Abstract
OBJECTIVES
Remote ischaemic preconditioning (RIPC) may protect distant organs against ischaemia-reperfusion injury. We investigated the impact of RIPC on kinin receptor expression in neutrophils following RIPC in patients undergoing coronary artery bypass grafting (CABG).
METHODS
Patients undergoing elective CABG with cardiopulmonary bypass (CPB) were randomized to RIPC (n = 15) or control (n = 15) groups. The study group underwent RIPC by inflation of a blood pressure cuff on the arm. Expression of kinin receptors, plasma concentrations of IL-6, IL-8, IL-10, TNF-α and neutrophil elastase were determined at baseline (before RIPC/sham), immediately before surgery (after RIPC/sham) and 30 min and 24 h after surgery. Plasma bradykinin levels were assessed before and after RIPC/sham, and at 30 min, 6, 12 and 24 h after surgery. Serum creatine kinase (CK), troponin I, C-reactive protein (CRP) and lactate levels were measured immediately prior to surgery and 30 min, 6, 12, 24 and 48 h after surgery.
RESULTS
Kinin B2 receptor expression did not differ between the groups at baseline (pre-RIPC), but was significantly lower in the RIPC group than in the control group after RIPC/sham (P < 0.05). Expressions of both kinin B1 and B2 receptors were significantly down-regulated in the RIPC group, and this persisted to 24 h after surgery (P < 0.001). Neutrophil elastase levels were significantly increased after surgery. There were no differences in CK, CRP, cytokine, lactate or troponin I levels between the groups.
CONCLUSIONS
RIPC down-regulated the expression of kinin B1 and B2 receptors in neutrophils of patients undergoing CABG.
Keywords: Bradykinin, Ischaemia, Ischaemic preconditioning, Kinin receptors, Cardiac surgery
INTRODUCTION
Ischaemic preconditioning (IPC) is a phenomenon in which brief cycles of ischaemia and reperfusion produce a protective response against subsequent prolonged periods of lethal ischaemia. In experimental settings, IPC provided powerful protection to target organs against subsequent ischaemia-reperfusion injury [1]. A more clinically applicable method of IPC is remote IPC (RIPC), in which a protective state is induced in a target organ, similar to the effect of local IPC, yet the brief cycles of IR are applied at a distance to the target organ (e.g. to a limb). RIPC was first identified by Przyklenk in 1993 [2], and recent randomized controlled clinical trials have demonstrated that RIPC has protective effects in patients undergoing cardiovascular surgery and angioplasty [3–7].
Although the precise mechanism underlying the phenomenon of RIPC remains unknown, it appears that neutrophils play a central role in the pathogenesis of IR injury, as well as the protective effect of RIPC [8]. We have previously demonstrated that RIPC modifies the expression of proinflammatory genes [9] and the functional responses of human neutrophils [10]. It now becomes apparent that bradykinin, a potent neutrophil activator, is directly involved in IR injury and preconditioning [11–13]. Interestingly, the infusion of low-dose bradykinin in patients undergoing percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) provided a cardioprotective effect similar to that of RIPC [14, 15]. Activation of kinin (B1 and B2) receptors on the neutrophil surface is a key step of bradykinin signalling [16, 17]. We previously demonstrated that RIPC decreased the expression of kinin receptors in neutrophils of normal healthy volunteers [16]. The present randomized, controlled study was designed to extend previous studies [13–16] to clinical surgical practice, and to determine if a similar down-regulation of kinin receptors in neutrophils occurred following RIPC in patients undergoing CABG.
METHODS
Patients
The study was approved by the Human Research Ethics Committee at Sir Charles Gairdner Hospital. This was a randomized, double-blind controlled prospective trial to assess the effects of RIPC in patients undergoing CABG surgery. Consecutive patients scheduled to undergo elective CABG with cardiopulmonary bypass (CPB) at Sir Charles Gairdner Hospital between February and December 2010 were randomly assigned to the RIPC (n = 15) or control (n = 15) groups. The perioperative characteristics of the patients are provided in Table 1. Patients with evolving myocardial infarction, those taking oral suphonyl urea medication or insulin for diabetes, those with a left ventricular ejection fraction <35% and those undergoing perioperative haemodialysis were excluded.
Table 1:
Baseline demographic, clinical and intraoperative details for the two groups
| Clinical data | Control group (n = 15) | RIPC group (n = 15) | P-value |
|---|---|---|---|
| Males/females (n) | 13/2 | 16/0 | 0.13 |
| Age (years) | 68.7 ± 7.8 | 65.1 ± 10.5 | 0.29 |
| BMI (kg/m2) | 28.34 ± 4.77 | 28.13 ± 4.03 | 0.89 |
| Smoking history (n) | |||
| Current smoker | 5 | 1 | 0.15 |
| Ex-smoker | 8 | 10 | |
| Non-smoker | 2 | 4 | |
| LVEF (%) | 54.7 ± 7.5 | 58.1 ± 6.2 | 0.2 |
| Peripheral vascular disease (n) | 3 | 1 | 0.25 |
| Diabetes mellitus (n) | 4 | 4 | 0.92 |
| COPD (n) | 3 | 2 | 0.57 |
| Number of grafts | 4.1 ± 0.5 | 4.4 ± 1.1 | 0.32 |
| CPB time (min) | 95.2 ± 26.7 | 89.6 ± 28.1 | 0.57 |
| Cross-clamp time (min) | 62.8 ± 17.3 | 67.5 ± 24.9 | 0.55 |
Data are mean ± SD or numbers of patients. BMI: body mass index; COPD: chronic obstructive pulmonary disease; CBP: cardiopulmonary bypass; LVEF: left ventricular ejection fraction.
Study protocol
Following the induction of anaesthesia, patients were assigned to either the RIPC or the control group. This was done by one of the clinicians who was not directly involved in the randomization process. For patients in the RIPC group, the forearm was rendered ischaemic for three 5-min periods, each separated by 5 min of reperfusion. This was achieved by inflation of a standard blood pressure cuff, placed on the upper arm, to a pressure exceeding systolic pressure by 20 mmHg. Interruption and restoration of blood flow were documented using a standard pulse oximeter applied to a finger on the same arm. For patients assigned to the control group, a blood pressure cuff was placed around the upper arm but was not inflated (i.e. sham protocol). All patients underwent standard CABG with cardioplegic heart arrest and CPB.
Creatine kinase (CK), C-reactive protein (CRP), lactate and troponin I levels were measured immediately prior to surgery and 30 min, 6, 12, 24 and 48 h after surgery. Plasma bradykinin levels were assessed at baseline (before RIPC/sham), immediately before surgery (after RIPC/sham) and 30 min, 6, 12 and 24 h after surgery. Plasma concentrations of IL-6, IL-8, IL-10, TNF-α, neutrophil elastase and expression of kinin receptors in neutrophils were determined at baseline (before RIPC/sham), immediately before surgery (after RIPC/sham) and 30 min and 24 h after surgery.
Neutrophil isolation and immunolabelling for kinin B1 and B2 receptors
Blood was collected in tubes containing acid–citrate dextrose, mixed with an equal volume of 6% dextran in phosphate-buffered saline (PBS, pH 7.4), and red blood cells were allowed to sediment for 20 min at room temperature. Neutrophils were isolated from the upper leucocyte layer by centrifugation on Percoll and resuspended in Hank's balanced salt solution. Neutrophils were spotted on poly-l-lysine-coated slides, allowed to air-dry and fixed in acetone–methanol (1:1). After rehydration (0.01 M PBS, pH 7.4), excess peroxidase activity was inhibited with peroxidase block (DAKO, Sydney, Australia) for 5 min. Non-specific protein binding was blocked with 10% human serum, 20% swine serum and serum-free protein block (DAKO) for 15 min each. Slides were then incubated for 3 h at room temperature with kinin B1 receptor or B2 receptor antibodies (Abcam, Cambridge, UK) at a dilution of 1/100 in 0.01 M PBS containing 1% bovine serum albumin. The slides were washed three times (0.01 M PBS, pH 7.4) and incubated with anti-rabbit horseradish peroxidase-conjugated polymer for 30 min at room temperature. After washing three times (0.01 M PBS, pH 7.4), labelling was visualized by incubating the slides with 3,3′-diaminobenzidine (DAB) and counter staining with Mayer's haematoxylin. The specificity of immune-labelling was verified using negative control slides from which the primary antibody was omitted. The intensity of immune-labelling of neutrophils for kinin B1 and B2 receptors was assessed by quantitative bright field microscopy using Image J software. The intensity of immunolabelling was determined for a minimum of 100 cells for each sample, and mean intensities were then calculated.
Measurement of cytokines and biochemical markers in plasma
Blood samples for the measurement of cytokines and neutrophil elastase were anticoagulated with acid–citrate dextrose and, after centrifugation, the plasma was stored at −80°C. Plasma concentrations of IL-6, IL-8, IL-10 and TNF-α were measured using specific ELISA kits (BD Biosciences, Sydney, Australia). Neutrophil elastase concentrations were measured using an ELISA kit (Bender MedSystems, Vienna, Austria). Heparinized plasma samples were used for the measurement of CRP and troponin I concentrations by routine immunoassays, and CK and lactate concentrations were measured by routine biochemical assays on an automated analyser (ARCHITECT ci16200, Abbott Diagnostics, Sydney, Australia).
Measurement of plasma bradykinin levels
Blood was collected in tubes containing a cocktail of protease and kininase inhibitors (0.4 mg/ml aprotinin, 4 mg/ml soybean trypsin inhibitor, 10 mM captopril, 10 mM phosphoramidon and 60 mM EDTA) to prevent proteolytic degradation of bradykinin. After centrifugation, the plasma was stored at −80°C until bradykinin levels were assayed, using a commercially available enzyme immunoassay kit (Bachem, Inc., Torrance, CA, USA).
Statistical analysis
Data for kinin receptor expression and neutrophil elastase concentrations are presented as medians with interquartile range. Data for cytokine, bradykinin, CK, troponin I, CRP and lactate levels are presented as mean values with 95% confidence intervals. Comparisons of mean values between the RIPC and control groups were performed using unpaired t-tests, while χ2-square tests were used for categorical data. Comparisons of the intensity of kinin receptor expression and other median values between the RIPC and control groups were performed using the Mann–Whitney U-test. Within-group comparisons of the intensity of kinin receptor expression at different time points were performed using the Kruskal–Wallis test followed by Dunn's multiple comparison test. A P-value <0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
RESULTS
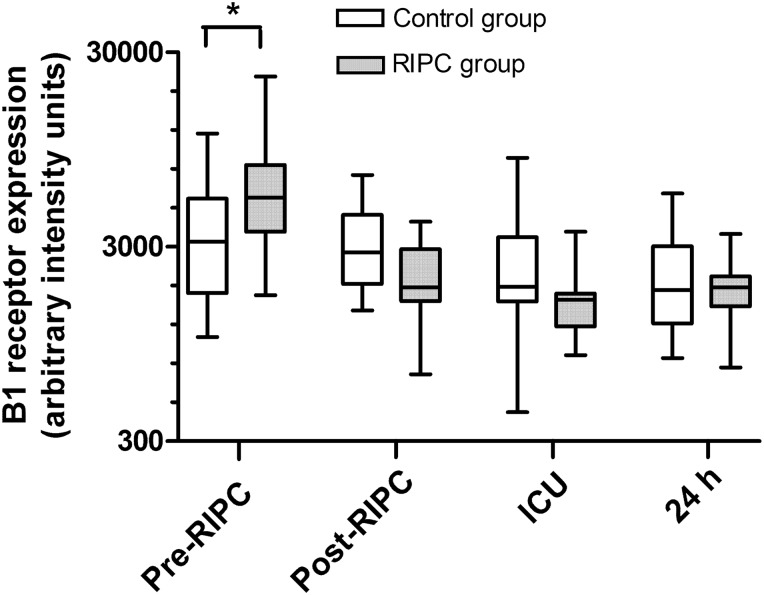
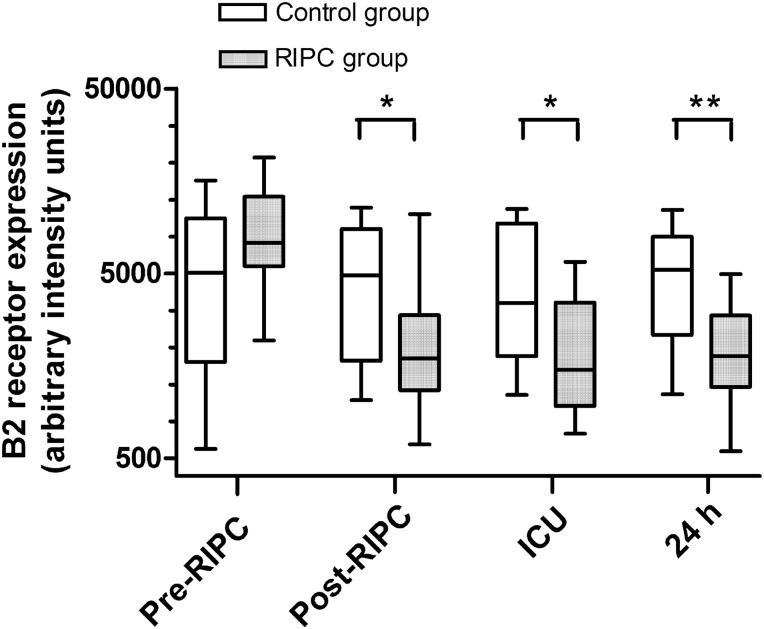
At baseline (pre-RIPC), kinin B1 receptor expression in neutrophils was significantly greater in the RIPC group than in the control group (P < 0.05) (Figs 1 and 2). At subsequent time points, kinin B1 receptor expression did not differ significantly between the groups. However, within-group analysis showed that in the RIPC group, kinin B1 receptor expression was significantly down-regulated after RIPC, and this decrease in B1 receptor expression persisted at the ICU and 24 h time points (P < 0.001). There were no significant within-group changes in B1 receptor expression in the control group. Kinin B2 expression did not differ between the groups at baseline (pre-RIPC) but was significantly lower in the RIPC group than in the control group post-RIPC (P < 0.05), in ICU (P < 0.05), and at the 24 h time point (P < 0.01) (Figs 3 and 4). In addition, in the RIPC group, kinin B2 receptor expression was significantly down-regulated after RIPC, and this decrease in B2 receptor expression persisted at the ICU and 24-h time points (P < 0.001). There were no significant within-group changes in B2 receptor expression in the control group.
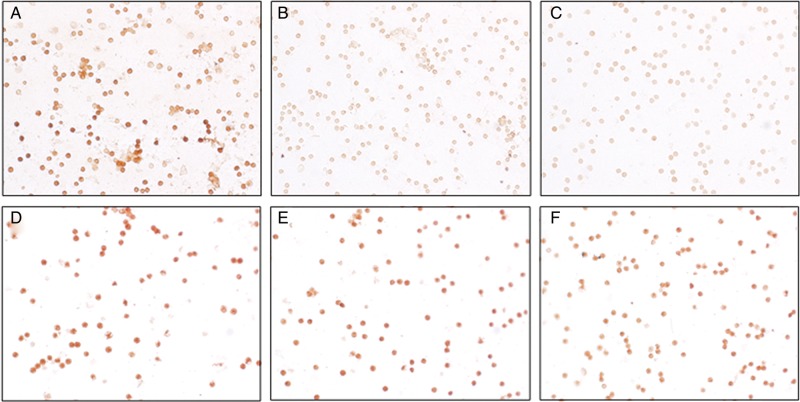
Figure 1:
Kinin B1 receptor expression on neutrophils. Representative images showing immunolabelling (DAB staining) of B1 receptors in neutrophils from a patient in the remote ischaemic preconditioning (RIPC) group (A–C) and the control group (D–F) before RIPC/sham (A and D), immediately post-RIPC/sham (B and E) and at 24 h after RIPC/sham (C and F).
Figure 2:
Quantitative image analysis of kinin B1 receptor expression on neutrophils of patients undergoing CABG surgery. Patients in the remote ischaemic preconditioning (RIPC) group (n = 15) received RIPC immediately prior to surgery, whereas patients in the control group (n = 15) did not. Expression of kinin B1 receptors on peripheral blood neutrophils was assessed immediately before RIPC (pre-RIPC), immediately after RIPC (post-RIPC), on admission to the intensive care unit (ICU) and 24 h after surgery (24 h). Data are median values with interquartile range (boxes) and range (whiskers). *P < 0.05 for comparisons between the groups. In the RIPC group, B1 receptor expression was significantly lower post-RIPC, in ICU and at 24 h compared with pre-RIPC (P < 0.001).
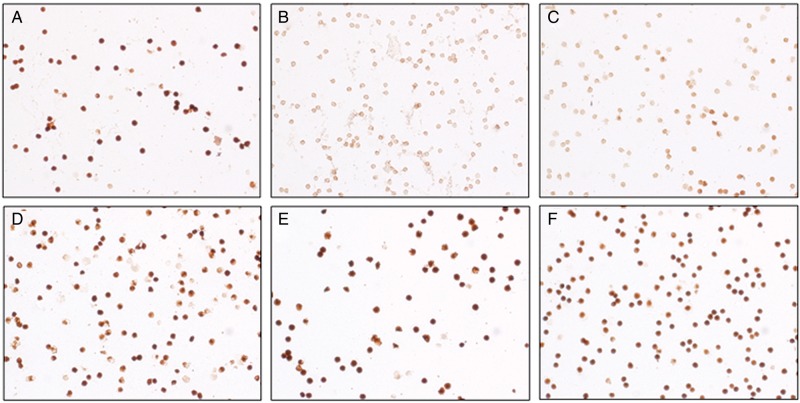
Figure 3:
Kinin B2 receptor expression on neutrophils. Representative images showing immunolabelling (DAB staining) of B2 receptors in neutrophils from a patient in the remote ischaemic preconditioning (RIPC) group (A–C) and the control group (D–F) before RIPC/sham (A and D), immediately post-RIPC/sham (B and E) and at 24 h after RIPC/sham (C and F).
Figure 4:
Quantitative image analysis of kinin B2 receptor expression on neutrophils. Patients in the remote ischaemic preconditioning (RIPC) group (n = 15) received RIPC immediately prior to surgery, whereas patients in the control group (n = 15) did not. Expression of kinin B2 receptors on peripheral blood neutrophils was assessed immediately before RIPC (pre-RIPC), immediately after RIPC (post-RIPC), on admission to the intensive care unit (ICU) and 24 h after surgery (24 h). Data are median values with interquartile range (boxes) and range (whiskers). **P < 0.01 and *P < 0.05 for comparisons between the groups. In the RIPC group, B2 receptor expression was significantly lower post-RIPC, in ICU and at 24 h compared with pre-RIPC (P < 0.001).
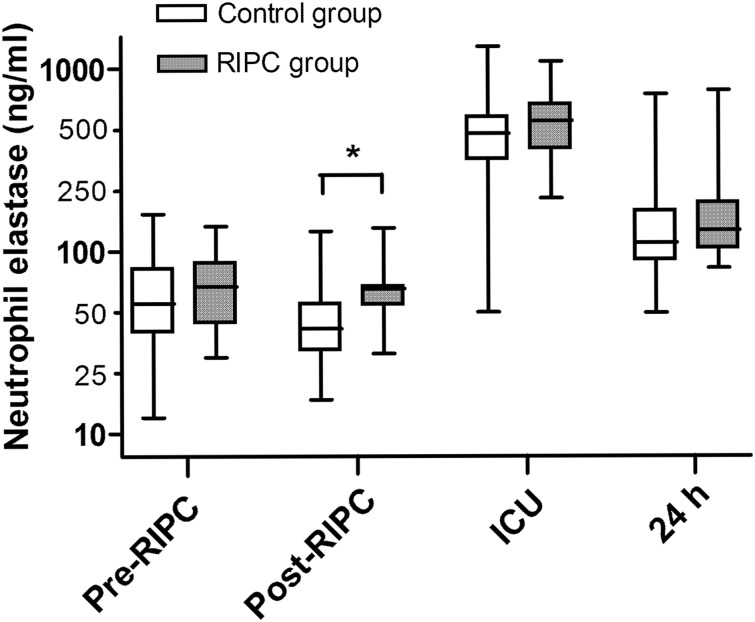
Neutrophil elastase concentrations were significantly elevated in all patients after surgery and tended to return to baseline during 24 h after surgery (Fig. 5). RIPC had no effect on post-surgery levels of neutrophil elastase. In all patients, the bradykinin levels remained stable at all time points and were not changed after surgery, although the RIPC patients had slightly higher bradykinin levels at 6 h (P = 0.03) (Table 2). There were no significant differences between the groups in the bradykinin levels at any other time point. There were no differences between the RIPC and control groups in the levels of IL-6, IL-8, IL-10 or TNF-α at any time point (Table 2). There were also no differences in gross clinical markers of IR injury (Table 3). Clinically, all patients did well and had an uncomplicated postoperative course. There were no differences between the groups in length of hospital stay.
Figure 5:
Neutrophil elastase levels in patients undergoing CABG surgery. Neutrophil elastase concentrations were measured immediately before remote ischaemic preconditioning (RIPC) (pre-RIPC), immediately after RIPC (post-RIPC), on admission to the intensive care unit (ICU) and 24 h after surgery (24 h). Data are median values with range (whiskers). P < 0.05 for comparisons between the group.
Table 2:
Plasma levels of cytokines and bradykinin in control patients and in patients who received remote ischaemic preconditioning before surgery
| Marker | Control group | RIPC group | P-value |
|---|---|---|---|
| IL-6 (pg/ml) | |||
| Baseline | 4.32 (3.03–5.61) | 4.17 (2.0–6.33) | 0.25 |
| Post-RIPC | 4.1 (2.89–5.31) | 3.61 (1.95–5.26) | 0.13 |
| 30 min | 147.1 (95.1–199.0) | 160.2 (110.4–210.1) | 0.63 |
| 24 h | 154.1 (112.2–196.1) | 119.2 (84.6–153.7) | 0.22 |
| IL-8 (pg/ml) | |||
| Baseline | 4.42 (2.49–6.35) | 3.61 (2.17–5.05) | 0.40 |
| Post-RIPC | 7.29 (1.23–15.8) | 5.1 (0.74–9.46) | 0.43 |
| 30 min | 47.28 (30.75–63.81) | 47.42 (31.57–63.26) | 1.0 |
| 24 h | 17.48 (12.57–22.4) | 12.76 (8.89–16.63) | 0.14 |
| IL-10 (pg/ml) | |||
| Baseline | 19.89 (17.7–57.48) | 2.62 (2.24–2.99) | 0.12 |
| Post-RIPC | 19.87 (17.37–57.1) | 2.69 (2.03–3.36) | 0.9 |
| 30 min | 141.7 (90.74–192.6) | 94.09 (55.13–133.1) | 0.11 |
| 24 h | 27.54 (3.1–51.98) | 13.75 (8.07–19.43) | 0.19 |
| TNF-α (pg/ml) | |||
| Baseline | 1.7 (0.88–2.53) | 2.11 (0.99–3.23) | 0.23 |
| Post-RIPC | 1.42 (0.75–2.09) | 1.45 (0.71–2.2) | 0.48 |
| 30 min | 1.45 (0.94–1.96) | 2.17 (1.21–3.13) | 0.41 |
| 24 h | 1.57 (0.9–2.24) | 1.74 (0.68–2.8) | 0.95 |
| Bradykinin (ng/ml) | |||
| Baseline | 0.64 (0.44–1.71) | 0.16 (0.11–0.2) | 0.71 |
| Post-RIPC | 0.98 (0.86–2.82) | 0.12 (0.09–0.14) | 0.83 |
| 30 min | 0.33 (0.1–0.57) | 0.27 (0.19–0.35) | 0.71 |
| 6 h | 0.28 (0.007–0.55) | 1.19 (0.24–2.62) | 0.03 |
| 12 h | 0.94 (0.27–2.15) | 0.46 (0.09–1.02) | 0.87 |
| 24 h | 0.82 (0.52–2.17) | 2.6 (0.39–5.58) | 0.75 |
Data are mean values with 95% confidence intervals in parentheses.
P-values were calculated using the Mann–Whitney U-test. RIPC: remote ischaemic preconditioning; IL: interleukin; TNF: tumor necrosis factor.
Table 3:
Plasma levels of C-reactive protein, creatine kinase, troponin I and lactate in control patients and in patients who received remote ischaemic preconditioning before surgery
| Marker | Control group | RIPC group | P-value |
|---|---|---|---|
| CK (U/l) | |||
| Post-RIPC | 97.64 (57.48–137.8) | 108.1 (51.51–164.8) | 0.91 |
| 30 min | 408.1 (301.7–514.6) | 381.5 (217.8–545.2) | 0.17 |
| 6 h | 522.0 (394.8–649.2) | 478.8 (323.9–633.6) | 0.24 |
| 12 h | 515.9 (416.3–615.6) | 477.1 (346.9–607.3) | 0.22 |
| 24 h | 576.7 (423.0–730.5) | 565.4 (350.4–780.4) | 0.49 |
| 48 h | 472.6 (345.5–599.7) | 465.8 (265.0–666.6) | 0.3 |
| Troponin I (µg/l) | |||
| Post-RIPC | 0.64 (0.63–1.92) | 0.09 (0.02–0.21) | 0.6 |
| 30 min | 6.54 (1.53–11.56) | 3.61 (1.4–5.82) | 0.27 |
| 6 h | 14.17 (5.19–23.16) | 10.14 (2.53–17.74) | 0.58 |
| 12 h | 6.55 (2.43–10.67) | 4.79 (1.91–7.68) | 0.39 |
| 24 h | 2.94 (1.57–4.32) | 2.28 (0.65–3.91) | 0.09 |
| 48 h | 1.75 (0.88–2.62) | 1.18 (0.35–2.01) | 0.05 |
| CRP (mg/l) | |||
| Post-RIPC | 5.2 (0.04–10.36) | 2.06 (0.94–3.18) | 0.65 |
| 30 min | 3.92 (0.25–7.59) | 1.73 (0.76–2.7) | 0.64 |
| 6 h | 13.15 (6.59–19.71) | 13.2 (9.4–17.0) | 0.47 |
| 12 h | 45.67 (34.91–56.42) | 52.31 (39.92–64.7) | 0.57 |
| 24 h | 122.3 (103.9–140.6) | 137.5 (112.7–162.3) | 0.61 |
| 48 h | 194.7 (166.2–223.1) | 199.4 (154.9–244.0) | 0.9 |
| Lactate (mmol/l) | |||
| Post-RIPC | 1.47 (1.13–1.81) | 1.57 (1.14–1.99) | 0.75 |
| 30 min | 1.8 (1.46–2.14) | 1.79 (1.37–2.22) | 0.77 |
| 6 h | 1.58 (1.24–1.92) | 1.61 (1.28–1.94) | 0.81 |
| 12 h | 1.94 (0.82–3.05) | 1.4 (1.09–1.71) | 0.28 |
| 24 h | 1.44 (1.25–1.64) | 1.43 (1.17–1.69) | 0.93 |
| 48 h | 1.75 (1.42–2.09) | 1.45 (1.21–1.7) | 0.11 |
Data are mean values with 95% confidence intervals in parentheses.P-values were calculated using the Mann–Whitney U-test. RIPC: remote ischaemic preconditioning; CK: creatine kinase; CRP: C-reactive protein.
DISCUSSION
During the last decade, RIPC has been applied in clinical practice following successful animal experiments. The exact mechanism of the RIPC is unknown. Our previous studies demonstrated that the RIPC modified genomics and proteomics of blood [9, 18] as well as neutrophil functional responses [10] and down-regulated of kinin receptors on the surface of neutrophils in normal healthy volunteers [16]. Thus, we aimed at assessing kinin receptor expression in patients undergoing cardiac surgery with CPB in the present study.
There is a close association between IR injury and the leucocyte-mediated inflammatory response [17, 19, 20]. Bradykinin is involved in the inflammatory response by regulating the expression of adhesion molecules and the infiltration of leucocytes into the tissues [21]. Bradykinin in low doses attenuates IR-induced leucocyte recruitment and microvascular dysfunction through B2 receptor complex-dependent nitric oxide production [22]. Shigematsu et al. [23] used a rat mesentery model to demonstrate that IR injury increased the numbers of rolling, adherent and emigrated neutrophils, and that these effects were prevented by bradykinin-mediated preconditioning. The dual role of bradykinin in causing IR injury and protecting against myocardial IR injury is well recognized. Bradykinin mediates ischaemic preconditioning, and the B2 receptor antagonist, HOE-140, abolishes this cardioprotective effect in animal models [24, 25]. We have previously demonstrated that in healthy human volunteers, an RIPC stimulus down-regulates the expression of B1 and B2 receptors for up to 24 h following RIPC [16] and discussed the concept of kinin receptor internalization via the signalosomes. Bradykinin binds to the kinin receptors in neutrophils and the receptor then rapidly internalizes within the cell through the formation of a signalosome [16]. The activation of this signalling pathway results in the formation of phosphatidylinositol 3-kinase, which is responsible for the activation of a protein kinase, as well as downstream activation of nitric oxide synthase. The NO that is produced then causes the activation of mitochondrial protein kinase G. This intracellular signalling may transfer to mitochondria and result in cytoprotection [16]. Therefore, decreased expression of kinin receptors may, in part, explain the multiorgan protective effect observed in patients who underwent RIPC [3–7]. The role of kinins in this setting appears to be 2-fold. Bradykinin is not only involved in inflammatory response through the induction of chemotaxis and the activation of neutrophils [11], but may also attenuate the proinflammatory response, resulting in protection against IR injury [22, 23]. The dual role of bradykinin in injury and cellular protection raises several questions regarding the pattern of bradykinin release and its protective effect, the interaction between kinins and other mediators involved in preconditioning induced protection, and how best to manipulate this pathway for wider applicability in the clinical setting.
Although the RIPC had demonstrated protective effects in an experimental setting, its clinical translation into surgical practise is still limited. Some of the most important changes following IR injury during cardiac surgery involve the cardiovascular and pulmonary systems. Cardiac surgery with CPB caused the activation of neutrophils as demonstrated by the immediate increase in neutrophil elastase levels in all patients in this study. We have previously demonstrated that activated neutrophils become adherent to vascular endothelium in the pulmonary capillaries and cause post-CPB systemic inflammatory response [17]. Here, we confirmed the down-regulation of kinin receptors in neutrophils after RIPC in patients undergoing CABG. Down-regulation of both B1 and B2 receptors occurred after RIPC, and this persisted for at least 24 h. However, whether this consistent down-regulation of the kinin receptors translates into clinically measurable decrease in post-CPB inflammatory response or not is yet to be established. It appears that kinin receptors might be involved in the mechanism of RIPC and further investigation of these inflammatory pathways may lead to a better understanding of RIPC-induced perioperative multiorgan protection.
LIMITATIONS
This study was not designed or powered to evaluate the effects of the RIPC on the systemic inflammatory response to CPB or clinical outcomes. It was powered to identify changes in kinin receptors expression and, although the markers of inflammatory responses were assessed, the study was not powered to identify the differences.
CONCLUSION
RIPC down-regulated the expression of kinin B1 and B2 receptors in neutrophils of patients undergoing CABG.
Funding
This study was supported by the National Health and Medical Research Council of Australia grant # 557507.
Conflict of interest: none declared.
REFERENCES
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Przyklenk K, Bauer B, Ovize M, Kloner R, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 3.Cheung MMH, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery. J Am Coll Cardiol. 2006;47:2277–82. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 4.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116(11 Suppl):I98–105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 5.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–7. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet. 2007;370:575–9. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 7.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–5. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–50. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158:155–61. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, et al. Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol. 2006;80:117–24. doi: 10.1189/jlb.1205744. [DOI] [PubMed] [Google Scholar]
- 12.Tio RA, van Gilst WH, Rett K, Wolters K, Dietze GJ, Wesseling H. The effects of bradykinin and the ischemic isolated rat heart. Horm Metab Res Suppl. 1990;22:85–9. [PubMed] [Google Scholar]
- 13.Pan HL, Chen SR, Scicli GM, Carretero OA. Cardiac interstitial bradykinin release during ischemia is enhanced by ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2000;279:H116–21. doi: 10.1152/ajpheart.2000.279.1.H116. [DOI] [PubMed] [Google Scholar]
- 14.Leesar MA, Stoddard MF, Manchikalapudi S, Bolli R. Bradykinin-induced preconditioning in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999;34:639–50. doi: 10.1016/s0735-1097(99)00297-1. [DOI] [PubMed] [Google Scholar]
- 15.Wei M, Wang X, Kuukasjärvi P, Laurikka J, Rinne T, Honkonen EL, et al. Bradykinin preconditioning in coronary artery bypass grafting. Ann Thorac Surg. 2004;78:492–7. doi: 10.1016/j.athoracsur.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Saxena P, Shaw OM, Misso NL, Naran A, Shehatha J, Newman MA, et al. Remote ischemic preconditioning stimulus decreases the expression of kinin receptors in human neutrophils. J Surg Res. 2011;171:311–6. doi: 10.1016/j.jss.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Ilton MK, Langton PE, Taylor ML, Misso NL, Newman M, Thompson PJ, et al. Differential expression of neutrophil adhesion molecules during coronary artery surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1999;118:930–7. doi: 10.1016/s0022-5223(99)70064-4. [DOI] [PubMed] [Google Scholar]
- 18.Hepponstall M, Ignjatovic V, Binos S, Monagle P, Jones B, Cheung MH, et al. Remote Ischemic Preconditioning (RIPC) modifies plasma proteome in humans. PLoS One. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol Gastrointestinal Liver Physiol. 1989;257:G683–8. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol Heart Circ Physiol. 1987;253:H699–703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 21.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Concentration-dependent effects of bradykinin on leukocyte recruitment and venular hemodynamics in rat mesentery. Am J Physiol. 1999;277:H152–60. doi: 10.1152/ajpheart.1999.277.1.H152. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Bradykinin prevents postischemic leukocyte adhesion and emigration and attenuates microvascular barrier disruption. Am J Physiol Heart Circ Physiol. 1999;277:H161–71. doi: 10.1152/ajpheart.1999.277.1.H161. [DOI] [PubMed] [Google Scholar]
- 23.Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Postischemic anti-inflammatory effects of bradykinin preconditioning. Am J Physiol Heart Circ Physiol. 2001;280:H441–54. doi: 10.1152/ajpheart.2001.280.1.H441. [DOI] [PubMed] [Google Scholar]
- 24.Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77:611–21. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- 25.Starkopf J, Bugge E, Ytrehus K. Preischemic bradykinin and ischemic preconditioning in functional recovery of the globally ischemic rat heart. Cardiovasc Res. 1997;33:63–70. doi: 10.1016/s0008-6363(96)00195-2. [DOI] [PubMed] [Google Scholar]