Abstract
OBJECTIVES
The present study investigated the potential of the failing myocardium of patients with ventricular assist devices (VAD) to respond to physiological growth stimuli, such as exercise, by activating growth signalling pathways. This may be of therapeutic relevance in identifying novel pharmacological targets for therapies that could facilitate recovery after VAD implantation.
METHODS
Twenty-two patients bridged to heart transplantation (HTx) with VAD were included in the study. A group of patients underwent moderate intensity aerobic exercise (GT), while another group of patients did not receive exercise training (CG). Thyroid hormone receptor alpha1 (TRα1) protein and total (t) and phosphorylated (p) protein kinase B (Akt) and c-Jun N-terminal kinase (JNK) kinase signalling were measured in myocardial tissue by western blotting at pre-VAD and pre-HTx period. In addition, Thyroid hormone (TH) levels were measured in plasma.
RESULTS
Peak oxygen consumption (VO2) at pre-HTx period was higher in patients subjected to training protocol [18.0 (0.8) for GT when compared with 13.7 (0.7) for CG group, P = 0.002]. N-terminal-prohormone of brain natriuretic peptide (NT-proBNP) levels were 1068 (148) for CG vs 626 (115) for GT group, P = 0.035. A switch towards up-regulation of physiological growth signalling was observed: the ratio of p-Akt/t-Akt was 2-fold higher in GT vs CG, P < 0.05 while p-JNK/t-JNK was 2.5-fold lower (P < 0.05) in GT vs CG, in pre-HTx samples. This response was accompanied by a 2.0-fold increase in TRα1 expression in pre-HTx samples with concomitant increase in circulating T3 in GT vs CG, P < 0.05. No differences in peak VO2, NT-proBNP, T3, TRα1, p/t-AKT and p/t-JNK were found between groups in the pre-VAD period.
CONCLUSIONS
The unloaded failing myocardium responded to physical training by enhancing thyroid hormone signalling. This response was associated with an up-regulation of Akt and suppression of JNK activation.
Keywords: Thyroid hormone, Heart failure, Exercise training, Thyroid hormone receptor alpha1, Kinase signalling
INTRODUCTION
Implantation of ventricular assist devices (VAD) is considered an effective treatment for end-stage heart failure, either as a bridge to transplantation or as destination therapy. Interestingly, this therapeutic approach has led to the recognition that the myocardium may retain the ability to improve its functional state even at terminal stages [1]. Thus, patients treated with clenbuterol or medications targeting the neurohormonal system activation were shown to improve cardiac function and even undergo VAD explantation [1, 2]. Along this line, a recent case report demonstrated a complete recovery in a young patient facilitated by increased thyroid hormone levels due to amiodarone use [3]. This may indicate a potential implication of thyroid hormone signalling in the pathophysiology of heart failure [4]. Indeed, there is a growing experimental work showing that thyroid hormone receptor alpha1 (TRα1) is critical for the growth response of the myocardium to ischaemic stress and/or mechanical loading [5, 6]. Pharmacological inhibition of this receptor eliminates compensatory hypertrophy and accelerates the transition to heart failure [6], while treatment with thyroid hormone results in physiological growth and enhanced functional recovery after experimental myocardial infarction [7, 8].
On the basis of this evidence, the present study investigated whether changes in thyroid hormone signalling can occur in the unloaded, failing myocardium in response to physical training. Identification of novel molecular drivers of stress-induced physiological growth may be of therapeutic relevance. Pharmacological mimicking of the exercise action may potentiate its effect and result in new effective treatments that may facilitate recovery in patients with end-stage heart failure and VAD.
MATERIALS AND METHODS
Patient population
This study included patients from a small prospective ongoing randomized study, which aims to identify molecular changes (potential pharmacological targets) induced by physical training in the myocardium of patients with VAD in the Department of Cardiology (Onassis Cardiac Surgery Center). Patients are randomly assigned to a training (GT) or a control group (CG), respectively. Postoperatively, patients in the GT group participate in an in-hospital rehabilitation programme which includes respiratory physiotherapy, early mobilization, progressive aerobic and resistance exercise. The clinical protocol was approved by the Hospital Ethics Committee and written informed consent was obtained from each patient after full explanation of the purpose and nature of all procedures used. From this ongoing study, the first 22 consecutive heart failure patients of a total of 26 patients implanted with either intracorporeal left ventricular assist device (LVAD) or with extracorporeal (EXCOR) LVAD or biventricular assist device (BiVAD) (Berlin Heart GmbH, Berlin, Germany) as a bridge to heart transplantation (HTx), were analysed. The EXCOR support system is a pulsatile volume displacement device, whereas intracorporeal support is a non-pulsatile continuous-flow rotary pump with axial configuration propelling the blood continuously to assist ventricular output. Four of 26 patients died in the period after VAD implantation and did not reach transplantation.
Training protocol
Patients in both groups were advised to walk every day for 30–45 min. In addition, patients in the GT group performed exercise training using a bicycle or treadmill at home for 45 min and at moderate intensity of 12–14 of the Borg scale, four times a week. They also underwent high-intensity inspiratory muscle training (IMT) at 60% of SPimax to exhaustion, three times a week in hospital using a computer running on a purpose-designed software programme (TRAINAIR, Project Electronics Ltd, Kent, UK) as previously described [9]. The training period lasted for 12 weeks. After this period and up to HTx, patients in this group continued to perform aerobic training as above while IMT was performed at home using an incentive spirometer (Spiro-ball, Leventon Co., Barcelona, Spain) for 15 min during most days of the week. All patients included in Group GT maintained training at home according to the provided instructions.
Cardiopulmonary exercise test
The patients performed a cardiopulmonary exercise test to evaluate their exercise capacity by measuring peak oxygen consumption (peak VO2) in millilitres per kilograms per min. Exercise testing with respiratory gas exchange measurements was performed using the Medgraphics CPX/MAX (Medical Graphics Corp., St. Paul, MN, USA) measuring system, with the patients exercising on a treadmill according to the Dargie protocol. Breath-by-breath respiratory gas analysis for the measurement of oxygen and carbon dioxide was obtained every 30 s online at rest, throughout exercise and during the recovery period. Peak VO2 during exercise was calculated as the mean value during the last minute of exercise.
Thyroid hormone measurements
Total plasma T3, T4 and thyroid stimulating hormone (TSH) levels were measured in all patients at pre-VAD period as well as at pre-HTx period. Blood samples were obtained from an antecubital vein and after centrifugation serum was collected and total T3 and T4 were determined using a Hitachi Modular E170 Chemistry Analyzer, while TSH was measured using ADVIA Centaur Immunoassay System (Siemens Healthcare, Erlangen, Germany). The normal values of thyroid hormones and TSH ranged from 0.8 to 1.6 ng/ml for total T3, 58 to 156 nmol/l (4.5–12 μg/dl) for total T4 and 0.3 to 3.8 mIU/l for TSH. Intra-assay and interassay precision (CV%) for thyroid hormone measurements were found to be 8 and 4%, respectively.
Molecular analysis of myocardial tissue samples
Myocardial tissue samples were obtained from the left ventricular apex of the heart at the time of VAD implantation (pre-VAD) and subsequently at HTx (pre-HTx) following VAD support. Left ventricular tissue was homogenized in ice-cold buffer (A) containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH: 7.8), 10 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM ethylene glycol tetraacetic acid (EGTA), 0.5 mM phenylmethanesulfonylfluoride (PMSF), 1 mM dithiothreitol (DTT) and 10 μg/ml leupeptin. Two hundred microlitres of 10% Igepal were added and samples were left in ice for 30 min. Homogenization was repeated and the homogenate was centrifuged at 1000 g for 5 min, 4°C. The pellet containing the nuclear fraction was washed again in buffer (A) with 1% Igepal, while the supernatant containing the cytosolic fraction was stored at −80°C. The final pellet was resuspended in 300 μl buffer (B) containing 20 mM HEPES (pH: 7.8), 420 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5 mM PMSF, 1 mM DTT, 10 μg/ml leupeptin and 10% glycerol and samples were incubated at 4°C for 60 min (under agitation) followed by centrifugation at 10 000 g for 5 min, 4°C. The supernatant containing the nuclear fraction was separated and stored at −80°C. Thyroid hormone receptors (TRs) protein expression was determined in nuclear fraction. Protein concentrations were determined by the bicinchoninic acid (BCA) method.
Samples were prepared for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) by boiling for 5 min in Laemmli sample buffer containing 5% 2-mercaptoethanol. Twenty micrograms (nuclear fraction) or 35 μg (cytosolic fraction) of total protein were loaded onto 7.5 or 10% (w/v) acrylamide gels and subjected to SDS–PAGE in a Bio-Rad Mini Protean gel apparatus. For western blotting, following SDS–PAGE, proteins were transferred electrophoretically to a nitrocellulose membrane (Hybond ECL, GE Healthcare Life Sciences, Buckinghamshire, UK) at 100 V and 4°C, for 1.5 h using Towbin buffer. After western blotting, filters containing the nuclear fraction were probed with specific antibodies against TRα1 (Abcam, ab53729, dilution 1:1000, o/n at 4°C), TRβ1 (Affinity Bioreagents, MA1–216, dilution 1:1000, o/n at 4°C) and histone H3 (Cell Signalling, #9715, dilution 1:1000, o/n at 4°C). In addition, filters containing the cytosolic fraction were probed with specific antibodies against total c-jun NH2-terminal kinases (JNKs), dual phospho-JNKs and total Akt and phospho-Akt (Ser473), (Cell Signalling Technology, dilution 1:1000) overnight at 4°C. Filters were incubated with appropriate anti-mouse (GE Healthcare Life Sciences, Buckinghamshire, UK) or anti-rabbit (New England Biolabs, Ipswich, MA 01938-2723, USA) horseradish peroxidase (HRP) secondary antibodies and immunoreactivity was detected by enhanced chemiluminescence using Lumiglo reagents (New England Biolabs, Ipswich, MA 01938-2723, USA). Two samples from each patient were analysed pre-VAD and pre-HTx. Normalization of nuclear protein loaded on the gel was performed by histone H3. Immunoblots were quantified using the Fluorchem HD2 Imaging Densitometer (Alpha Innotech Corporation, 14 743, Catalina Street, San Leandro, CA, USA). This study was performed according to the guidelines of the Declaration of Helsinki. All procedures involving human tissue use were approved by our Institutional Review Board.
Statistical analysis
Continuous variables are expressed as mean (standard error, SE), whereas categorical variables are reported as the number of patients per total group number or percentages (%). Independent-sample t-test or non-parametric Mann–Whitney U-test (for continuous variables) and χ2 test or Fisher's exact test (for dichotomized variables) were used to assess differences in parameters between groups. All tests were two-sided. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed using the statistical software package SPSS 17.0.
RESULTS
Patients characteristics
Baseline characteristics of patients before VAD implantation were similar in both groups. Mean age was 40.9 (4.9) years for CG vs 39.7 (4.3) for GT, P > 0.05. In addition, male patients represented 81.8% in CG vs 90.9% in GT, P > 0.05. Concerning the medical history, diabetes was present only in 1 of 11 patients in GT and in 0 of 11 patients in CG group. Hypertension and dyslipidemia were both found in 2/11 patients in CG when compared with 3/11 and 4/11 in GT, respectively, P > 0.05. In the majority of patients (8/11 in CG vs 9/11 in GT, P > 0.05), the aetiology of heart failure was non-ischaemic dilated cardiomyopathy. Standard laboratory values were not different between groups before VAD implantation (pre-VAD). In fact, creatinine values were 1.1 (0.13) mg/dl for CG vs 1.04 (0.07) mg/dl for GT group, P > 0.05. Haemoglobin levels were also found to be 11.4 (0.32) g/dl for CG vs 12.3 (0.42) g/dl for GT group, P > 0.05. No significant difference existed in medication regimen between the groups.
Six patients in the CG group and 7 in the GT group were implanted with LVAD while 5 in CG and 4 in GT were implanted with BiVAD. Concerning the type of flow, 8 patients were implanted with pulsatile flow VAD and three with continuous-flow VAD in each group. The duration of the VAD implantation was comparable among groups [363 (70) days for CG vs 434 (66) for GT group, P > 0.05]. At pre-HTx, no difference in standard laboratory values and medication regimen was observed between groups.
Functional response in trained patients with ventricular assist device implantation
Functional capacity as assessed by cardiopulmonary exercise test was not different between groups in the pre-VAD period. In fact, the mean values of peak VO2 before VAD implantation were 12.0 (0.8) ml/kg/min in CG when compared with 12.9 (1.2) in GT group, P > 0.05. In the pre-HTx period, peak VO2 was higher in patients subjected to training protocol [18.0 (0.8) for GT when compared with 13.7 (0.7) for CG group, P = 0.002].
Changes in N-terminal-prohormone of brain natriuretic peptide levels
In pre-VAD, NT-proBNP levels were 4780 (945) for CG vs 3700 (970) for GT group, P > 0.05. In pre-HTx, NT-proBNP levels were 1068 (148) for CG vs 626 (115) for GT group, P = 0.035.
Thyroid hormone levels in plasma
Total T3, T4 and TSH levels in the pre-VAD period were not different between groups. However, after VAD implantation, a significant increase in T3 levels was found in trained patients while T4 and TSH levels were similar between the two groups. Thus, in pre-VAD, total T3, T4 and TSH levels were found to be 0.65 (0.07) ng/ml, 8.1 (0.5) μg/dl and 2.03 (0.5) mIU/l, respectively, for group CG and 0.75 ng/ml (0.05), 7.8 (0.54) μg/dl and 2.01 (0.4) mIU/l, respectively, for group GT, P > 0.05. In pre-HTx, T3 levels were 0.92 (0.05) ng/ml for group CG when compared with 1.15 (0.03) ng/ml for group GT, P < 0.01. Levels of T4 and TSH were 8.9 (0.4) μg/dl and 1.8 (0.3) mIU/l, respectively, for group CG when compared with 8.3 (0.5) μg/dl and 1.4 (0.2) mIU/l, respectively, for group GT, P > 0.05.
MOLECULAR ANALYSIS
Thyroid hormone receptor protein expression
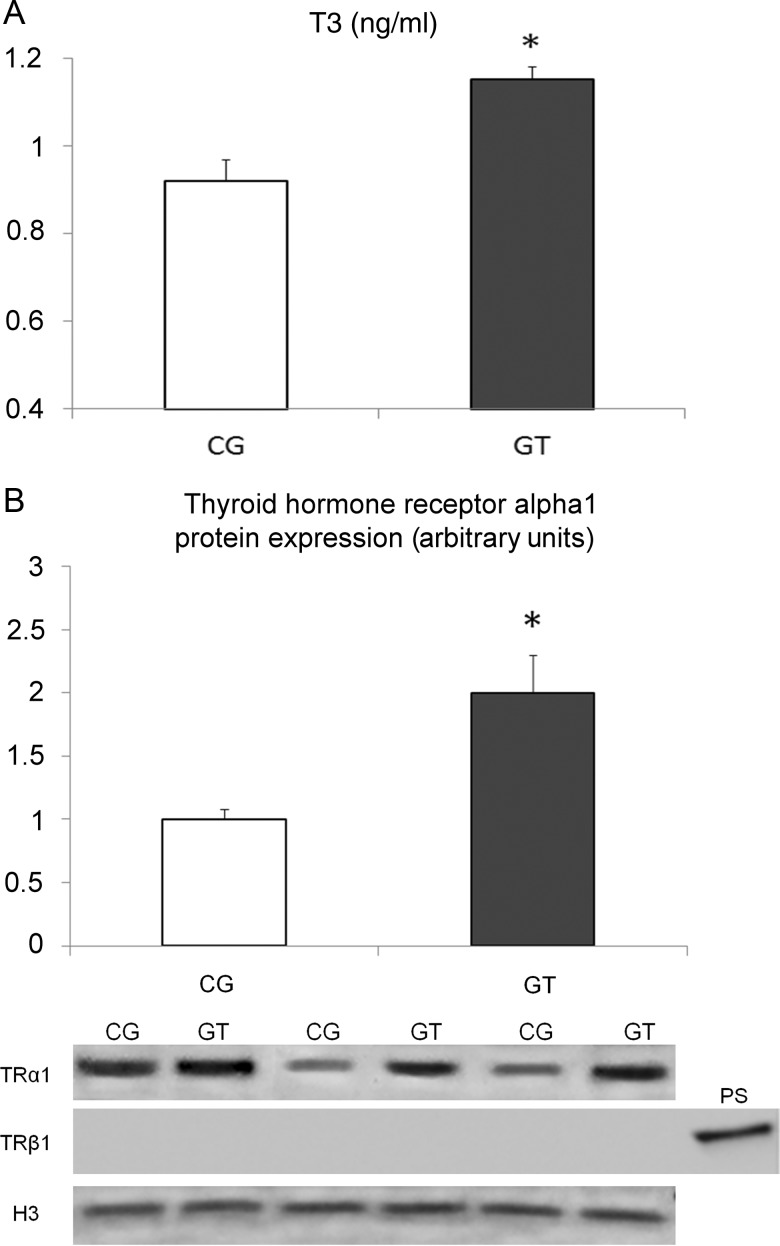
Protein expression analysis of the TRα1 in the nucleus showed a 2-fold increase in myocardial samples of trained patients obtained during HTx (P < 0.05 vs group CG, Fig. 1), while no statistical difference was observed between groups in samples obtained during VAD implantation. Thyroid hormone receptor beta1 (TRβ1) was nearly undetectable in all samples obtained both during VAD implantation, as well as during HTx (Fig. 1).
Figure 1:
Levels of total T3 (A) and densitometric assessment-representative western blot of TRα1 expression (B) in end-stage heart failure patients with VAD implantation subjected, or not, to exercise training (Group GT and Group CG, respectively). Serum samples and myocardial samples were obtained at HTx. It is interesting to note that levels of TRβ1 were undetectable. *P < 0.05 vs Group CG.
Protein kinase B and c-jun N-terminal kinases signalling in the myocardium
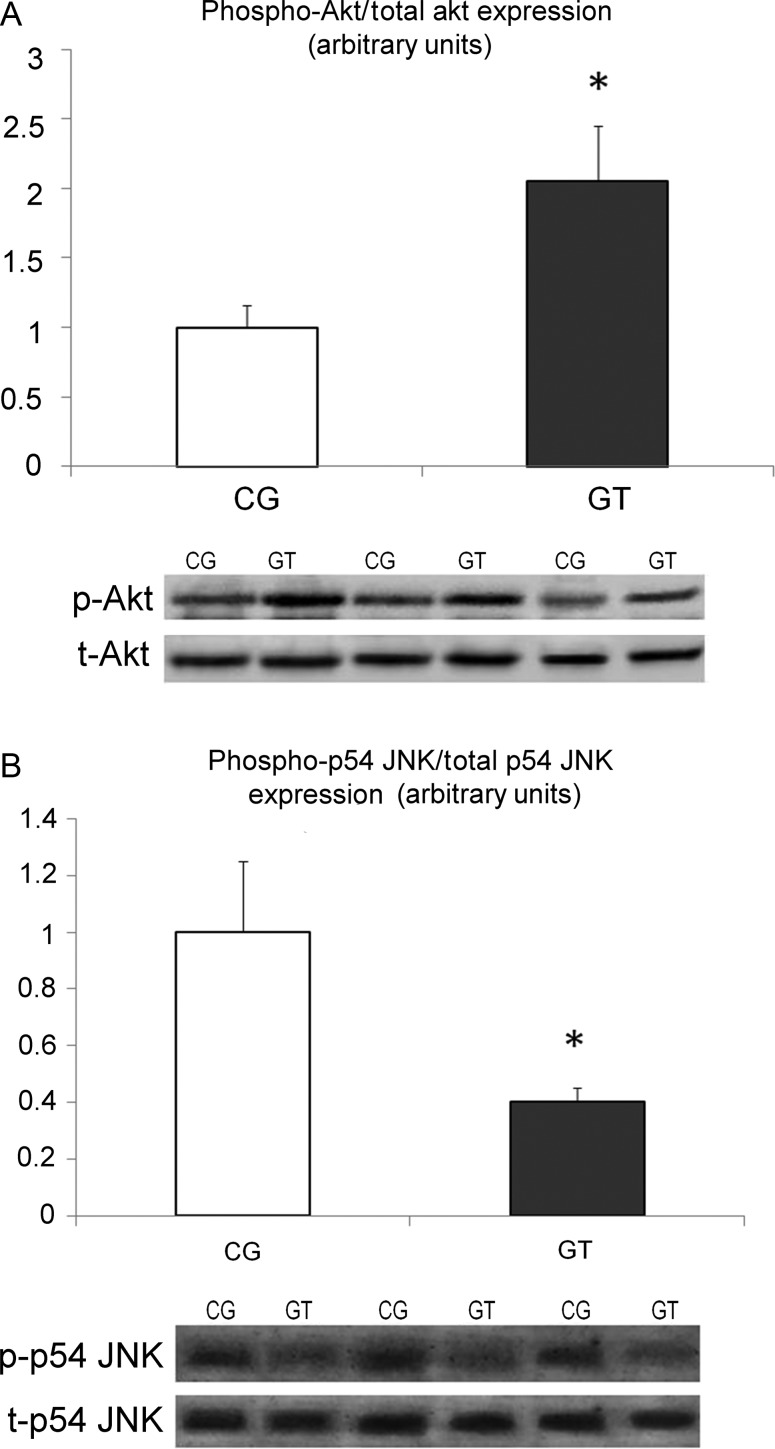
Analysis of myocardial samples obtained during VAD implantation revealed no differences in the ratio of p-Akt/total Akt and p-JNK/total JNK between groups (data not shown). In samples obtained during transplantation, the ratio of p-Akt/total Akt was increased 2-fold in Group GT, when compared with Group CG, P < 0.05 (Fig. 2). Furthermore, the ratio of p-JNK/total p54 JNK was found to be reduced 2.5-fold in Group GT when compared with Group CG, P < 0.05 (Fig. 2).
Figure 2:
Densitometric assessment and representative western blots of p-Akt/total Akt (A), and p-p54/total p54 JNK (B) are shown in end-stage heart failure patients with VAD implantation subjected, or not, to exercise training (Group GT and Group CG, respectively). Myocardial samples were obtained during HTx. *P < 0.05 vs Group CG.
DISCUSSION
The present study has provided some evidence showing that the failing myocardium has the ability to respond to physiological stimuli, such as physical training by activating growth signalling pathways. In this context, distinct changes of novel molecules, such TRs, were identified in the myocardium. Thyroid hormone signalling appears to be an important component of the adaptive response of the myocardium to stress [4]. Changes in TRs have been observed in the injured myocardium, which are of physiological relevance [10]. In particular, TRα1, the most abundant thyroid receptor in the myocardium, is shown to be over-expressed during pathological hypertrophy and down-regulated during the transition to heart failure [10]. Pharmacological inhibition of this receptor abolishes stress-induced growth response and accelerates myocardial infarction induced heart failure [6]. Furthermore, studies in cell-based models show that TRα1 is regulated by stress-activated intracellular kinase signalling and acts as a molecular switch for the conversion of pathological to physiological cellular growth depending on the thyroid hormone availability [11]. In accordance with this evidence, the present study found an increased expression of TRα1 in the myocardium with concomitant increase in the circulating T3 in the trained patients. The latter was probably due to the increased conversion of T4 to T3, since T4 levels were not found to be elevated. Activation of thyroid hormone signalling may act as a biological driver for the up-regulation of physiological growth signalling pathways. In fact, thyroid hormone is shown to activate the prosurvival signalling Akt in cardiomyocytes [12] and inactivate the antihypertrophic JNK [13] leading to physiological growth. Along the same line, a similar pattern of activation of those kinases was also observed in the trained myocardium. Akt activation was found to be significantly increased, whereas JNK activation was significantly suppressed. Here, it should be noted that controlled over-expression of Akt has been shown to result in moderate cardiac hypertrophy with preserved systolic function [14]. Furthermore, JNK is activated in heart failure, modulates the transcription factor FOXO3a and via this mechanism, can induce cardiac atrophy [15].
Taken together, these data show that physiological growth kinase signalling can be up-regulated in the failing myocardium by physiological stimuli, such as exercise. However, this response may not be sufficient to lead to complete recovery. In fact, although exercise improved peak oxygen consumption and significantly reduced NT-proBNP levels, patients still needed to be transplanted. Identification of important molecules implicated in growth response may allow new pharmacological treatments to emerge, aiming at potentiating the growth response and facilitating recovery after VAD implantation. In this context, thyroid analogues (e.g. targeting TRα1) may prove to be novel therapies for the management of heart failure. This issue is of important clinical and therapeutic relevance and probably merits further investigation. Intriguingly, the potentiation of thyroid hormone signalling by amiodarone use facilitated bridge-to-recovery in a patient with end-stage heart failure and VAD [3].
Limitations of the study
The present study has focused on the molecular signature imprinted on the human myocardium by physical training. This study has not explored the potential predictors for complete recovery after VAD implantation, such as parameters related to exercise response or changes in circulating thyroid hormone levels and other neurohormonal systems. Furthermore, due to the small number of patients, we did not explore whether a link between circulating thyroid hormone levels and cardiac function exists.
In conclusion, the unloaded failing myocardium responded to physical training by enhancing thyroid hormone signalling. This response was associated with an up-regulation of Akt and suppression of JNK activation. Physical training improved the functional status of patients with VAD, but did not result in complete recovery.
Funding
This work was supported by the Alexander S. Onassis Public Benefit Foundation
Conflict of interest: none declared.
REFERENCES
- 1.Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and molecular effects. Circ Heart Fail. 2011;4:224–33. doi: 10.1161/CIRCHEARTFAILURE.110.959684. doi:10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SR, Saeed O, Murthy S, Bhatia V, Shin JJ, Wang D, et al. Combining neurohormonal blockade with continuous-flow left ventricular assist device support for myocardial recovery: a single-arm prospective study. J Heart Lung Transplant. 2013;32:305–312. doi: 10.1016/j.healun.2012.11.019. doi:10.1016/j.healun.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Letsou GV, Reverdin S, Frazier OH. Thyrotoxicosis-facilitated bridge to recovery with a continuous-flow left ventricular assist device. Eur J Cardiothorac Surg. 2013;44:573–4. doi: 10.1093/ejcts/ezt106. [DOI] [PubMed] [Google Scholar]
- 4.Pantos C, Mourouzis I, Cokkinos DV. Thyroid hormone and cardiac repair/regeneration from Prometheus myth to reality? Can J Physiol Pharmacol. 2012;90:977–87. doi: 10.1139/y2012-031. doi:10.1139/y2012-031. [DOI] [PubMed] [Google Scholar]
- 5.Belke DD, Gloss B, Swanson EA, Dillmann WH. Adeno-associated virus-mediated expression of thyroid hormone receptor isoforms-alpha1 and -beta1 improves contractile function in pressure overload-induced cardiac hypertrophy. Endocrinology. 2007;148:2870–7. doi: 10.1210/en.2007-0009. doi:10.1210/en.2007-0009. [DOI] [PubMed] [Google Scholar]
- 6.Mourouzis I, Kostakou E, Galanopoulos G, Mantzouratou P, Pantos C. Inhibition of thyroid hormone receptor alpha1 impairs post-ischemic cardiac performance after myocardial infarction in mice. Mol Cell Biochem. 2013;379:97–105. doi: 10.1007/s11010-013-1631-9. [DOI] [PubMed] [Google Scholar]
- 7.Mourouzis I, Mantzouratou P, Galanopoulos G, Kostakou E, Roukounakis N, Kokkinos AD, et al. Dose-dependent effects of thyroid hormone on post-ischemic cardiac performance: potential involvement of Akt and ERK signalings. Mol Cell Biochem. 2012;363:235–43. doi: 10.1007/s11010-011-1175-9. doi:10.1007/s11010-011-1175-9. [DOI] [PubMed] [Google Scholar]
- 8.Pantos C, Mourouzis I, Markakis K, Dimopoulos A, Xinaris C, Kokkinos AD, et al. Thyroid hormone attenuates cardiac remodeling and improves hemodynamics early after acute myocardial infarction in rats. Eur J Cardiothorac Surg. 2007;32:333–9. doi: 10.1016/j.ejcts.2007.05.004. doi:10.1016/j.ejcts.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Laoutaris ID, Dritsas A, Adamopoulos S, Manginas A, Gouziouta A, Kallistratos MS, et al. Benefits of physical training on exercise capacity, inspiratory muscle function, and quality of life in patients with ventricular assist devices long-term postimplantation. Eur J Cardiovasc Prev Rehabil. 2011;18:33–40. doi: 10.1097/HJR.0b013e32833c0320. [DOI] [PubMed] [Google Scholar]
- 10.Pantos C, Mourouzis I, Galanopoulos G, Gavra M, Perimenis P, Spanou D, et al. Thyroid hormone receptor alpha1 downregulation in postischemic heart failure progression: the potential role of tissue hypothyroidism. Horm Metab Res. 2010;42:718–724. doi: 10.1055/s-0030-1255035. doi:10.1055/s-0030-1255035. [DOI] [PubMed] [Google Scholar]
- 11.Pantos C, Xinaris C, Mourouzis I, Perimenis P, Politi E, Spanou D, et al. Thyroid hormone receptor alpha 1: a switch to cardiac cell ‘metamorphosis’? J Physiol Pharmacol. 2008;59:253–69. [PubMed] [Google Scholar]
- 12.Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006;281:20666–72. doi: 10.1074/jbc.M512671200. doi:10.1074/jbc.M512671200. [DOI] [PubMed] [Google Scholar]
- 13.Pantos C, Xinaris C, Mourouzis I, Malliopoulou V, Kardami E, Cokkinos DV. Thyroid hormone changes cardiomyocyte shape and geometry via ERK signaling pathway: potential therapeutic implications in reversing cardiac remodeling? Mol Cell Biochem. 2007;297:65–72. doi: 10.1007/s11010-006-9323-3. doi:10.1007/s11010-006-9323-3. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–901. doi: 10.1074/jbc.M200347200. doi:10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 15.Chaanine AH, Jeong D, Liang L, Chemaly ER, Fish K, Gordon RE, et al. JNK modulates FOXO3a for the expression of the mitochondrial death and mitophagy marker BNIP3 in pathological hypertrophy and in heart failure. Cell Death Dis. 2012;3:265. doi: 10.1038/cddis.2012.5. doi:10.1038/cddis.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]