Abstract
OBJECTIVES
The occurrence of intra-abdominal hypertension (IAH), as well as its promoting factors in cardiac surgery, has been poorly explored. The aim of the present study was to characterize intra-abdominal pressure (IAP) variations in patients undergoing cardiac surgical procedures, and to identify the risk factors for IAH in this setting.
METHODS
All consecutive adult patients requiring postoperative intensive care unit admission for >24 h were enrolled. Demographic data, pre-existing comorbidities, type and duration of surgery, cardiopulmonary bypass (CPB) use and duration, perioperative IAP, organ function and fluid balance were recorded. IAH was defined as a sustained increase in IAP >12 mmHg. Multivariate logistic regression and stepwise analyses identified the baseline and perioperative variables associated with IAH.
RESULTS
Of 69 patients, 22 (31.8%) developed IAH. In the logistic model, baseline IAP, high central venous pressure, vasoactive drugs administration, positive fluid balance, AKI, CPB, total sequential organ failure assessment score and age were all promoting factors for IAH (Hosmer–Lemeshow χ2 = 7.23; P = 0.843). Baseline IAP, high central venous pressure and positive fluid balance were independent risk factors for IAH in the stepwise analysis. The ROC curve analysis, obtained by plotting the occurrence of IAH vs the IAP baseline value, showed an AUC of 0.75 (SE 0.064; 99% CI 0.62–0.87; P < 0.0001). The best IAP cut-off value was at 8 mmHg (sensitivity 63% and specificity 76%). Considering on- and off-pump surgery groups, fluid balance and vasoactive drugs use were significantly higher in the on-pump group. Linear regression analysis showed a positive correlation (P = 0.0001) between IAP changes and fluid balance only in the on-pump group.
CONCLUSIONS
IAH develops in one-third of cardiac surgery patients and is strongly associated with higher baseline IAP values, higher central venous pressure, positive fluid balance, extracorporeal circulation, use of vasoactive drugs and AKI. Determinants of IAH should be accurately assessed before and after surgery, and patients presenting risk factors must be monitored properly during the perioperative period. In this context, the baseline value of IAP may be a valuable and early warning parameter for IAH occurrence.
Keywords: Intra-abdominal pressure, Abdominal hypertension, Cardiac surgery, Cardiac surgical patients, Acute kidney injury
INTRODUCTION
Intra-abdominal pressure (IAP) is the steady-state pressure concealed within the abdominal cavity. In physiological conditions, IAP ranges from subatmospheric to 0 mmHg. A prolonged pathological elevation of IAP ≥12 mmHg, defined as intra-abdominal hypertension (IAH) [1], could lead to the dysfunction and failure of virtually all body organ systems [1, 2]. This value derives from the deleterious effects on renal, cardiac and gastrointestinal functions witnessed at IAP levels between 10 and 15 mmHg [3–7]. Over the past decade, IAH has been increasingly recognized as a cause of morbidity and mortality in critically ill patients [1, 2], being associated with severe organ dysfunction when already present on ICU admission, and being an independent outcome predictor when developed during ICU stay [4]. Whereas early studies on this topic regarded mainly abdominal surgical and trauma patients [8–10], recently the focus has moved to other categories of critically ill patients related to the observation that not only local insults (such as peritonitis, abdominal surgery, ileus, volvulus and pancreatitis), but also systemic conditions such as massive fluid resuscitation, polytransfusion, hypothermia and severe coagulation disorders are critical determinants of IAH [3, 4]. In cardiac surgery, both cardiopulmonary bypass (CPB) and off-pump procedures are associated with factors potentially predisposing to IAH [11–13]. Compromised bowel capillary endothelium by ischaemia-reperfusion, inflammatory mediators injury or splanchnic hypoperfusion may enhance third space losses and cause IAH.
Despite the high frequency of these predisposing conditions, the incidence of IAH in cardiac surgery remains unclear, and the risk factors for IAH in cardiac surgery are poorly understood [14].
The aim of the present study was to analyse IAP variations and to identify their promoting factors in patients undergoing cardiac surgical procedures.
MATERIALS AND METHODS
Patients
The local ethics committee approved the study protocol, and all patients gave their informed consent. Over a 3-month period, patients >18 years old, undergoing elective cardiac surgical procedures in general anaesthesia and admitted for >24 h in ICU were enrolled. Exclusion criteria were: contraindications to intravesical pressure measurement (neurogenic bladder, haematuria etc.), minithoracotomy, chronic renal failure requiring haemodialysis, an IAP value ≥12 mmHg at baseline, and participation in other clinical trials.
Anaesthesia
Oral premedication was administered with lorazepam (1 mg) on call to the operating room. Anaesthesia was induced with diazepam (0.1 mg/kg), fentanyl (3–5 mg/kg) and sodium thiopenthal (2–4 mg/kg). Muscle relaxation was achieved with cis-atracurium (0.1 mg/kg). Thereafter, anaesthesia was maintained with sevoflurane (1–1.5 minimum alveolar concentration), fentanyl (15–20 mg/kg total dose) and cis-atracurium (0.08 mg/kg/h). During CPB, anaesthesia maintenance was performed with sodium thiopenthal (5 mg/kg), followed by midazolam (5 mg) or propofol (60–100 mg/kg/min) on rewarming.
Cardiopulmonary bypass and off-pump procedures management
CPB was carried out with an open system and non-coated CPB lines and oxygenator (D 903 Avant, Sorin Group, Italy). Cardiac arrest and protection were achieved by means of intermittent hyperkalemic cold (8–10°C) blood cardioplegia. CPB temperature was maintained at 34–35°C. Prime volume was composed by ringer solution (50%), 18% mannitol solution (8%), Thamesol® (LDB Laboratori Diaco Biomedicali, Italy) (12%) and colloids (30%), for a total volume of priming of 1200–1500 ml. Target haemoglobin levels were >7.5 g/dl. In off-pump procedures, leg elevation and fluids administration (0.9% saline solution or 6% hydroxyethyl starch 130/0.4) were performed to maintain cardiac preload. When hypotension or low cardiac output persisted despite circulating blood volume optimization, vasoactive drugs or inotropic drugs were administered. Target haemoglobin levels were >8 g/dl.
In both on- and off-pump procedures, a cell-salvage device was used and salvaged blood was reinfused to the patient before the end of surgery. At chest closure, morphine (0.1–0.15 mg/kg) was administered for early postoperative analgesia.
Respiratory weaning
Upon ICU admission, patients were mechanically ventilated with pressure-controlled ventilation (tidal volume 5–8 ml/kg, PEEP 5 cmH2O, respiratory rate of 10/min), adjusted to maintain normocapnia. When patients achieved extubation criteria (awake and cooperative, fully rewarmed, haemodynamically stable, with blood gases within the normal ranges), extubation was accomplished. Chest tubes were removed when drainage was <10 ml/h for at least 4 h, which generally occurred within 24–48 h.
Data collection
For each patient, demographic data (age, sex and BMI), pre-existing comorbidities and kind and duration of surgical procedure were recorded. Intra-abdominal pressure (IAP), mean arterial pressure (MAP), central venous pressure (CVP), PaO2/FiO2, pH, PaCO2, haemoglobin, haematocrit, platelet count, bilirubin, creatinine, fluid replacement (blood components, colloids and crystalloids), urine output, total fluid balance, ileum, use of vasoactive drugs, Risk, Injury, Failure, Loss and End-stage kidney failure (RIFLE) classification and Sequential Organ Failure Assessment (SOFA) score were collected at five time points: after anaesthesia induction (baseline value), and 2, 6,12 and 24 h after surgery (time points 0, 1, 2, 3 and 4, respectively). Length of ICU stay and duration of mechanical ventilation were also recorded.
Definitions and intra-abdominal pressure measurement technique
Acute kidney injury (AKI) was defined according to the risk class definition of the RIFLE criteria [15]. Liver dysfunction was defined as a bilirubin value >2 mg/dl [16]. IAH was defined as a sustained or repeated elevation of IAP at or above 12 mmHg [1], in at least two consecutive measurements.
IAP was measured via a Foley bladder catheter, according to the standardized technique [1, 2]. A three-way stopcock was connected to a pressure transducer attached to a standard invasive pressure measurement device. A 16-gauge needle-less cannula was inserted into the sampling port of the urinary drainage tubing and the needle removed, using an aseptic technique. The cannula was attached to the stopcock via pressure tubing. After flushing the system with saline and zeroing it at the level of the mid-axillary line at the iliac crest, the urinary drainage tubing was clamped immediately distal to the sampling port. The stopcock was turned off to the patient and the pressure transducer, and 25 ml of sterile saline was aspirated from the IV bag and instilled into the bladder. Then the stopcock was turned off to the syringe and IV tubing. After a stabilization period of 30–60 s to allow for equilibrium to occur, with the patient in the complete supine position and after ensuring that abdominal muscle contractions were absent, IAP was measured at end-expiration on the bedside monitor [1, 2]. After IAP determination, the clamp was removed, the bladder allowed to drain, and the volume of saline utilized subtracted from the patient's urinary output for that hour.
Statistical analysis
Continuous normally distributed data are expressed as mean ± confidence intervals (CIs) and compared by using unpaired Student's t-test or two-way analysis of variance for multiple comparisons. Non-normally distributed data are expressed as median and 25–75% inter-quartile range and compared using the Mann–Whitney U-test. Categorical data are expressed as the number of events and percentage and compared using χ2 (when the number of observations was >5) or the Fisher's exact (when the number of observations was <5) tests. A logistic regression analysis was performed with IAH as the dependent outcome variable. Potential promoting factors with a P-value of <0.10 in univariate analysis and a prevalence of at least 10% in the IAH group were included in the model. Multivariate analysis results were summarized by estimating odds ratios (ORs) and respective 95% CIs. The power of the model was tested by the Hosmer–Lemeshow goodness-of-fit test. The effect of potential confounding factors was determined by introducing each factor independently in the final model and considering the variation in the model fit. To evaluate the role of each variable as an independent risk factor, all variables associated with IAH at the <0.05 level of risk in logistic analysis were introduced in a backward stepwise logistic regression model with an α to remove of 0.05. A Pearson's regression analysis was also performed to further clarify the association between variables. A receiver operator characteristic (ROC) curve was plotted to identify the threshold value of IAP at baseline that optimized variable sensitivity (the ability to identify true positives) and specificity (the ability to identify true negatives) for predicting IAH development. In all comparisons, a P-value of <0.05 was considered statistically significant. Data were analysed using a Statistical Package from Social Sciences (SPSS, release 5.0.1 for Windows, Chicago, IL, USA) software.
RESULTS
Of 76 enrolled patients, 7 were excluded due to an IAP value >12 mmHg at baseline. Thus, 69 patients were included in the final analysis. Twenty-two patients (31.8%) developed the IAH group. Patient and surgical procedure characteristics in the IAH and control groups are reported in Table 1.
Table 1:
Demographic and clinical characteristics of the study population
| IAH group, n = 22 | Control group, n = 47 | P-value | |
|---|---|---|---|
| Age (years) | 70 ± 8 | 66 ± 10 | 0.12 |
| Male sex (%) | 15 (68.2) | 28 (59.6) | 0.49 |
| BMI (kg/m2) | 26.7 ± 4 | 25.6 ± 3 | 0.21 |
| Diabetes mellitus (%) | 6 (27.3) | 10 (21.3) | 0.58 |
| Hypertension (%) | 15 (68.2) | 33 (70.2) | 0.86 |
| COPD (%) | 3 (13.6) | 5 (10.6) | 1 |
| Peripheral artery disease (%) | 1 (4.5) | 5 (10.6) | 0.65 |
| CABG (%) | 16 (72.7) | 36 (76.6) | 0.73 |
| Valve replacement (%) | 5 (22.7) | 9 (19.1) | 0.75 |
| Valve replacement plus CABG (%) | 1 (4.5) | 2 (4.3) | 1 |
| Surgery time (min) | 324 ± 88 | 271 ± 68 | 0.008 |
| On-pump (%) | 19 (86.3) | 28 (59.6) | 0.03 |
| Off-pump (%) | 3 (14.7) | 19 (41.4%) | |
| CPB time (min) | 132 ± 53 | 118 ± 40 | 0.29 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; CABG: coronary artery bypass grafting: CPB: cardiopulmonary bypass.
Intra-abdominal hypertension group vs control group
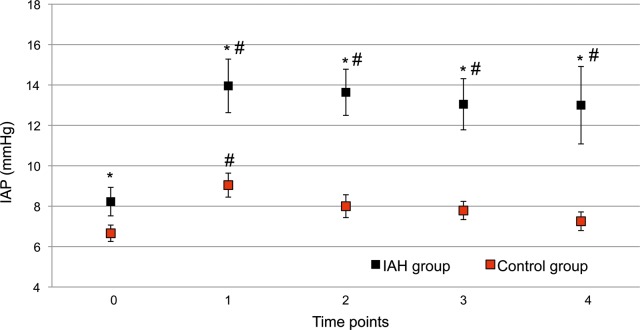
In univariate comparisons, no differences were found with regard to age, gender, comorbidities and type of surgical procedure. In the IAH group, duration of surgery was longer and more patients underwent on-pump procedures, while CPB duration did not differ between groups (Table 1). Baseline values of IAP were significantly higher in patients who subsequently developed IAH, when compared with those who did not (8.2 ± 1.7 vs 6.6 ± 1.4 mmHg; P = 0.0001) (Fig. 1). IAP values peaked at 2 h after surgery and remained significantly higher in the IAH group compared with the control group, throughout the whole study period (Fig. 1).
Figure 1:
Intra-abdominal pressure (IAP) values in intra-abdominal hypertension (IAH) and control groups. Black dots: IAH group; red dots: control group. Data are expressed as means and 95% CIs. *P < 0.05 between groups; #P < 0.05 within group vs time point 0.
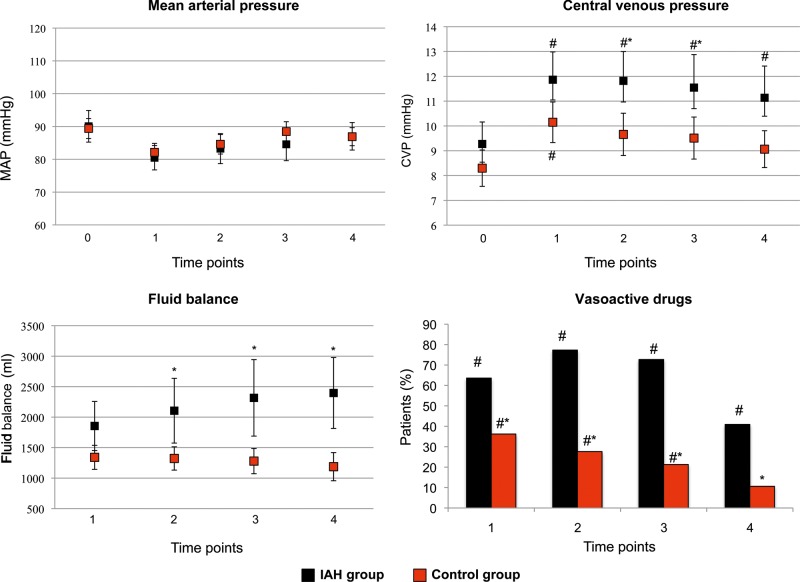
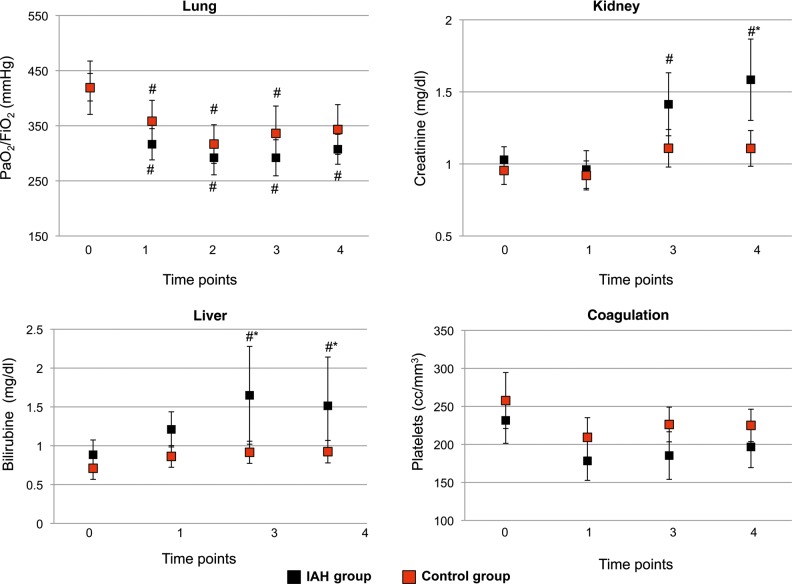
No differences were found in MAP between groups, while CVP was significantly higher at time points 2 and 3, and fluid balance at time points 2, 3 and 4 in the IAH group when compared with the control group. The number of patients requiring vasopressors was significantly higher in the IAH group in all the measurement time points, starting from time point 1 (Fig. 2). Lung function did not differ between groups, while in the IAH group, renal function worsened on time point 4, and liver function worsened on time points 3 and 4. Platelets count was not different between groups (Fig. 3). AKI was more frequent in the IAH group (36.3 vs 12.7%; P = 0.03). Significantly longer mechanical ventilation duration and ICU length of stay were observed in the IAH group (12 ± 6 h and 3 ± 1 days, respectively; P < 0.001), when compared with the control group (6 ± 3 h and 2 ± 1 days, respectively; P < 0.001).
Figure 2:
Haemodynamics and fluid balance in intra-abdominal hypertension (IAH) and control groups. Black dots/bars: IAH group; red dots/bars: control group. Data are expressed as means and 95% CIs. *P < 0.05 between groups; #P < 0.05 within group vs time point 0.
Figure 3:
Organ function in intra-abdominal hypertension (IAH) and control groups. Black dots: IAH group; red dots: control group. Data are expressed as means and 95% CI. *P < 0.05 between groups; #P < 0.05 within group vs time point 0.
Promoting factors of intra-abdominal hypertension
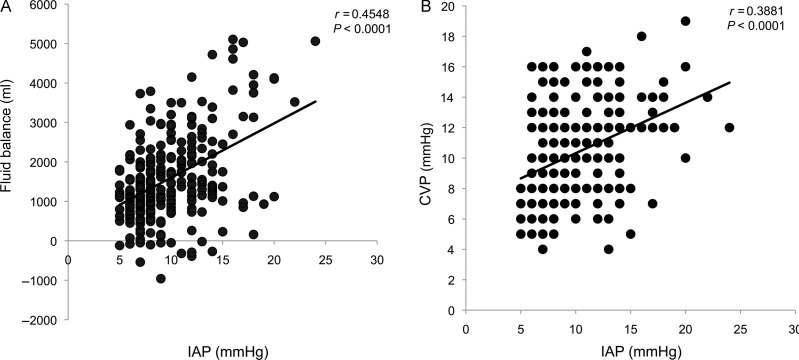
In the logistic regression model, baseline IAP values, CVP values, use of vasoactive drugs, fluid balance, AKI, CPB, total SOFA score and age were all promoting factors for IAH development. In the stepwise analysis, baseline IAP value was the strongest independent predictor of IAH, followed by CVP, and positive fluid balance (Table 2). The ROC curve analysis obtained by plotting the presence or absence of IAH vs the IAP baseline value showed an AUC of 0.75 (SE 0.064; 99% CI 0.62–0.87; P < 0.0001). The best cut-off value was at 8 mmHg, with a sensitivity of 63% and a specificity of 76%. Significant positive correlations between IAP and fluid balance (y = 137.7x + 223.81; r = 0.4548; P < 0.0001) and between IAP and CVP (y = 0.3312x + 7.0063; r = 0.3881; P < 0.0001) were observed (Fig. 4).
Table 2:
Predictive factors of intra-abdominal hypertension in the study population in both binomial and stepwise analyses
| Predictors | Binomial analysisa |
Stepwise analysis | ||
|---|---|---|---|---|
| OR | 95% CI | P-value | P-value | |
| Baseline IAP value | 4.58 | 2.76–5.72 | 0.002 | 0.009 |
| CVP | 3.35 | 1.68–5.37 | 0.012 | 0.014 |
| Vasoactive drugs | 4.81 | 1.57–6.9 | 0.029 | – |
| Fluid balance | 4.31 | 1.68–5.54 | 0.033 | 0.041 |
| Acute kidney injury | 2.27 | 1.12–4.71 | 0.035 | – |
| CPB | 2.73 | 1.92–5.12 | 0.037 | – |
| SOFA score | 2.68 | 1.85–3.93 | 0.049 | – |
aHosmer–Lemeshow χ2 = 7.23; P = 0.843.
IAP: intra-abdominal pressure; CVP: central venous pressure; CPB: cardiopulmonary bypass; SOFA: sequential organ failure assessment.
Figure 4:
Linear regression analysis between fluid balance and intra-abdominal pressure (IAP) (A), and between central venous pressure and IAP (B).
On-pump vs off-pump group
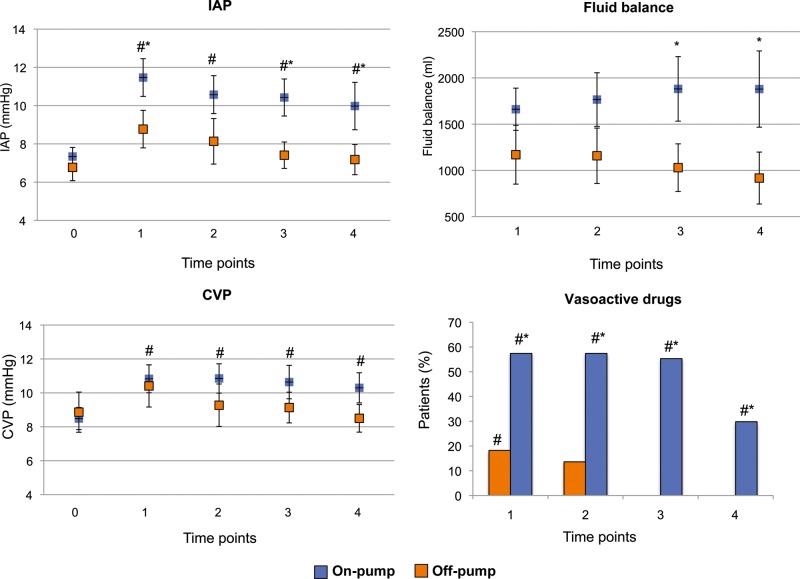
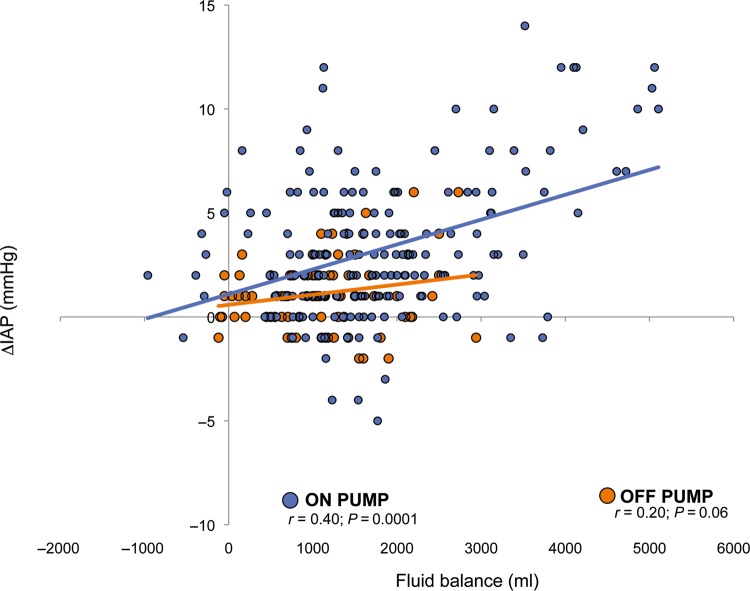
Comparing patients who underwent CPB (on-pump group) with those who underwent off-pump procedures (off-pump group), in the on-pump group the use of vasoactive drugs was significantly higher at each measurement point, and fluid balance was significantly higher at time points 3 and 4, while IAP was higher at time points 1, 3 and 4 (Fig. 5). The linear regression analysis between fluid balance and IAP variations showed a strong positive correlation between IAP changes and fluid balance in the on-pump group, whereas no relationship was found in the off-pump group (Fig. 6).
Figure 5:
Haemodynamics, intra-abdominal pressure (IAP) and fluid balance at each measurement point in on-pump and off-pump groups. Blue dots/bars: Intra-abdominal hypertension (IAH) on pump group; orange dots/bars: off pump group. Data are expressed as means and 95% CIs. *P < 0.05 between groups; #P < 0.05 within group, vs time point 0.
Figure 6:
Overall correlation between intra-abdominal pressure (IAP) changes and fluid balance in on-pump and off-pump groups. Blue dots: on-pump group; orange dots: off-pump group.
DISCUSSION
IAH develops in one-third of cardiac surgery patients and is already evident 2 h after the end of surgery. IAH is strongly associated with baseline IAP and CVP, and a more positive fluid balance. A direct relationship between fluid balance and IAP is particularly significant in patients undergoing CPB. Moreover, IAH is associated with a higher occurrence of postoperative AKI and longer duration of mechanical ventilation and ICU stay.
Although cardiac surgery patients exhibit most of IAP-predisposing factors identified in other patient populations, knowledge about IAH in cardiac surgery is still scarce. The only available data [12, 17], showed IAH in 44% of patients undergoing CPB for coronary artery bypass grafting. In that report, IAH was dependent on the degree of normovolemic haemodilution [12], and was related to body mass index and CVP [17].
Besides injury or disease involving the abdomino-pelvic region, other conditions have been associated with IAH. In surgical and medical patients, IAH may develop following intense volume resuscitation, with consequent acute ascites and splanchnic visceral oedema [17]. Several authors have found a strong correlation between positive fluid balance and increased IAP in surgical and trauma patients [8, 10], and net fluid balance has been recognized as the only causative factor of abdominal compartment syndrome in critically ill surgical patients [18]. In a recent study, cumulative fluid balance and shock were the main independent predictors of IAH [7]. On the other side, a negative fluid balance, obtained by means of aggressive ultrafiltration, has been recently proposed among the conservative strategies to decrease IAP in patients with IAH [19]. The results of the present study corroborate these findings and suggest that also in cardiac surgical patients, a positive fluid balance is a strong causative factor of IAH.
The association between IAP variations and fluid balance was statistically significant only in patients undergoing CPB. Comparing this group to off-pump patients, it can be noticed that the relationship between fluid balance and IAP variation is steeper, i.e. at the same fluid balance, CPB patients present larger IAP variations than the off-pump group. Therefore, CPB plays a critical role in this relationship. CPB produces a generalized and vigorous inflammatory response that, associated with splanchnic ischaemia-reperfusion, may compromise bowel capillary endothelium, promoting third space losses [20–24]. Moreover, the initiation of CPB results in decreased colloid osmotic pressure, which leads to an increase in microvascular permeability, and subsequent hypothermia and rewarming, with the release of substances causing vasoconstriction at the microcirculatory level and the gut mucosa, which may further lead to bowel oedema. Therefore, a first hit represented by CPB increases permeability that, if associated with positive fluid balance, may promote gut oedema and increase in IAP. IAH, by raising CVP, may further decrease lymph outflow (lymph oedema) and increase capillary pressure (stasis oedema), leading to a vicious cycle that perpetuates IAH itself.
IAH occurrence produces deleterious effects on organ function. The clinical impact of IAH in the present study is evidenced by the worst renal and liver parameters in IAH patients. Biancofiore et al. [8] reported a linear relationship between IAH and severity of decreased kidney function. Dalfino et al. [7] studied 123 consecutive patients admitted to a general ICU for at least 24 h, finding that an IAP of 12 mmHg was the best cut-off value for AKI, defined using the RIFLE classification. In this cohort of unselected ICU patients, IAH also was an independent risk factor for the development of AKI (OR 2.44). Renal impairment in IAH is considered a multifactorial process, related to haemodynamic, endocrine and local effects. Direct hydrostatic effects by mechanical compression, increasing renal vascular resistance, coupled with a decrease in cardiac output are the most likely causes. Similarly to multiple organ failure patients [3], due to the association between IAH and postoperative AKI, in cardiac surgical patients the presence of IAH should be carefully evaluated in patients at risk of renal injury.
Together with the need for more vasopressor drugs, all this might have been responsible for longer mechanical ventilation duration and ICU stay in patients with IAH. Moreover, Dabrowski et al. [25] have found a strong, inverse relationship between IAP and coronary arterial perfusion pressure, measured as the difference between MAP and pulmonary capillary wedge pressure.
Interestingly, the baseline value of IAP differed between IAH and control groups. The IAH group presented higher, albeit within the normal range, values of baseline IAP, to indicate a reduced opportunity to face increases in abdominal volume, without developing IAH. Therefore, the baseline value of IAP may be a precious and early warning parameter for IAH occurrence, and patients identified as at high or moderate risk for IAH should be accurately assessed before and after surgery. This may be a critical issue, since IAP monitoring is not continuous and not frequent in cardiac surgical protocols. IAP monitoring and early diagnosis of IAH could improve prognosis, avoiding the occurrence of organ failure.
This study has several features that may limit the generalizability of the findings. First, the patient sample was not large enough to compare the predictive value of all the known potential promoting factors of IAH, and we cannot exclude the potential role of uncontrolled confounding variables; however, it is encouraging that our findings do not conflict with previous studies on the same subject. Secondly, it may be difficult to exactly discriminate the individual role of IAH and CPB, since a high number of IAH patients had undergone CPB. However, although predictive in binomial analysis, CPB lost its association with IAH in the stepwise analysis. Moreover, the two factors may not be mutually exclusive, but may interact each other, and IAH may be one critical factor conditioning clinical outcomes in on-pump patients. Finally, therapeutic interventions to reduce IAP were not investigated.
These findings encourage further studies to define the role of continuous IAP monitoring and therapeutical strategies to prevent the development of IAH in order to improve the clinical outcome of patients undergoing cardiac procedures.
Funding
Support was provided solely from departmental sources.
Conflict of interest: none declared.
REFERENCES
- 1.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–32. doi: 10.1007/s00134-006-0349-5. doi:10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 2.Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–62. doi: 10.1007/s00134-007-0592-4. doi:10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 3.Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822–9. doi: 10.1007/s00134-004-2169-9. doi:10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 4.Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–22. doi: 10.1097/01.ccm.0000153408.09806.1b. doi:10.1097/01.CCM.0000153408.09806.1B. [DOI] [PubMed] [Google Scholar]
- 5.Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 2002;33:279–82. doi: 10.1097/00005373-199208000-00019. doi:10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Diebel LN, Dulchavsky SA, Wilson RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:45–8. doi: 10.1097/00005373-199207000-00010. doi:10.1097/00005373-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Dalfino L, Tullo L, Donadio I, Malcangi V, Brienza N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34:707–13. doi: 10.1007/s00134-007-0969-4. doi:10.1007/s00134-007-0969-4. [DOI] [PubMed] [Google Scholar]
- 8.Biancofiore G, Bindi ML, Romanelli AM, Boldrini A, Consani G, Bisà M, et al. Intra-abdominal pressure monitoring in liver transplant recipients: a prospective study. Intensive Care Med. 2003;29:30–6. doi: 10.1007/s00134-002-1552-7. [DOI] [PubMed] [Google Scholar]
- 9.Sugrue M, Jones F, Deane SA, Bishop G, Bauman A, Hillman K. Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg. 1999;134:1082–5. doi: 10.1001/archsurg.134.10.1082. doi:10.1001/archsurg.134.10.1082. [DOI] [PubMed] [Google Scholar]
- 10.Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138:637–42. doi: 10.1001/archsurg.138.6.637. doi:10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- 11.Andrási TB, Buhmann V, Soós P, Juhász-Nagy A, Szabó G. Mesenteric complications after hypothermic cardiopulmonary bypass with cardiac arrest: underlying mechanisms. Artif Organs. 2002;26:943–6. doi: 10.1046/j.1525-1594.2002.07116.x. doi:10.1046/j.1525-1594.2002.07116.x. [DOI] [PubMed] [Google Scholar]
- 12.Czajkowski M, Dabrowski W. Changes in intra-abdominal pressure during CABG with normovolemic hemodilution. Med Sci Monit. 2006;12:487–92. [PubMed] [Google Scholar]
- 13.Fiore G, Brienza N, Cicala P, Tunzi P, Marraudino N, Schinosa de Luca Tupputi L, et al. Superior mesenteric artery blood flow modifications during off-pump coronary surgery. Ann Thorac Surg. 2006;82:62–8. doi: 10.1016/j.athoracsur.2006.02.012. doi:10.1016/j.athoracsur.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 14.De Wolf A, Poelaert J, Herck I, De Waele JJ. Surgical decompression for abdominal compartment syndrome after emergency cardiac surgery. Ann Thorac Surg. 2008;85:2133–5. doi: 10.1016/j.athoracsur.2007.12.041. doi:10.1016/j.athoracsur.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, et al. Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. doi: 10.1186/cc9960. doi:10.1186/cc9960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med. 2006;32:267–74. doi: 10.1007/s00134-005-0023-3. doi:10.1007/s00134-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 17.Dabrowski W, Rzecki Z. Intra-abdominal and abdominal perfusion pressure in patients undergoing coronary artery bypass graft surgery. Acta Clin Belg. 2009;64:216–24. doi: 10.1179/acb.2009.038. [DOI] [PubMed] [Google Scholar]
- 18.Maerz L, Kaplan LJ. Abdominal compartment syndrome. Crit Care Med. 2008;36:S212–5. doi: 10.1097/CCM.0b013e318168e333. doi:10.1097/CCM.0b013e318168e333. [DOI] [PubMed] [Google Scholar]
- 19.McNelis J, Marini CP, Jurkiewicz A, Fields S, Caplin D, Stein D, et al. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch Surg. 2002;137:133–6. doi: 10.1001/archsurg.137.2.133. doi:10.1001/archsurg.137.2.133. [DOI] [PubMed] [Google Scholar]
- 20.Malbrain ML, Deeren D, De Potter TJR. Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care. 2005;11:156–71. doi: 10.1097/01.ccx.0000155355.86241.1b. doi:10.1097/01.ccx.0000155355.86241.1b. [DOI] [PubMed] [Google Scholar]
- 21.Tassani P, Schad H, Winkler C, Bernhard A, Ettner U, Braun SL, et al. Capillary leak syndrome after cardiopulmonary bypass in elective, uncomplicated coronary artery bypass grafting operations: does it exist? J Thorac Cardiovasc Surg. 2002;123:735–41. doi: 10.1067/mtc.2002.120348. doi:10.1067/mtc.2002.120348. [DOI] [PubMed] [Google Scholar]
- 22.Holmes JHT, Connolly NC, Paull DL, Hill ME, Guyton SW, Ziegler SF, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579–86. doi: 10.1007/pl00012432. doi:10.1007/PL00012432. [DOI] [PubMed] [Google Scholar]
- 23.Heltne JK, Koller M-E, Lund T, Bert J, Rynning SE, Stangeland L, et al. Dynamic evaluation of fluid shifts during normothermic and hypothermic cardiopulmonary bypass in piglets. Acta Anaesthesiol Scand. 2000;44:1220–5. doi: 10.1034/j.1399-6576.2000.441006.x. doi:10.1034/j.1399-6576.2000.441006.x. [DOI] [PubMed] [Google Scholar]
- 24.Farstad M, Heltne JK, Rynning SE, Lund T, Mongstad A, Eliassen F, et al. Fluid extravasation during cardiopulmonary bypass in piglets—effect of hypothermia and different cooling protocols. Acta Anaesthesiol Scand. 2003;47:397–406. doi: 10.1034/j.1399-6576.2003.00103.x. doi:10.1034/j.1399-6576.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 25.Dabrowski W, Wacinski P, Visconti J. Abdominal perfusion pressure and coronary arterial perfusion pressure in patients undergoing coronary artery bypass graft surgery. Exp Clin Cardiol. 2009;4:e84–8. [PMC free article] [PubMed] [Google Scholar]