Abstract
OBJECTIVES
Lung transplant recipients were reviewed to compare our early and current experience of vascular complications. Since 1995, we have had a policy of early identification and intervention.
METHODS
We undertook a retrospective review of all adult lung transplants performed at our centre. Patients with pulmonary vascular complications before and after 1995 were identified and reviewed to determine changes in management and outcome.
RESULTS
We identified a total of 13 patients with either pulmonary artery or venous obstruction out of a total of 720 adult lung transplants (1.8%). There were 9 females and 4 males with an age range of 25–64 years. Complications were more common in patients with fibrotic lung disease and involved 15 vascular anastomoses, most commonly the pulmonary arterial anastomosis. Prior to 1995, 5 cases were identified, all postoperatively. In this group, the mean time for identification of the complication was 9.4 (range 4–14) days. Only 1 patient survived to discharge. After 1995, vascular complications were identified intraoperatively in 4 cases and corrected immediately. Four cases were identified postoperatively (at <1–17 days) by a computed tomography pulmonary angiogram. Three were treated surgically within 24 h of diagnosis (using cardiopulmonary bypass with cold preservation). One patient was managed conservatively. Among patients identified after 1995, 5 survived to discharge.
CONCLUSIONS
Though rare, pulmonary vascular complications after lung transplantation carry high mortality. In our opinion, early identification and intervention improves outcome. Intraoperative assessment by pressure gradient measurement and transoesophageal echocardiography is recommended. Despite this, mortality remains high and prevention is better than cure.
Keywords: Lung transplantation, Vascular stenosis
INTRODUCTION
Vascular complications after lung transplantation occur in 1.75% of vascular anastomoses [1]. Only a limited number of series have been reported in the literature [1–3]. Vascular anastomotic complications are associated with high morbidity and mortality [1]. Donor–recipient size mismatch, surgical technique, twisting, stricture or thrombosis at the arterial or venous anastomosis have been cited as causes.
Pulmonary vascular compromise should be suspected when the patient experiences unexplained hypoxia, particularly in conjunction with pulmonary hypertension. This may be accompanied by haemodynamic compromise. Pulmonary venous obstruction additionally causes pulmonary oedema (clinical and radiographic) due to venous congestion. The differential diagnosis includes primary graft dysfunction, infection, rejection and myocardial dysfunction.
The risk of pulmonary infarction is greatest in the immediate postoperative period, because the newly transplanted lung does not have an alternate pathway for bronchial circulation. Four–six hours of warm ischaemia may be sufficient to cause irreversible damage to the allograft [3]. Based on this understanding, after publication of our early experience up to 1995, we have adopted a proactive policy of early identification and intervention for vascular anastomotic complications. We sought to examine and update our experiences in the management of vascular complications in lung transplantation.
MATERIALS AND METHODS
A retrospective review of all adult lung transplant recipients at the Freeman Hospital from the programme inception in 1987 to 31st November 2011 was performed. Patients with pulmonary vascular complications were identified and reviewed. Fisher's exact test was used to perform univariate analysis comparing lung transplant recipients who had vascular complications with recipients who did not.
Both pulmonary arterial and venous anastomoses are fashioned using a continuous suture technique. The arterial anastomosis is fashioned with 5-0 monofilament suture, and the pulmonary artery is divided on both recipient and donor proximal to the first arterial branch except in the case of dilated arteries with size mismatch. The venous anastomosis is constructed with 4-0 monofilament suture and utilizes a standard left atrial cuff technique to create a wide confluent anastomosis.
The reports of the 8 cases since 1995 follow. A full description of the cases prior to 1995 has already been published [1].
Patient 1
A 25-year old man underwent bilateral sequential lung transplantation for bronchiectasis. At the end of the implantation, the left pulmonary artery and the left pulmonary vein anastomoses were found to be compromised with distortion and kinking. This was confirmed by intraoperative pressure gradient measurement. Both left-sided vascular anastomoses were refashioned on cardiopulmonary bypass. The total ischaemic time was 504 min, with a cardiopulmonary bypass time of 312 min. The patient did very well postoperatively with a critical care stay of 2 days and no major complications. He survived for a further 15 years after transplantation.
Patient 2
A 40-year old female with cystic fibrosis underwent bilateral sequential lung transplantation. Intraoperative imaging with transoesophageal echocardiography (TOE) together with inspection and direct pressure measurement revealed a right pulmonary artery anastomotic stricture. The right pulmonary artery anastomosis was refashioned with the aid of cardiopulmonary bypass. Her postoperative course was difficult. She required re-exploration for haemorrhage and went on to develop evidence of right heart failure. An intra-aortic balloon pump was placed. She developed significant renal dysfunction and required renal replacement therapy in the form of continuous veno-venous haemofiltration. Her stay in critical care extended to 7 days. She was discharged home and has been followed up for nearly 10 years.
Patient 3
A 55-year old female with fibrotic lung disease underwent right single-lung transplantation. The ischaemic time was 330 min. The patient made a good initial recovery from her operation and was discharged from critical care after 48 h. She subsequently developed a right-sided pneumothorax after removal of her chest drains and required re-admission to the critical care unit for a short period of time. Her progress was slow but satisfactory. She went on to have a computed tomography (CT) pulmonary angiogram performed on Day 17 that demonstrated minor narrowing of the right pulmonary anastomosis. This was felt to be not significant and she was managed conservatively with no further intervention required. She survived for a total of 14 months post-transplantation.
Patient 4
A 51-year old man with fibrosing alveolitis underwent bilateral sequential lung transplantation. Intraoperative imaging with TOE combined with inspection and direct pressure measurement confirmed the presence of distortion and kinking of the left pulmonary artery anastomosis. The anastomosis was surgically refashioned. He developed sepsis and multiorgan failure. A tracheostomy was performed, but despite all supportive measures, the patient passed away on the 14th postoperative day.
Patient 5
A 46-year old female with fibrotic lung disease underwent bilateral lobar lung transplantation. The operation was performed with cardiopulmonary bypass, and the ischaemic times for the lungs were 138 and 80 min, respectively, for the right and left sides. After weaning from bypass, pulmonary hypertension was noted along with a significant gradient across both the left and right pulmonary venous anastomoses on direct pressure measurement. The left and then the right were sequentially refashioned. The patient remained unwell on the intensive care unit and died on the 12th postoperative day with pulmonary oedema and right heart failure.
Patient 6
A 64-year old female with fibrotic lung disease underwent left single-lung transplantation. Her condition had deteriorated while on the waiting list. The operation was complicated by donor–recipient mismatch, and the donor lung was therefore reduced to allow a good fit and adequate expansion. The ischaemic time for the lungs was acceptable at 254 min, and the operation was performed through a sternotomy with the aid of cardiopulmonary bypass. At the end of the operation, the sternum was initially left open. The patient was stabilized and returned to theatre after 36 h to have the chest closed. Intraoperative TOE appeared satisfactory. Her progress remained suboptimal, with poor gas exchange and she developed infiltrates on the chest X-ray. A bronchoscopy and biopsy demonstrated a satisfactory appearance of the bronchial anastomosis and no evidence of acute rejection. She remained ventilator dependent and a CT pulmonary angiogram was organized on Day 10. This demonstrated a kink in the tortuous left pulmonary artery reducing the lumen to 6 mm. The patient was returned to theatre the same day for reconstruction of the left pulmonary artery anastomosis. This was performed without complication. The patient then went on to make a good recovery and has been followed up successfully after transplantation for a total of 58 months.
Patient 7
A 37-year old female with pulmonary artery hypertension underwent bilateral sequential lung transplantation. Her operation was performed with a clamshell incision on cardiopulmonary bypass. The cardiopulmonary bypass time was 228 min with an ischaemic time of 320 min. The procedure was uncomplicated. However, on return to the critical care unit, there was a decline in gas exchange and rising airway pressures on the ventilator. A CT pulmonary angiogram performed the next morning, demonstrating a kink in the left pulmonary artery anastomosis. The patient was returned to theatre immediately and the left pulmonary artery anastomosis was reconstructed. Her progress was subsequently unremarkable and she made a good recovery allowing her discharge from hospital. She has been followed up for just under a year.
Patient 8
A 52-year old male with idiopathic pulmonary fibrosis underwent right single-lung transplantation. The operation was performed without cardiopulmonary bypass and the ischaemic time was 226 min. The patients' initial progress allowed extubation on the second postoperative day with an arterial oxygen level of 12.2 kPa and a satisfactory chest X-ray. Increasing hypoxia was noted with the development of infiltrative changes on the right side of the chest X-ray. The patient was reintubated on the fifth postoperative day, and a TOE was performed to exclude vascular anastomotic complications. This appeared satisfactory with good pulmonary venous flow. He failed to improve and a CT pulmonary angiogram on Day 7 demonstrated a critical right pulmonary artery stenosis (Fig. 1). Surgical revision of the anastomosis was preferred to pulmonary artery stenting due to the short time interval from the transplant and the anatomy of the stenosis. The patient returned to theatre and underwent a median sternotomy with cardiopulmonary bypass and revision of the right pulmonary artery anastomosis. Gas exchange remained poor and a tracheostomy was performed on the 10th postoperative day. He remained critical, eventually developing a systemic inflammatory response and died from multiorgan failure on Day 21 post-transplantation.
Figure 1:
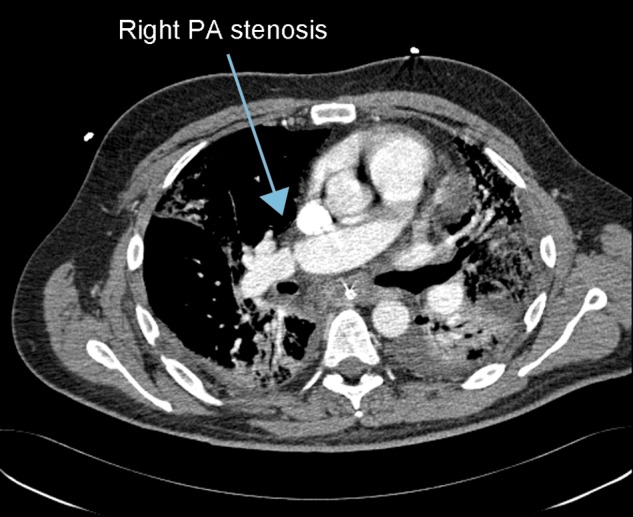
Computed tomogram angiography demonstrating significant narrowing and stenosis of the right pulmonary artery.
RESULTS
A total of 720 lung transplants were performed with 386 males (54%). Two hundred and eighty-nine patients had single-lung transplants, 425 had bilateral lung transplants, 3 had bilateral lobar lung transplants, 2 had bilateral lung and liver transplants and 1 had a double-lung transplant with a tracheal anastomosis. The underlying diagnosis leading to transplantation was cystic fibrosis in 35%, obstructive airway disease in 29% and fibrotic lung disease in 19% with other aetiologies making up the remaining 17%. Among these 720 patients, we identified a total of 13 patients with either pulmonary artery or venous obstruction (1.8%). There were 9 females and 4 males in the age range 25–64 years. Fibrotic lung disease was the diagnosis in 8 of these patients (62%) and this was highly significant on univariate analysis (P = 0007).
The following lung transplant operations had anastomotic complications: 7 single, 5 bilateral and 1 bilateral lobar lung transplant. We found vascular anastomotic compromise in the following sites: 5 right pulmonary arteries, 5 left pulmonary arteries, 1 left pulmonary vein, 1 with both right and left pulmonary veins and 1 combined left pulmonary artery and vein.
Prior to 1995, all the 5 cases were identified postoperatively. Radio-nucleotide perfusion scans suggested the diagnosis in 3 cases. Pulmonary angiogram was used to confirm these diagnoses and in the other 2 cases, it was used as the primary diagnostic modality. The mean time for identification of the complication was 9.4 (range 4–14) days. Interventions included surgical revision of the pulmonary stenosis in 3 cases (warm ischaemia in 1 case and cardiopulmonary bypass with cold flush in 2) with subsequent reintervention, angioplasty and stent insertion in 1 of these cases. Two patients died before any intervention. Only 1 patient with multiple interventions survived to discharge.
Since 1995, there have been another 8 cases. Four of these were identified intraoperatively with inspection, TOE and/or direct pressure measurement across the anastomosis. These anastomoses were corrected immediately. Four cases were identified postoperatively (range <1–17 days) by CT pulmonary angiogram. Three were treated surgically with revision of the anastomosis within 24 h of diagnosis (cardiopulmonary bypass with cold flush). One patient with minor narrowing of the pulmonary artery anastomosis, identified on Day 17, was managed conservatively. Five of these patients survived to hospital discharge.
DISCUSSION
We report a large series of 720 patients undergoing lung transplantation with an overall incidence of vascular complications of 1.8%. Griffith et al. [2] reported seven vascular complications in 134 patients (5.2%) in their early experience. More recently, Schulman et al. [4] prospectively studied 87 lung transplant recipients and reported a 15% incidence of venous thrombosis. Notably, they reported no cases of anatomic obstruction that comprise our experience. Imaging with TOE in their study was performed within 48 h of the operation, and functional compromise of the graft was not present in all cases. In addition, when graft compromise was present, they were uncertain which occurred first—venous thrombosis or graft failure. It is unclear why the incidence and type of complications reported by Schulman et al. are so different from ours, but differences in study design, surgical technique, timing of investigation and clinical significance likely play a role. In our experience, the incidence of these complications has not changed dramatically from the period before 1995 to date. However, after 1995, we were able to identify a number of patients with complications intraoperatively with the routine use of TOE and direct pressure measurements across the anastomosis.
It is conceivable that conditions and approaches that make the anastomoses technically more challenging would increase the incidence of anastomotic complications [5–7]. In our series, 8 patients had restrictive pathologies due to fibrotic lung disease and this finding was significant. There was also a trend towards a higher incidence of vascular complications in female patients (data not shown). This has been suggested previously and may be related to a smaller thoracic cavity and smaller vascular structures making anastomoses more challenging to construct [1]. Additionally, in our previous series, the surgery was noted to be particularly challenging in 1 patient due to size and kyphoscoliosis, and in this series, there was one instance (Patient 5) of venous anastomotic obstruction in a bilateral lobar recipient and one instance (Patient 6) of donor–recipient size mismatch [1]. At the time we had limited experience with the technical challenges of lobar lung transplantation and this early experience contributed to our abandonment of the technique. There have been several reports of vascular complications in the setting of lobar transplantation [8–10].
Patients receiving bilateral transplants may have a degree of unilateral vascular obstruction that is undetected due to compensation by the other lung, while single-lung transplant recipients do not have a contralateral functional lung to compensate for a failure in the allograft. In theory, this may increase the clinical incidence of vascular complications in single-lung recipients; however, we did not demonstrate a significant difference between single and bilateral recipients in this series.
Complications involving the pulmonary artery are more common in our series than those involving the pulmonary venous anastomosis. This can be attributed to technical aspects and differences between the construction of the two anastomoses. The arterial anastomosis is more prone to poor orientation, narrowing or kinking due to excessive length. The use of an atrial cuff for the venous anastomosis makes it easier to orient and also leads to a large anastomosis that is more forgiving and less likely to impact on individual venous drainage.
We have adopted intraoperative TOE as standard practice to check the anastomoses and venous drainage. Although TOE has aided in intraoperative detection of vascular complications, it is not failsafe. It is accepted that the left pulmonary anastomosis is sometimes poorly visualized on TOE, and in one reported series the left pulmonary anastomoses could not be seen in any of the 18 cases [8, 11]. In 2 cases in this series (Patients 6 and 8), the TOE was deemed satisfactory yet CT scan subsequently diagnosed pulmonary artery stenosis. Pulmonary venous anastomoses and flow may also be difficult to identify and quantitate with TOE. To overcome these difficulties Felten et al. have recently advocated the use of intraoperative contact echocardiography [12]. Instead, as a simple, quick and cost-effective alternative, we would advocate direct pressure gradient measurement across the anastomosis using a needle manometry line. These must be measured at a period of haemodynamic stability and could be incorporated into routine practice. It should certainly be considered in any technically difficult anastomosis or in single-lung transplants in which there is more at stake if an anastomotic complication arises. Intraoperative detection with these techniques allows immediate surgical correction. While routine use of ventilation/perfusion (V/Q) scans within the first 24 h has been employed by some centres, this does not allow for intraoperative correction, and may be unnecessary for the majority of patients and incurs more cost.
Persistent hypoxaemia in the early postoperative period, especially if accompanied by pulmonary hypertension, necessitates further investigation. This may take the form of V/Q scanning, CT imaging or pulmonary angiography. In our practice, CT pulmonary angiography is the investigation of choice. High-resolution CT imaging has evolved during the course of this series, with post-processing techniques—multiplanar reformations, volume rendering, virtual bronchoscopy and minimum-intensity-projection and maximum-intensity-projection images allowing more accurate postoperative evaluation [13]. It defines the extent and degree of stenoses and also provides information about the common differential diagnoses for hypoxaemia by visualizing the lung parenchyma and pleural spaces. It is readily obtained and interpreted and is less invasive in comparison with angiography. Although used routinely prior to 1995, pulmonary angiography is now reserved for instances in which a catheter-based intervention may be required.
In our experience, vascular anastomotic complications have been of three types. Type I may be described as a kinking of the anastomosis leading to obstruction and is due to excessive length of the vascular structure or hilar misalignment. Type II is a problem of orientation and can result from inversion of the donor vessel with respect to the recipient. Type III is a true stricture of the anastomosis caused by overzealous tightening of the suture line or misplacement of sutures to narrow the anastomosis. A fourth and fifth types may be included from the literature. Intraluminal obstruction to flow may occur from thrombosis (IVa) or dissection (IVb) [3, 4, 7]. Type V is due to an extraluminal mass lesion and has been reported with the use of omental pedicles [14].
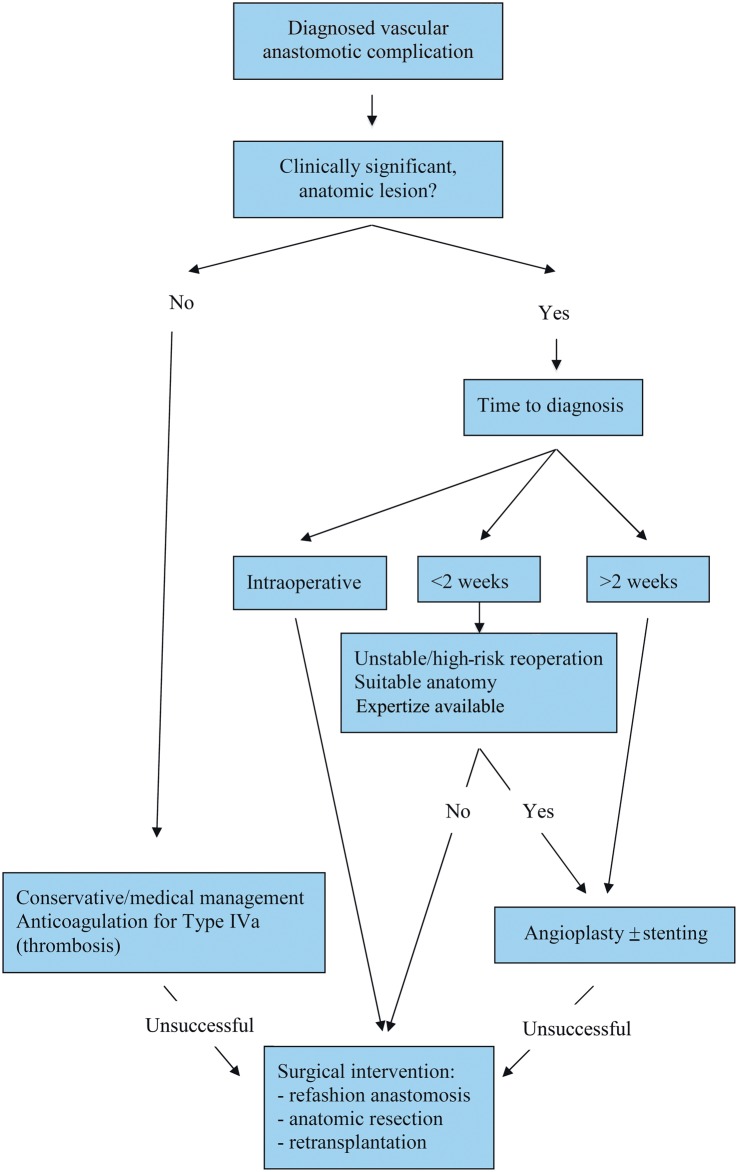
Current treatments for vascular complications include conservative or medical management, catheter-based interventions and surgical revision. At present, there is no strong evidence base to formulate concrete recommendations; however, certain guidelines for treatment can be inferred from our and others experiences (Fig. 2). Mild obstructions to flow may be treated without intervention, particularly in the setting of a bilateral transplant in which only one of the transplanted lungs is affected. For an anastomotic thrombosis, the initial treatment should consist of anticoagulation if tolerated, unless infarction of the graft due to severe thrombosis is anticipated. In that setting, surgical intervention may be warranted, though it carries a poor prognosis [4, 15].
Figure 2:
Algorithm for the management of vascular anastomotic complications.
Once identified, a significant anatomic lesion in the arterial or venous anastomosis accompanied by functional compromise of the graft necessitates urgent intervention. The type of intervention, catheter-based or surgical, will be guided by the time that has elapsed from implantation to diagnosis, the anatomy of the lesion, the perceived likelihood of the patient to tolerate a reoperation and the available expertize. Clearly, cases identified intraoperatively can be corrected while in the operating room. Those identified after several weeks are best treated with endovascular techniques, and several groups have reported successful interventions in this setting [16–20]. Although angioplasty without prosthesis has been reported by Shoji et al. [9], commonly pulmonary arterial lesions require stent placement due to the elasticity of the lesions. Stent placement has also been reported for venous stenoses utilizing a trans-septal approach but, to our knowledge, not in the setting of lung transplantation [21].
A grey area exists for patients indentified with anastomotic complications within the first 2 weeks after transplant. We and others have been hesitant to pursue early catheter-based interventions in these patients out of concern for the integrity of the anastomosis [22, 23]. However, reports do exist of successful stent placement in this early postoperative period [6, 10]. In both cases, the authors were cognizant of the proximity to surgery; additional care was taken at the time of the procedure and this influenced the stent employed. Conversely, the potential for stent-related complications such as migration, thrombosis, restenosis and embolization does exist and needs to be considered [24]. Patients diagnosed at this early stage may have severe respiratory and haemodynamic compromise, and this may tip the balance in favour of an attempt at endovascular intervention if the anatomy seems amenable and the expertize is available. A Type II or misorientation lesion (as in Patient 8) may necessitate surgical correction.
If surgical revision is considered, protection of the transplanted lung from warm ischaemia is recommended. This is best achieved by cooling on cardiopulmonary bypass and lung preservation with cold pulmoplegic solution as previously described [1]. Occasionally, in the setting of prolonged ischaemia with infarction, anatomic resection may be necessary and retransplantation has also been reported in this setting [14].
Our study is limited by the infrequency with which vascular complications occur and this has affected our ability to perform an analysis of predisposing factors and best interventions. Indeed, the low incidence coupled with a possible reluctance to report adverse events means that it is unlikely that there will be a series sufficiently large to enable definitive conclusions to be drawn. Accordingly, there is a paucity of guidance for surgeons on the practicalities of intra- and postoperative assessments and interventions for vascular complications.
In summary, vascular anastomotic complications at the time of lung transplantation are associated with significant morbidity and mortality and this warrants their consideration. In our series, fibrotic lung disease was associated with the occurrence of these complications and early identification appeared to improve the outcome. Further developments in technology, such as flow metres or contact echocardiography, may aid in intraoperative diagnosis. Strict adherence to the best surgical technique, direct pressure measurements across the anastomosis, TOE and early investigation with CT should result in the prevention and early detection of these complications and facilitate their successful treatment.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr J. Bekkers (Rotterdam, Netherlands): You describe a large series of lung transplantation with a 2.1% overall incidence of vascular anastomotic complications, so this is not a frequent complication. On the other hand, once this complication occurs, the chance of a bad outcome is high. In your series, the total mortality of these patients is more than 50%, indicating the high likelihood of a bad outcome. Therefore, you are to be commended for your studies on this subject and for bringing it to our attention again.
You mentioned that in 1995 you introduced intraoperative evaluation of vascular anastomoses by TOE and by intraoperative pressure measurement as a routine. By means of these techniques, you were able to identify these complications in four patients. However, since the introduction of these routine measurements, in four other patients the vascular complications were detected postoperatively, and of those four patients in whom they were detected postoperatively, three eventually underwent reoperation.
I have three questions for you. Since 1995, have you routinely evaluated all of your patients with this technique, and as there were still four patients in whom you did not detect this complication intraoperatively, did you see any reasons or any factors to explain why you couldn't identify this in those four patients?
Dr Ozalp: There are a few reasons for that. One is that TOE is very operator-dependent and it doesn't always show all the anastomoses we want to see. The left pulmonary artery, and sometimes also the right pulmonary artery, is particularly difficult to visualize on TOE. That is number one. During the reperfusion time after transplantation, the transvenous pressures across the anastomoses are quite low, and it is then difficult for a stenosis to make itself significant when the pressures are quite low at this initial period. Those are the two main reasons why they are under-diagnosed. In those series it is 50%. But if you look at the overall number of transplants and the complications, before 1995 we operated on very few. I don't have the exact figures, but the percentage of complications is much higher, 3%, 4%. After 1995, the complication rate is around 1%, and also the survival is so much better. Before 1995, it was 80% mortality (1 in 5 survived), and after 1995, only three out of eight died.
Dr Bekkers: That brings me to my second question. You indicated that before 1995, almost all of your patients died, all patients in whom it was detected postoperatively. Of the four patients where you detected the complication intraoperatively, from your manuscript I read that two of those died. Of the four patients where you detected it postoperatively, three patients survived. So were there perhaps other factors contributing to this improved survival for those patients where you still detected these anastomotic complications postoperatively?
Dr Ozalp: If you detect them as early as possible and operate on them as soon as you detect, they tend to do better.
Dr Bekkers: And that was not the case before 1995 perhaps?
Dr Ozalp: Well, the mean time of detection before 1995 was 9 days, and the patients would probably have sustained enough damage to not survive any further intervention.
Dr Bekkers: I have one final question. Do you see any relationship between the technique of lobar transplantation and the possibility of getting these complications?
Dr Ozalp: As we are not doing lobar transplantation very often these days, our experience in that is quite limited, so I would not be able to comment on that.
Dr L. Voltolini (Sienna, Italy): When you bring the patient back to the operating room, what is your surgical strategy? Do you prefer either to cool the lungs again, to flush the lung with the preservation solution, or to put the patient on bypass and cool the lungs, or do you accept a period of warm ischaemia? I am not sure.
Dr Ozalp: No, we do not like to accept warm ischaemia. We like to put them back on bypass and cool the lungs and have the cold flush.
Dr Voltolini: The other thing, the venous problem, I think it is different in terms of results, because you probably find that the lung is infarcted, and even if you can fix the problem, you could still have problems with the function. Is this true? Have you observed different results depending on which one was the problem, the PA or the atrial cuff?
Dr Ozalp: The atrium is usually not a big problem because you are stitching a big cuff of both the atrium and the pulmonary veins, and in our experience, only two cases out of the 13 were PVs. The pulmonary artery is a bit more difficult technically, especially if there is any kind of mismatch between donor and recipient. In our experience, we have also seen that these complications appear more often in patients who have a very tight chest, for example fibrotic patients, and therefore technically more difficult. The pulmonary artery can distort and kink very easily, its tissues are more friable and can become narrowed much more readily.
REFERENCES
- 1.Clark SC, Levine AJ, Hasan A, Hilton CJ, Forty J, Dark JH. Vascular complications of lung transplantation. Ann Thorac Surg. 1996;61:1079–82. doi: 10.1016/0003-4975(96)00003-3. doi:10.1016/0003-4975(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 2.Griffith BP, Magee MJ, Gonzalez IF, Houel R, Armitage JM, Hardesty RL, et al. Anastomotic pitfalls in lung transplantation. J Thorac Cardiovasc Surg. 1994;107:743–53. [PubMed] [Google Scholar]
- 3.González-Fernández C, González-Castro A, Rodríguez-Borregán JC, López-Sánchez M, Suberviola B, Francisco Nistal J, et al. Pulmonary venous obstruction after lung transplantation. Diagnostic advantages of transesophageal echocardiography. Clin Transplant. 2009;23:975–80. doi: 10.1111/j.1399-0012.2009.01078.x. doi:10.1111/j.1399-0012.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 4.Schulman LL, Anandarangam T, Leibowitz DW, Ditullio MR, McGregor CC, Galantowicz ME, et al. Incidence of pulmonary vein complications after lung transplantation: a prospective transesophageal echocardiographic study. J Am Soc Echocardiogr. 2001;14:806–12. doi: 10.1067/mje.2001.111855. doi:10.1067/mje.2001.111855. [DOI] [PubMed] [Google Scholar]
- 5.Fadel BM, Abdulbaki K, Nambiar V, Al Amri M, Shahid M, Khouqeer F, et al. Dual thrombosis of the pulmonary arterial and venous anastomotic sites after single lung transplantation: role of transesophageal echocardiography in diagnosis and management. J Am Soc Echocardiogr. 2007;20:438. doi: 10.1016/j.echo.2006.10.024. e9–12. [DOI] [PubMed] [Google Scholar]
- 6.Hearne SE, O'Laughlin MP, Davis RD, Baker WA, Bashore TM, Harrison JK. Total pulmonary artery occlusion immediately after lung transplantation: successful revascularization with intravascular stents. J Heart Lung Transplant. 1996;15:532–5. [PubMed] [Google Scholar]
- 7.Sakamaki Y, Minami M, Ohta M, Takahashi T, Matsumiya G, Miyoshi S, et al. Pulmonary artery dissection complicating lung transplantation for primary pulmonary hypertension. Ann Thorac Surg. 2006;81:360–2. doi: 10.1016/j.athoracsur.2004.08.041. doi:10.1016/j.athoracsur.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Miyaji K, Nakamura K, Maruo T, Morita H, Saito H, Emori T, et al. Effect of a kink in unilateral pulmonary artery anastomosis on velocities of blood flow through bilateral pulmonary vein anastomoses in living-donor lobar lung transplantation. J Am Soc Echocardiogr. 2004;17:998–9. doi: 10.1016/j.echo.2004.04.014. doi:10.1016/j.echo.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Shoji T, Hanaoka N, Wada H, Bando T. Balloon angioplasty for pulmonary artery stenosis after lung transplantation. Eur J Cardiothorac Surg. 2008;34:693–4. doi: 10.1016/j.ejcts.2008.06.005. doi:10.1016/j.ejcts.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Tazaki J, Shibata T, Miwa S, Yamazaki K, Ishii H. Stent angioplasty for a kink in the pulmonary artery anastomosis soon after living-donor lobar lung transplantation. Ann Thorac Surg. 2011;92:e105–6. doi: 10.1016/j.athoracsur.2011.05.049. doi:10.1016/j.athoracsur.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 11.Michel-Cherqui M, Brusset A, Liu N, Raffin L, Schlumberger S, Ceddaha A, et al. Intraoperative transesophageal echocardiographic assessment of vascular anastomoses in lung transplantation. A report on 18 cases. Chest. 1997;111:1229–35. doi: 10.1378/chest.111.5.1229. doi:10.1378/chest.111.5.1229. [DOI] [PubMed] [Google Scholar]
- 12.Felten ML, Michel-Cher qui M, Sage E, Fischler M. Transesophageal and contact ultrasound echographic assessments of pulmonary vessels in bilateral lung transplantation. Ann Thorac Surg. 2012;93:1094–100. doi: 10.1016/j.athoracsur.2012.01.070. doi:10.1016/j.athoracsur.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 13.Gill RR, Poh AC, Camp PC, Allen JM, Delano MT, Jacobson FL, et al. MDCT evaluation of central airway and vascular complications of lung transplantation. Am J Roentgenol. 2008;191:1046–56. doi: 10.2214/AJR.07.2691. doi:10.2214/AJR.07.2691. [DOI] [PubMed] [Google Scholar]
- 14.Malden ES, Kaiser LR, Gutierrez FR. Pulmonary vein obstruction following single lung transplantation. Chest. 1992;102:645–7. doi: 10.1378/chest.102.2.645. doi:10.1378/chest.102.2.645. [DOI] [PubMed] [Google Scholar]
- 15.Sarsam MA, Yonan NA, Beton D, McMaster D, Deiraniya AK. Early pulmonary vein thrombosis after single lung transplantation. J Heart Lung Transplant. 1993;12:17–9. [PubMed] [Google Scholar]
- 16.Berger H, Steiner W, Schmidt D, Forst H, Dienemann H. Stent-angioplasty of an anastomotic stenosis of the pulmonary artery after lung transplantation. Eur J Cardiothorac Surg. 1994;8:103–5. doi: 10.1016/1010-7940(94)90102-3. doi:10.1016/1010-7940(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 17.Najafizadeh K, Daneshvar A, Dezfouli AA, Kashani BS, Ahmadi ZH, Shadmehr MB, et al. Pulmonary artery stenosis shortly after lung transplantation: successful balloon dilation and stent insertion in one case. Ann Transplant. 2009;14:52–5. [PubMed] [Google Scholar]
- 18.Waurick PE, Kleber FX, Ewert R, Pfitzmann R, Bruch L, Hummel M, et al. Pulmonary artery stenosis 5 years after single lung transplantation in primary pulmonary hypertension. J Heart Lung Transplant. 1999;18:1243–5. doi: 10.1016/s1053-2498(99)00091-1. doi:10.1016/S1053-2498(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee SK, Santhanakrishnan K, Shapiro L, Dunning J, Tsui S, Parmar J. Successful stenting of anastomotic stenosis of the left pulmonary artery after single lung transplantation. Eur Respir Rev. 2011;20:59–62. doi: 10.1183/09059180.00009610. doi:10.1183/09059180.00009610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferretti G, Boutelant M, Thony F, Carpentier F, Pison C, Guignier M. Successful stenting of a pulmonary arterial stenosis after a single lung transplant. Thorax. 1995;50:1011–2. doi: 10.1136/thx.50.9.1011. doi:10.1136/thx.50.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle TP, Loyd JE, Robbins IM. Percutaneous pulmonary artery and vein stenting: a novel treatment for mediastinal fibrosis. Am J Respir Crit Care Med. 2001;164:657–60. doi: 10.1164/ajrccm.164.4.2012132. doi:10.1164/ajrccm.164.4.2012132. [DOI] [PubMed] [Google Scholar]
- 22.Gaubert JY, Moulin G, Thomas P, Reynaud-Gaubert M, Noirclerc M, Bartoli JM. Anastomotic stenosis of the left pulmonary artery after lung transplantation: treatment by percutaneous placement of an endoprosthesis. Am J Roentgenol. 1993;161:947–9. doi: 10.2214/ajr.161.5.8273631. doi:10.2214/ajr.161.5.8273631. [DOI] [PubMed] [Google Scholar]
- 23.Bousamra M, II, Mewissen MW, Batter J, Presberg KW, Schlueter DP, Haasler GB. Pulmonary artery thrombolysis and stenting after a bilateral sequential lung transplantation. J Heart Lung Transplant. 1997;16:678–80. [PubMed] [Google Scholar]
- 24.Lumsden AB, Anaya-Ayala JE, Birnbaum I, Davies MG, Bismuth J, Cheema ZF, et al. Robot-assisted stenting of a high-grade anastomotic pulmonary artery stenosis following single lung transplantation. J Endovasc Ther. 2010;17:612–6. doi: 10.1583/10-3208R.1. doi:10.1583/10-3208R.1. [DOI] [PubMed] [Google Scholar]