Abstract
OBJECTIVES
The St. Jude Medical Trifecta aortic bioprosthesis (St. Jude Medical, Inc., St. Paul, MN, USA) is a new stented pericardial tissue heart valve. The aim of the study was to evaluate the clinical and haemodynamic performance of the Trifecta bioprosthesis in the early postoperative period.
METHODS
From July 2010 to September 2012, a total of 200 consecutive patients underwent aortic valve replacement with the Trifecta valve in our institution. All intraoperative and postoperative data were prospectively collected. Mean EuroSCORE II was 3.98%. Echocardiography was performed at discharge in all patients.
RESULTS
The mean age was 71.2 ± 7.7 (range 39–89 years). Extubation in the operating theatre was successfully performed in 96% of patients. Mean hospital stay was 8.5 days. The prosthesis sizes were 19 mm (n = 33), 21 mm (n = 81), 23 mm (n = 59), 25 mm (n = 23) and 27 mm (n = 4). Mean systolic pressure gradients ranged from 9.4 mmHg (size 19 valve) to 4.8 mmHg (size 27 valve). Mean effective orifice area (EOA) ranged from 1.61 cm2 (size 19 valve) to 2.5 cm2 (size 27 valve). Severe mismatch (<0.65 cm2/m2) did not occur in any patient. Of note, 99.5% of patients had mild or no aortic insufficiency at discharge. The early (30-day) mortality was 2.5% (n = 5).
CONCLUSIONS
The Trifecta valve offers good clinical results and excellent haemodynamic performance. Special care must be taken to avoid oversizing, which can lead to difficulty in implantation and can produce gradient increases due to an excess of prosthetic leaflet tissue.
Keywords: Aortic valve, Trifecta bioprosthesis, Mean systolic pressure gradients, Mean effective orifice area
INTRODUCTION
The Trifecta aortic bioprosthesis (St. Jude Medical, Inc., St. Paul, MN, USA) is a stented pericardial tissue heart valve designed for supra-annular placement.
Its valve design includes pericardial tissue leaflets attached to the exterior of the valve stent to improve the opening area. The pericardial-covered stent for tissue-to-tissue contact is intended to reduce the risk of abrasion and structural valve deterioration. The elimination of a tacking suture at the top of the commissure aims to reduce decrease the risk of tearing. The fatigue-resistant high-strength titanium stent was designed to reduce the stress on the leaflets during the cardiac cycle.
The objective of this study was to evaluate the clinical and haemodynamic performances of the Trifecta bioprosthesis in the early postoperative period and analyse the technique of implantation.
METHODS
A consecutive series of patients undergoing aortic valve replacement from July 2010 to September 2012 was analysed prospectively. The only inclusion criterion was the need for a biological aortic valve replacement; there were no exclusion criteria. All stented biological aortic valves implanted in this period were Trifecta valves.
The primary study endpoint was the clinical and haemodynamic performances of the Trifecta bioprosthesis in the early postoperative period. It included major adverse prosthesis-related events according to the guidelines for reporting mortality and morbidity after cardiac valve intervention [1]. Moreover, we also reported transvalvular gradients, effective orifice area (EOA) and prosthesis-patient mismatch (PPM) determined by echocardiography.
The secondary study endpoint was to evaluate the surgical implant technique.
Echocardiography was performed at discharge on all patients. Three experienced echocardiographers performed all echocardiographic examinations transthoracically.
Patients were included consecutively into the study, and all patient data were collected prospectively. Data analysis was performed using commercially available statistical software packages (SPSS Version 20.0/SPSS, Inc., Chicago, IL, USA).
OPERATIVE TECHNIQUE
Cardiopulmonary bypass was established. We used aortic cannulation near the aortic arch, and cavoatrial venous cannula and ventricular vent through the right superior pulmonary vein. In all patients, myocardial protection was done with cold blood antegrade and retrograde cardioplegia with mild hypothermia. The aortotomy should be high, ∼1–2 cm above the sinotubular junction. To compensate for the difficulties arising from the high-aortotomy approach, a wide aortotomy is recommended, done transversally or in a hockey-stick way.
We proceeded to the resection of the native valve, with decalcification of the ring in the usual manner. The size of the prosthesis was measured with standard Trifecta sizers. The intra-annular end of the sizer should enter into the aortic annulus tightly. The supra-annular end of the sizer is designed to reproduce the valve in order to check the supra-annular position regarding the coronary ostia. As explained later, it is important to avoid oversizing.
We used a supra-annular non-everted suture technique, with pledgets on the ventricular aspect, with an interrupted horizontal mattress suture (2-0 Ethibond, Ethicon, Inc., Somerville, NJ, USA). As the prosthesis is lowered towards the valvular plane, we recommend first fitting the left coronary sinus, then the right and finally the non-coronary sinus. It is important not to distort the prosthesis during positioning and knotting. Once knotted, it is important to check proper placement and the coronary ostia, avoiding the presence of leaks with a hook. The aorta is closed in the usual manner, in our case with double layer of 5-0 polypropylene monofilament.
Transoesophageal echocardiography was performed intraoperatively in all patients to assess the correct positioning and normal function of the prosthesis. Postoperative anticoagulation therapy consisted only of acetylsalicylic acid (100 mg). Oral anticoagulation (vitamin K antagonist) was administered only if indicated by other pathology.
RESULTS
Between July 2010 and September 2012, a total of 200 patients (85 females, 42.5%, 115 males, 57.5%) underwent aortic valve replacement with the Trifecta valve at our institution. More than half, 56.5% (n = 113), did not have any other associated procedure.
As given in Table 1, concomitant surgical procedures were performed in 43.5% of patients (n = 87). Urgent surgery was performed only in 3.5% (n = 7). Mean EuroSCORE II was 3.98%.
Table 1:
Preoperative data
| Age, years ± SD (range) | 71.18 ± 7.7 (39–89) |
| <70 | 54 (27%) |
| 70–79 | 126 (63%) |
| >80 | 20 (10%) |
| Gender, M/F | 115/85 (57.5/42.5%) |
| BMI ± SD (range) | 28.41 ± 4.8 (18.1–43.8) |
| BSA, m2 ± SD (range) | 1.86 ± 0.2 (1.5–2.6) |
| NYHA class | |
| I | 2 (1%) |
| II | 48 (24%) |
| III | 118 (59%) |
| IV | 32 (16%) |
| Angina pectoris | 72 (36%) |
| Syncope | 6 (3%) |
| LVEF | |
| <20% | 3 (1.5%) |
| 21–30% | 8 (4%) |
| 31–50% | 48 (24%) |
| >50% | 141 (70.5%) |
| Urgent procedures | 7 (3.5%) |
| Hypertension | 144 (72%) |
| Diabetes | 80 (40%) |
| Dyslipidemia | 74 (37%) |
| COPD | 20 (10%) |
| Chronic renal disease | 22 (11%) |
| Chronic atrial fibrillation | 34 (17%) |
| Coronary artery disease | 70 (35%) |
| Redo | 13 (6.5%) |
| Aortic lesion | |
| Stenosis | 129 (64.5%) |
| Insufficiency | 27 (13.5%) |
| Mixed | 40 (20%) |
| Prosthetic failure | 4 (2%) |
| Aetiology | |
| Degenerative | 110 (55%) |
| Congenital | 57 (28.5%) |
| Rheumatic | 12 (6%) |
| Endocarditis | 13 (6.5%) |
| Prosthetic failure | 4 (2%) |
| Undefined | 4 (2%) |
| Valve gradient (mmHg) ± SD | |
| Mean | 48.1 ± 3.3 |
| Peak | 73.9 ± 5.4 |
| Surgery | |
| Valve size (mm) | |
| 19 mm | 33 (16.5%) |
| 21 mm | 81 (40.5%) |
| 23 mm | 59 (29.5%) |
| 25 mm | 23 (11.5%) |
| 27 mm | 4 (2%) |
| Concomitant procedures | 87 (43.5%) |
| Coronary artery bypass grafting | 55 (27.5%) |
| Mitral valve replacement | 9 (4.5%) |
| Mitral valve repair | 8 (4%) |
| Tricuspid annuloplasty | 4 (2%) |
| Ascending aorta aneurysm | 11 (5.5%) |
M/F: male/female; BMI: body mass index; BSA: body surface area; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease.
The mean age was 71.2 years ± 7.7 (range 39–89 years). Preoperative characteristics are described in Table 1. The most frequent aortic valve disease was stenosis for calcific degeneration, followed by mixed lesions and aortic insufficiency.
Extubation in the operating theatre was successfully performed in 96% (192 patients). Mean intensive care unit length of stay was 36 h. Mean hospital stay was 8.5 days.
The prosthesis sizes were 19 mm (n = 33), 21 mm (n = 81), 23 mm (n = 59), 25 mm (n = 23) and 27 mm (n = 4). We observed a marked gender distribution concerning valve size, with the largest valve sizes more frequent in males. Only the 21-mm size had an almost equal gender distribution. In terms of percentage, 96.97% of size 19-mm valve patients were female, with only 1 male (3.03%). In the 21-mm size, the gender distribution was 49.4% female and 50.6% male. In the 23-mm size, 22% female and 78% male; in valves 25 and 27-mm, 100% were male. Body surface area ranged from 1.6 m2 (size 19) to 2.5 m2 (size 27).
Echocardiography was performed at discharge in 100% of patients. Mean and maximal pressure gradients were calculated with the simplified Bernoulli equation.
The peak and mean systolic pressure gradients for each valve size (Fig. 1) were 19 mm (17 ± 6.4; 9.4 ± 4 mmHg), 21 mm (16.2 ± 5.5; 8.7 ± 3.4 mmHg), 23 mm (12.8 ± 3.8; 6.9 ± 2.3 mmHg), 25 mm (12.6 ± 3.6; 6.9 ± 2.3 mmHg) and 27 mm (9.6 ± 1.1; 4.8 ± 0.9 mmHg).
Figure 1:
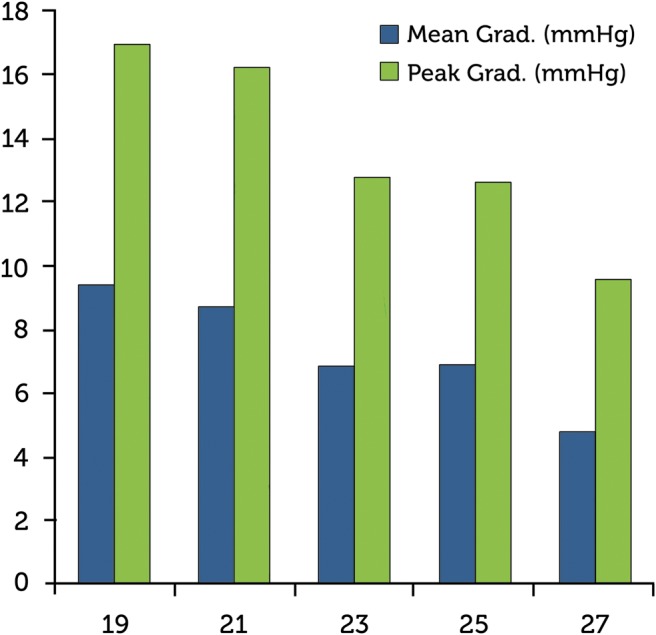
Trifecta valve mean and peak gradients. Valve size (mm) in the x-axis.
Mean EOA was 1.6 ± 0.3 cm2 (size 19 valve), 1.94 ± 0.5 cm2 (size 21 valve), 2.3 ± 0.5 cm2 (size 23 valve), 2.3 ± 0.4 cm2 (size 25 valve) and 2.5 ± 0.1 cm2 (size 27 valve).
The indexed valve effective orifice areas (iEOA) were 1.01 ± 0.2 cm2/m2 (size 19 valve), 1.1 ± 0.3 cm2/m2 (size 21 valve), 1.19 cm2/m2 ± 0.3 (size 23 valve), 1.05 cm2/m2 ± 0.1 (size 25 valve) and 1.15 ± 0.03 cm2/m2 (size 27 valve). These EOAs were slightly higher than those from St. Jude Medical data (Fig. 2).
Figure 2:
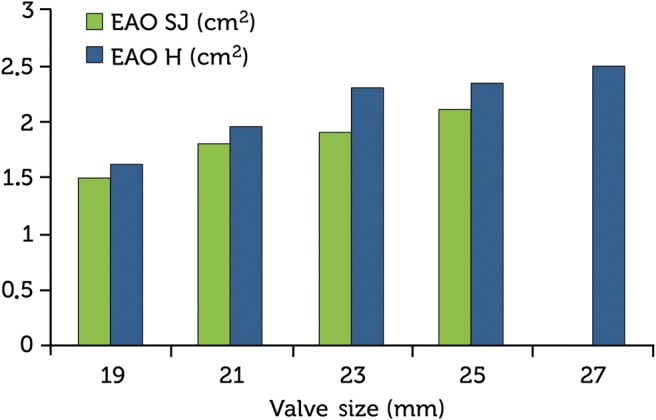
Trifecta valve effective orifice areas (EOA) comparing the results from St. Jude Medical and our institution, Hospiten Rambla.
PPM is defined by Blais et al. [2]. According to these criteria, at discharge severe mismatch (iEOA < 0.65 cm2/m2) did not occur in any patient and only 6 patients (3%) had mild-to-moderate.
Only 1 patient (0.5%) had significant aortic insufficiency because of prosthetic endocarditis. The remaining 99.5% had trivial or no aortic insufficiency. At discharge, 185 patients (92.5%) were in New York Heart Association (NYHA) class I to II.
Clinically or echocardiographically relevant structural valve deterioration (structural valve deterioration-related symptoms or mean trans-prosthetic gradient >40 mmHg) was not present in any patient.
Early mortality at 30 days was 2.5% (n = 5). All postoperative unexplained or undefined events or deaths were considered valve-related. Causes of death were considered valve-related in only 1 patient, who died 7 days after discharge for unknown reasons. The other 4 patients died because of non-valve-related events: mesenteric ischaemia (n = 1), pancreatitis (n = 1), right ventricle failure secondary to severe pulmonary hypertension (n = 1) and sepsis (n = 1).
The major complications registered during hospitalization were atrial fibrillation (n = 36; 18%), atrioventricular block with pacemaker implantation (n = 4; 2%), renal failure requiring haemofiltration (n = 7; 3.5%), stroke (n = 3; 1.5%), respiratory infection (n = 2; 1%) and endocarditis (n = 1; 0.5%). Reoperation was required only in this patient.
DISCUSSION
Our primary study endpoint was to evaluate the clinical and haemodynamic performance of the Trifecta bioprosthesis in the early postoperative period.
The Trifecta aortic bioprosthesis is a stented bovine pericardial tissue heart valve created exclusively for the aortic position. It is designed for supra-annular placement.
Supra-annular placement of an aortic bioprosthesis is one approach to optimize the haemodynamic result of an aortic valve replacement. With this concept, the internal valve diameter should theoretically be equal to the tissue annulus diameter and thus achieve an optimal haemodynamic performance with no obstruction of the blood flow.
Supra-annular placement cannot completely prevent high-pressure gradients or PPM, but the choice of a bovine prosthesis can optimize haemodynamic performance [3]. Comparisons of bovine and porcine heart valves showed a superiority of bovine tissue prostheses, particularly in small annulus sizes [4, 5]. Thus far, no consistent data show the superiority of any valve material as far as valve durability is concerned [3].
The Trifecta valve is made with bovine pericardium and is designed for supra-annular placement, obtaining the benefits mentioned above. Beyond that, its valve design includes pericardial tissue leaflets attached to the exterior of the valve stent intending to increase the opening area.
In our opinion, the results reported exhibit a promising haemodynamic performance. The mean gradients were single digit, ranging from 9.4 mmHg in 19-mm size to 3.6 mmHg in 25-mm size. An interesting fact is that the mean systolic gradient of the 27 mm prosthesis is slightly higher than that of the 25-mm size. This is most likely due to oversizing in the 27-mm sized patients. The effective areas that achieved the prosthesis are also very good, ranging from 1.6 cm2 (size 19 valve) to 2.5 cm2 (size 27 valve). These EOAs are slightly higher than those reported from St. Jude Medical, but caution should be taken because the difference could be the variability of the aortic valve area at echocardiography during the early postoperative period. In our study, the gradients at discharge were comparable and even lower than those reported for other stented biological valves [4, 6, 7]. Likewise, the Trifecta valve has shown even better haemodynamic results than those of its predecessor, the St. Jude Medical Epic supra-annular valve [8, 9]. It is important to remember that this is not and cannot be a comparative study. Our data also indicated that the Trifecta valve might have a slightly better performance than recently reported by Dell'Aquila et al. [10].
From the clinical point of view, early mortality at 30 days was 2.5% (n = 5), which is a low rate compared with other studies [6, 11, 12]. Causes of death were considered valve-related only in 1 patient, who died 7 days after discharge, of unknown reasons. All postoperative unexplained or undefined events or deaths were considered valve-related. Only 1 patient (0.5%) had significant aortic insufficiency because of prosthetic endocarditis. After antibiotic treatment, the prosthesis was replaced with another Trifecta valve. The remaining 99.5% had trivial or no aortic insufficiency. In our experience, the clinical performance of the Trifecta valve is perfectly comparable with the other stented biological valves.
The secondary study endpoint was to evaluate the surgical implantation technique.
After 200 implants, the Trifecta valve allowed a relatively simple implant, and the technique is not much different from that of other supported biological valves. In our opinion, there are two key points. The first is a high and wide aortotomy. The prosthesis has a high profile, so it is advisable to make a high aortotomy, ∼1–2 cm above the sinotubular junction. To compensate for the difficulties arising from the high-aortotomy approach, a wide aortotomy is recommended. The second key point and the most important, is to properly size the valve. The intra-annular sizer must fit in the aortic annulus, but it should not be very tight. Oversizing increases the difficulty of implantation, particularly when placing the prosthesis in the valvular plane and when knotting. Oversizing could also produce gradient increases due to an excess of prosthetic leaflet tissue, which could be the reason why our EOAs were slightly higher than the St. Jude Medical data. Care must also be taken not to distort the valve stent when lowering the valve into the aortic annulus.
Although haemodynamic and clinical outcomes at discharge are promising, this study had an important limitation that should be taken into account. Our results are from the early postoperative period, which is a short follow-up time. The results presented here offer an approach to the behaviour of this prosthesis, but it will be essential to re-examine the patients included in this study to test their functional status, observe the incidence of complications and do a new echocardiography at least at 1 year.
CONCLUSION
The St. Jude Medical Trifecta aortic valve is easy to implant, but special care must be taken to avoid oversizing, which can lead to difficulty in implantation and produce gradient increases due to an excess of prosthetic leaflet tissue.
The Trifecta valve offers a good alternative to other biological stented aortic valves. This study establishes excellent early clinical and haemodynamic performance at discharge, but further evaluation is needed during the follow-up.
Conflicts of interest: Rafael Llorens: St. Jude Medical Lecture fees. The other authors: none declared.
REFERENCES
- 1.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg. 2008;135:732–8. doi: 10.1016/j.jtcvs.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003;108:983–8. doi: 10.1161/01.CIR.0000085167.67105.32. [DOI] [PubMed] [Google Scholar]
- 3.Ruzicka DJ, Hettich I, Hutter A, Bleiziffer S, Badiu CC, Bauernschmitt R, et al. The complete supraannular concept: in vivo hemodynamics of bovine and porcine aortic bioprostheses. Circulation. 2009;120(11 Suppl):S139–45. doi: 10.1161/CIRCULATIONAHA.109.844332. [DOI] [PubMed] [Google Scholar]
- 4.Chambers JB, Rajani R, Parkin D, Rimington HM, Blauth CI, Venn GE, et al. Bovine pericardial versus porcine stented replacement aortic valves: early results of a randomized comparison of the Perimount and the Mosaic valves. J Thorac Cardiovasc Surg. 2008;136:1142–8. doi: 10.1016/j.jtcvs.2007.12.086. [DOI] [PubMed] [Google Scholar]
- 5.Eichinger WB, Botzenhardt F, Keithahn A, Guenzinger R, Bleiziffer S, Wagner I, et al. Exercise hemodynamics of bovine versus porcine bioprostheses: a prospective randomized comparison of the mosaic and perimount aortic valves. J Thorac Cardiovasc Surg. 2005;129:1056–63. doi: 10.1016/j.jtcvs.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 6.ISTHMUS Investigators. The Italian study on the Mitroflow postoperative results (ISTHMUS): a 20-year, multicentre evaluation of Mitroflow pericardial bioprosthesis. Eur J Cardiothorac Surg. 2011;39:18–26. doi: 10.1016/j.ejcts.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 7.Wong SP, Legget ME, Greaves SC, Barratt-Boyes BG, Milsom FP, Raudkivi PJ. Early experience with the Mosaic bioprosthesis: a new generation porcine valve. Ann Thorac Surg. 2000;69:1846–50. doi: 10.1016/s0003-4975(00)01167-x. [DOI] [PubMed] [Google Scholar]
- 8.Ruzicka DJ, Eichinger WB, Hettich IM, Bleiziffer S, Bauernschmitt R, Lange R. Hemodynamic performance of the new St. Jude Medical Epic Supra porcine bioprosthesis in comparison to the Medtronic Mosaic on the basis of patient annulus diameter. J Heart Valve Dis. 2008;17:426–33. [PubMed] [Google Scholar]
- 9.Maitland A, Hirsch GM, Pascoe EA. Hemodynamic performance of the St. Jude Medical Epic Supra aortic stented valve. J Heart Valve Dis. 2011;20:327–31. [PubMed] [Google Scholar]
- 10.Dell'Aquila AM, Schlarb D, Schneider SRB, Sindermann JR, Hoffmeier A, Kaleschke G, et al. Clinical and echocardiographic outcomes after implantation of the Trifecta aortic bioprosthesis: an initial single-centre experience. Interact CardioVasc Thorac Surg. 2013;16:112–5. doi: 10.1093/icvts/ivs460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichinger WB, Hettich IM, Ruzicka DJ, Holper K, Schricker C, Bleiziffer S, et al. Twenty-year experience with the St. Jude Medical Biocor bioprosthesis in the aortic position. Ann Thorac Surg. 2008;86:1204–10. doi: 10.1016/j.athoracsur.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson WRE, Lewis CTP, Sakwa MP, Cooley DA, Kshettry VR, Jones KW, et al. St Jude Medical Epic porcine bioprosthesis: results of the regulatory evaluation. J Thorac Cardiovasc Surg. 2010;58:69–75. doi: 10.1016/j.jtcvs.2010.05.055. [DOI] [PubMed] [Google Scholar]


