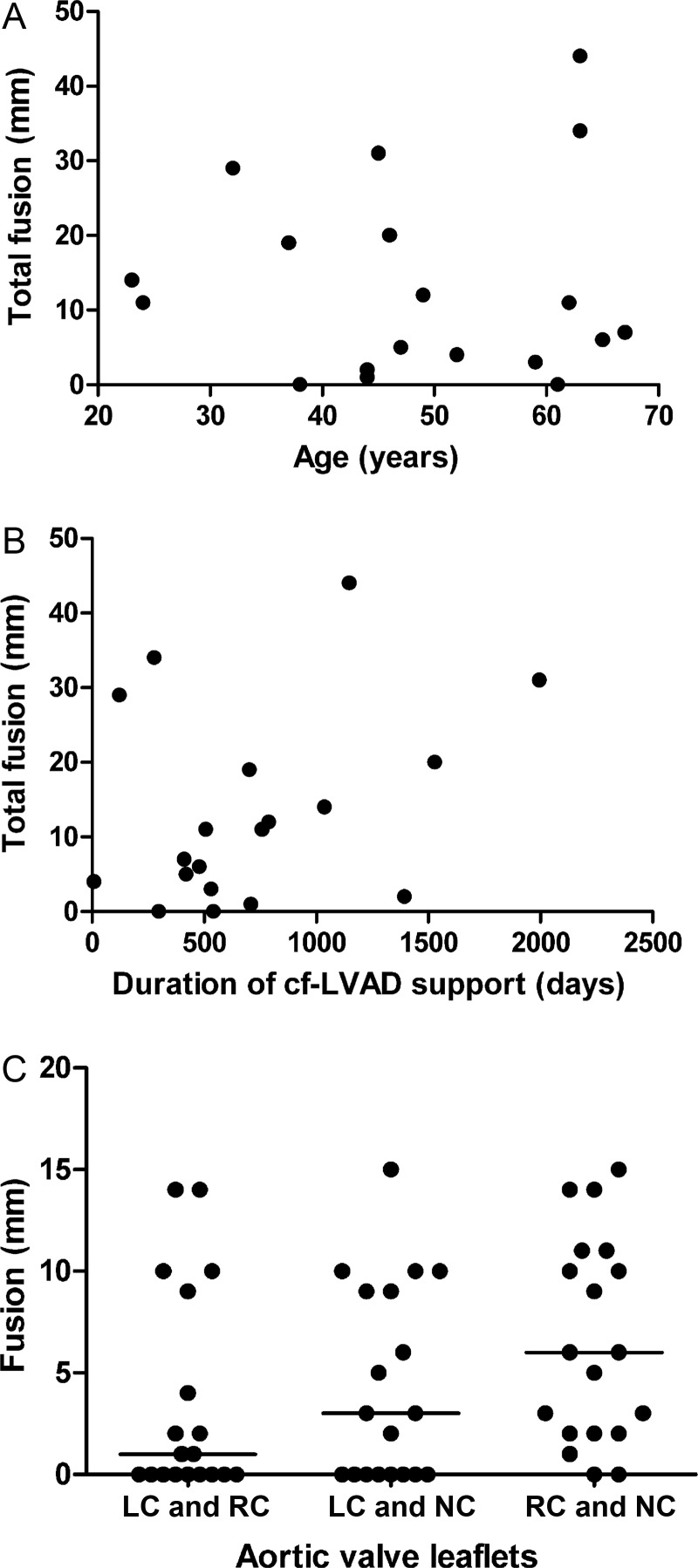
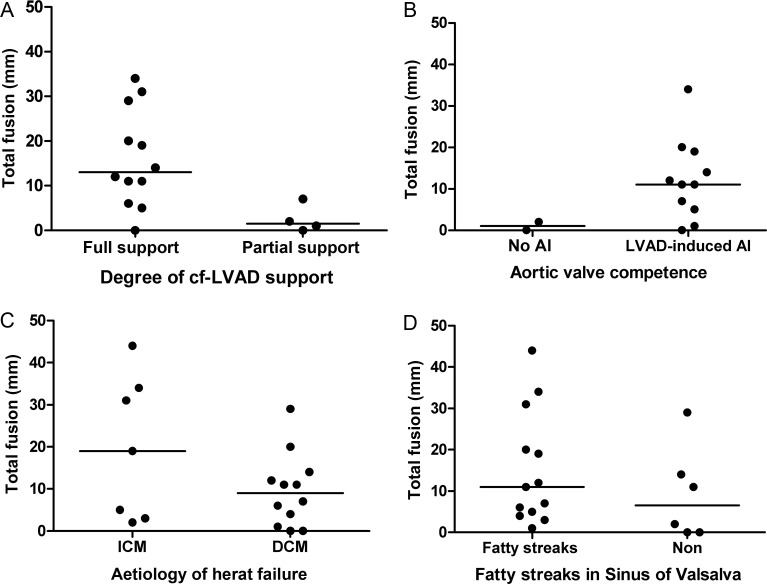
Linear regression analysis showed no statistical relationship between the total length of commissural fusion (total fusion) and age (R2 = 0; P = 0.94) or duration of LVAD support (R2 = 0.1; P = 0.18) (Fig. 4). Also, fusion was of similar length in all three commissures (P = 0.06). Patients on full support had a statistically larger total fusion compared with those on partial support (P = 0.03), although only 4 patients had partial support (Fig. 5). Cf-LVAD-induced AI was observed in 8 of the 8 patients with commissural fusion and in 3 of the 5 patients without fusion (low sample size did not allow statistical analysis). Total fusion was not statistically different between patients with DCM and ICM (P = 0.25). Also, total fusion was not statistically different in the presence or absence of histologically established fatty streaks in the sinus of Valsalva (P = 0.25).
Figure 4:
Correlation between total fusion and selected characteristics. Relationship between total fusion (mm) and (A) age (years) (R2 = 0; P = 0.94) and (B) duration of cf-LVAD support (days) (R2 = 0.1; P = 0.18). (C) The length of fusion (mm) for three different commissures (P = 0.06). Horizontal line indicates the median value.
Figure 5:
Clinical characteristics and total fusion. Total fusion (mm) of aortic valve commissural fusion stratified for: (A) degree of cf-LVAD support (P = 0.03); (B) aortic valve competence•; (C) aetiology of heart failure (P = 0.25); (D) fatty streaks in sinus (P = 0.25). Horizontal lines indicate the median values.•Low sample size did not allow statistical analysis.
Fourteen of 19 patients had undergone at least one pump speed change procedure, to clinically assess opening of the aortic valve at minimum pump speed settings. The aortic valve remained closed in one patient at the lowest pump speed and was partially opening in 3 other patients. In the other 10 patients, echocardiography evaluation at minimum pump speed showed good opening of the valve (Table 1). Fusion was confirmed in all cases with partial opening and no aortic valve opening at minimum pump speed, but also in 4 cases who had revealed good aortic valve opening by echocardiographic at minimum speed. The aortic valves did not show increased echo density in any of the cases.
In retrospect, none of the patients who had undergone heart transplantation had any clinical adverse events that could be related to the aortic valve pathology, other than mild LVAD-induced AI. Of the 5 patients who died during cf-LVAD support, three had pump obstruction probably due to thrombus formation. In these 3 cases, commissural fusion was an additional finding, which potentially might have played a role in the cause of death. Of the other 2 patients, 1 died of right ventricular failure and the other patient died of ischaemic stroke. Both cases could not be associated with aortic valve commissural fusion. An overview of all aortic valves examined is shown in Supplementary Fig. S2.
DISCUSSION
We demonstrate that aortic valve commissural fusion occurred in 58% of patients after support with the newest generation of cf-LVADs in this small cohort. Although LVAD-induced aortic valve fusion has been described after support with pulsatile devices, literature on the incidence after support with the newest generation cf-LVADs is limited. Morphological and histological analysis demonstrated that aortic valve commissural fusion manifested along with varying changes in valve layer structure, including the endothelial and connective tissue layers, without evidence of inflammatory infiltration on the site of fusion. In addition, we demonstrated for the first time that patients on full support of the cf-LVAD in which the aortic valve remains continuously closed have a significantly higher risk of pathologically confirmed commissural fusion than patients on partial support. Aortic insufficiency had often developed following cf-LVAD implantation in patients that in retrospect revealed aortic valve commissural fusion. The amount of commissural fusion was not significantly associated with age, duration of cf-LVAD support, aetiology of heart failure and the presence of fatty streaks in the sinus of Valsalva. The fusion was similar among the three leaflets.
As there is no evidence in literature associating commissural fusion with chronic heart failure, this commissural fusion must be induced by LVAD support. In contrast to degenerative aortic disease, aortic valve commissural fusion in cf-LVAD patients does not show a correlation with age [1,5]. Aortic valve commissural fusion has been reported in association with P-LVADs, but is less well reported for cf-LVADs patients. Rose et al. [8] first described aortic valve commissural fusion in 4 of 6 patients with a P-LVADs. Subsequently, Letsou et al. [12] reported 17 of 33 patients with aortic valve fusion. Other authors have also reported cases of aortic valve commissural fusion, all based on P-LVADs [11–13, 16]. In all these studies, aortic valve commissural fusion occurred in ∼50% of patients with P-LVADs, which was similar to our study based on 19 patients with cf-LVADs. In contrast, Mudd et al. [7] reported that 8 (89%) of 9 patients with the HeartMate II cf-LVAD had aortic valve commissural fusion, which was an even higher percentage. Yet, none of previous papers had shown the statistical effect of full support vs partial support on the development of aortic valve commissural fusion.
The mechanism associated with the development of commissural fusion is not well understood. The changes of aortic valve morphology appeared to progress in multiple stages, from minor to more extensive fusion. In cases with minor fusion, the endothelial layer was absent and the ventricularis layer of both leaflets simply seemed to be grown together. Probably, this fusion process is induced by the fact that the aortic valve leaflets lie constantly against each other for extended time. Under these circumstances, both endothelial cell layers that cover this area of the leaflets are not subjected to shear stress. As a result, this may induce a stimulus initiating fusion, after which the endothelial cell layers would disappear. Accordingly, a significant association was found between full LVAD support without valve opening and commissural fusion, which supports this theory. However, when the aortic valve displayed more extensive fusion, miscellaneous disruption of the elastin layer of the leaflet was observed. Also, an increase in extracellular matrix of the site of fusion was found, suggesting a mild fibrotic reaction in more advanced stages. No inflammatory infiltrates were seen at the site of fusion, suggesting that the fusion process is not driven by inflammation. Therefore, the susceptibility of the immobilized aortic valve may be an important substrate for tissue remodelling causing leaflets to fuse. In addition, the valves are also exposed to continuous pressure with reduced pulsatility during cf-LVAD support. The effect of such a biomechanical stimulus may play an additional role in inducing or accelerating commissural fusion, as suggested previously by May-Newman et al. [17]
Acquired commissural fusion may have important clinical consequences throughout cf-LVAD support. In the first place, commissural fusion may lead to valve disruption and dysfunction, resulting in valve incompetence. LVAD-induced AI may cause recirculation of flow through the leaking valve, leading to less peripheral flow to body organs. To compensate for this, the pump speed may have to be increased. Aortic valve commissural fusion may also cause functional aortic stenosis. Such a stenosis limits flow through the valve during exercise, when the valve is most likely to open during LVAD support [18]. In addition, commissural fusion may hamper output of the native heart after device removal following myocardial recovery [1]. Finally, in the possible event of a flow obstruction of the cf-LVAD, temporary pump stop or failure, it also may limit effective output by the native ventricle [19].
It has been recommended that the function of the aortic valve be assessed regularly, through either transthoracic or transoesophageal echocardiography [10]. In our study, transthoracic echocardiography assessment did not always lead to suspicion of valve fusion, despite the presence of fusion after gross examination. We performed echo-guided pump speed change procedures to assess aortic valve function at minimum pump speed of the cf-LVAD. Because of a different structural consistency, fusion may not be identifiable during echocardiography, unlike calcific aortic valve stenosis. If valve opening is not observed or less pronounced at such low pump speeds, suspicion of aortic valve commissural fusion may rise. However, partially fused aortic valves may still open, as seen in a clinical case with an obstructed cf-LVAD (see Supplementary Fig. S1). Furthermore, rather as a result of fusion, the aortic valve may also remain closed due to extremely poor ventricular function at the lowest pump speed.
Our results for the first time demonstrate that partial support is statistically associated with the absence of aortic valve commissural fusion. Therefore, periodic decrease of cf-LVAD pump speed and aortic valve reopening may reduce the likelihood of aortic valve commissural fusion. The application of intermittent low speed on the cf-LVAD, as recently evaluated in animal experiments, promoted aortic valve opening and might prevent complications associated with leaflet fusion [20]. The efficacy of such an algorithm on the development of aortic valve commissural fusion has yet to be demonstrated in patients.
We acknowledge the following limitations of the current study. First of all, this study is limited by the small sample size, which makes it difficult to draw substantial conclusions. Due to increasing amount of patients who remained on cf-LVAD support for larger periods of time sample collection is time consuming. Due to this small sample size, we were not able to perform multivariate testing. Macroscopic inspection took place after fixation with formaldehyde, which may have affected anatomical tissue structures. Occasionally, aortic valve functional properties of some patients could not be judged by echocardiography due to unavailability or poor image quality. Patients with a total fusion of ≤3 mm where not included in the group of patients with aortic valve commissural fusion, so we might have underestimated this phenomenon. Also, a pump speed change procedure was performed at fixed intervals after support and long periods between echocardiography evaluation and heart transplantation or autopsy remained in some cases. Thus, results regarding aortic valve dynamics at minimal pump speed may not always reflect macroscopic and histological findings after heart transplantation or autopsy. Finally, we did not consider the effect of biomechanical properties and cardiovascular haemodynamics on the incidence of aortic valve commissural fusion.
In summary, cf-LVAD support induces aortic valve commissural fusion in >50% of patients due to a non-inflammatory process leading to changes in valve layer structure. The development of commissural fusion is significantly associated with full support while the aortic valve is continuously closed. Clinical consequences may involve reduced efficacy of cardiac and circulatory support on cf-LVADs. Therefore, improved in vivo assessment of commissural fusion is warranted, and strategies improving aortic valve mobility, like alternating pump speeds, may prevent commissural fusion.
Supplementary material
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
REFERENCES
- 1.Birks EJ, George RS, Hedger M, Bahrami T, Wilton P, Bowles CT, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–90. doi: 10.1161/CIRCULATIONAHA.109.933960. [DOI] [PubMed] [Google Scholar]
- 2.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–21. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: patient selection and outcomes. Curr Opin Cardiol. 2011;26:232–6. doi: 10.1097/HCO.0b013e328345aff4. [DOI] [PubMed] [Google Scholar]
- 4.Popov AF, Hosseini MT, Zych B, Mohite P, Hards R, Krueger H, et al. Clinical experience with HeartWare left ventricular assist device in patients with end-stage heart failure. Ann Thorac Surg. 2012;93:810–5. doi: 10.1016/j.athoracsur.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 5.Lahpor JR. State of the art: implantable ventricular assist devices. Curr Opin Organ Transplant. 2009;14:554–9. doi: 10.1097/MOT.0b013e3283303750. [DOI] [PubMed] [Google Scholar]
- 6.Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010;3:668–74. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mudd JO, Cuda JD, Halushka M, Soderlund KA, Conte JV, Russell SD. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J Heart Lung Transplant. 2008;27:1269–74. doi: 10.1016/j.healun.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Rose AG, Park SJ, Bank AJ, Miller LW. Partial aortic valve fusion induced by left ventricular assist device. Ann Thorac Surg. 2000;70:1270–4. doi: 10.1016/s0003-4975(00)01929-9. [DOI] [PubMed] [Google Scholar]
- 9.Rose AG. Etiology of valvular heart disease. Curr Opin Cardiol. 1996;11:98–113. doi: 10.1097/00001573-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Banchs JE, Dawn B, Abdel-Latif A, Qureshi A, Agrawal N, Bouvette M, et al. Acquired aortic cusp fusion after chronic left ventricular assist device support. J Am Soc Echocardiogr. 2006;19:1401–3. doi: 10.1016/j.echo.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 11.May-Newman K, Mendoza A, Abulon DJ, Joshi M, Kunda A, Dembitsky W. Geometry and fusion of aortic valves from pulsatile flow ventricular assist device patients. J Heart Valve Dis. 2011;20:149–58. [PubMed] [Google Scholar]
- 12.Letsou GV, Connelly JH, Delgado RM, III, Myers TJ, Gregoric ID, Smart FW, et al. Is native aortic valve commissural fusion in patients with long-term left ventricular assist devices associated with clinically important aortic insufficiency? J Heart Lung Transplant. 2006;25:395–9. doi: 10.1016/j.healun.2005.11.451. [DOI] [PubMed] [Google Scholar]
- 13.Connelly JH, Abrams J, Klima T, Vaughn WK, Frazier OH. Acquired commissural fusion of aortic valves in patients with left ventricular assist devices. J Heart Lung Transplant. 2003;22:1291–5. doi: 10.1016/s1053-2498(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 14.Gallen TB, Lau WT, Mehta AR. Complete aortic valve fusion After HeartMate II left ventricular assist device support. J Cardiothorac Vasc Anesth. 2012;26:1060–2. doi: 10.1053/j.jvca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura M, Ohtake S, Sawa Y, Fukushima N, Matsumiya G, Matsuda H. Severe aortic valve fusion after nearly three years of support with the Novacor left ventricular assist system. J Thorac Cardiovasc Surg. 2002;124:179–80. doi: 10.1067/mtc.2002.122349. [DOI] [PubMed] [Google Scholar]
- 17.May-Newman K, Enriquez-Almaguer L, Posuwattanakul P, Dembitsky W. Biomechanics of the aortic valve in the continuous flow VAD-assisted heart. ASAIO J. 2010;56:301–8. doi: 10.1097/MAT.0b013e3181e321da. [DOI] [PubMed] [Google Scholar]
- 18.Andersen M, Gustafsson F, Madsen PL, Brassard P, Jensen AS, Secher N, et al. Hemodynamic stress echocardiography in patients supported with a continuous-flow left ventricular assist device. JACC Cardiovasc Imaging. 2010;3:854–9. doi: 10.1016/j.jcmg.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Feldman CM, Silver MA, Sobieski MA, Slaughter MS. Management of aortic insufficiency with continuous flow left ventricular assist devices: bioprosthetic valve replacement. J Heart Lung Transplant. 2006;25:1410–2. doi: 10.1016/j.healun.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Tuzun E, Gregoric ID, Conger JL, Golden K, Jarvik R, Frazier OH, et al. The effect of intermittent low speed mode upon aortic valve opening in calves supported with a Jarvik 2000 axial flow device. ASAIO J. 2005;51:139–43. doi: 10.1097/01.mat.0000155708.75802.c7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.