Abstract
Parathyroid carcinoma is a rare endocrine malignancy. The reported incidence is from 0.5 to 5% of primary hyperparathyroidism cases in various series. The cause is unknown, but clinical correlations with different genetic syndromes exist. Mutations in the HPRT2 gene seem to play a significant role in the pathogenesis of this disease. Men and women are equally affected, usually in the fourth or fifth decade of life. Most patients will present with signs and symptoms of hypercalcaemia. Cases of non-functioning carcinoma are exceedingly rare. Surgical resection is the most effective method of treatment and palliation. A significant proportion of patients will experience recurrence, and will need further surgical and, eventually, medical management of hypercalcaemia. The disease is progressive but slow growing. Most patients will require multiple operations to resect recurrent disease. The main cause of morbidity and mortality is the sequela of uncontrolled chronic hypercalcaemia rather than tumour burden. The current paper will review the epidemiology, pathogenesis, clinical presentation and diagnostic work-up of this disease. Surgical management in different scenarios is reviewed in detail, followed by other types of treatment and management of incurable disease.
Keywords: Parathyroid carcinoma, surgical management
Statement of Search Strategies Used and Sources of Information
A search was made on Medline, using PubMed clinical queries, of English-language literature using parathyroid carcinoma, parathyroid cancer, parathyroid malignancy and human in the following categories: epidemiology, cause, diagnosis and management. The full text of relevant papers was obtained and reviewed. Additional specific searches were made as necessary. Both original papers and reviews were examined, and those with the most reliable methodologies and highest citation rates were selected.
Introduction
Parathyroid carcinoma is a rare disease. Fewer than 1000 cases have been reported in the entire English-language literature [1]. Patients usually present with severe symptoms of hypercalcaemia, as if they are afflicted with ‘hyperparathyroidism on steroids’. Although clinically similar to adenoma, parathyroid carcinoma has its own unique features. Parathyroid adenomas are much more common in women, but carcinoma occurs with equal frequency in men and women [2–4]. On average, patients with carcinoma are 1 decade younger than patients with adenoma [2,5]. Both benign and malignant parathyroid cells are fairly resilient and can take hold in almost any tissue. In spite of this survival advantage, the disease commonly has an indolent and slow course, and most patients succumb to complications of relentless hypercalcaemia rather than tumour invasion and spread. None of these, however, are strict rules. Indeed, the very first documented case of parathyroid cancer was a non-functioning carcinoma reported by The Swiss surgeon, Fritz De Quervain, in 1904 [6]. His patient was a 68-year-old man who presented with a large neck mass and soon died of local recurrence and pulmonary metastases. De Quervain did not mention any signs or symptoms of hypercalcaemia. Uncommon presentations and courses continue to be reported in published studies. However, even in common scenarios, it is not always easy to differentiate between parathyroid carcinoma and adenoma. These features can mislead the managing practitioner and have a negative effect on treatment strategies and approaches. A less-than-optimal approach will deprive patients from receiving the best chance of cure, which to this date remains complete surgical excision [3–5,7]. Although no breakthroughs have been made in curative options, understanding of molecular pathogenesis of parathyroid carcinoma has expanded in recent years. New markers are helpful in making the diagnosis more certain in ambiguous cases. In addition, more medications have become available in management of incurable scenarios. The present brief overview will focus on the current principles of management of parathyroid cancer.
Epidemiology
By all accounts, parathyroid cancer is a rare entity [2,3]. The incidence and prevalence of disease are usually reported as the percentage of patients presenting with primary hyperparathyroidism, because prevalence in the general population is extremely low. The largest series comes from the USA, based on the National Cancer Data Base. Over the years, many different reports have estimated the incidence of parathyroid cancer as <1% in patients diagnosed with primary hyperparathyroidism [8–12]. Exceptions are reports from Japan, which report an incidence of about 5% [13,14]. Parathyroid carcinoma occurs equally in men and women, and the average patient is in the fourth or fifth decade of life, although patients as young as 8 years of age have been reported [15].
Pathogenesis and Molecular Biology
The cause of parathyroid cancer is unknown. However, a few revealing associations exist between cancer development and a number of clinical scenarios and genetic mutations. Prior neck radiation and end-stage renal disease have been associated with increased incidence of parathyroid cancer [16–19]. The correlation of radiation, however, is quite weak [3] and not as prominent as in the development of benign adenomas. Parathyroid cancer has also been reported in cases of MEN 1 [20–22] and MEN 2A [23], but not in any cases of MEN 2B. Increased risk is also observed in the rare autosomal-dominant disorder, familial hyperparathyroidism [24–27]. The most common chromosomal imbalances are losses of 1p and 13q. Gains are seen in 19p, Xc-q13, 9p33qter, 1q and 16p [28]. In contrast to adenoma, loss of 11q13 is not seen in carcinoma [28]. Several different mutations have been implicated in the pathogenesis of parathyroid cancer. These include the retinoblastoma (Rb) [29,30], p53 [31], breast carcinoma susceptibility (BRCA2) [32,33] and cyclin Dl/parathyroid adenomatosis gene 1 (PRAD1) genes [34,35]. None of these have been assigned a primary role in pathogenesis. The most extensive evidence comes from study of a rare genetic syndrome called ‘hereditary hyperparathyroidism–jaw tumour syndrome’ [36–38]. In this syndrome, patients develop primary hyperparathyroidism and mandibular and maxillary fibro-osseous lesions. In addition, up to 15% of patients can develop parathyroid cancer. The responsible gene is known as HRPT2 and codes for a nuclear protein named ‘parafibromin’ [22,38–49]. This mutation is found much more commonly in cases of sporadic parathyroid cancer than in benign adenomas (up to 76% of carcinomas vs 0.8–1.8% of adenomas) [41,50,51]. This evidence points to an important role for this mutation in the pathogenesis of parathyroid carcinoma. Parafibromin is mainly a nuclear protein [52–58] and acts as a regulator of transcription. Overexpression of this protein causes inhibition of cell proliferation and G1 phase arrest [59–61]. Non-functional isoforms, such as mutations in hereditary hyperparathyroidism–jaw tumour syndrome, have anti-apoptotic effects. More studies are underway to elucidate further mechanisms of tumourigenesis and growth modulation related to this protein.
Pathology
The diagnosis of parathyroid carcinoma can be challenging. These tumours are usually solitary and arise from a single gland; however, there have been reports of multi-glandular involvement [62]. Both right and left inferior glands have been counted as the most common location [3,8,63]. Ectopic tumours usually arise from glands in the mediastinum, but ectopic location does not increase the chance of malignant transformation of a gland. Cases of synchronous parathyroid carcinoma and adenoma have been reported [20]. Grossly, tumours are usually quite large with an average diameter of >3 cm and can weigh between 2 and 10 g. They are often irregular, firm to hard, and have a greyish-to-white colour [25]. Carcinomas are tenaciously adherent to adjacent tissues [64]. Tumour cells can infiltrate the ipsilateral lobe of the thyroid, the strap muscles and even the recurrent laryngeal nerve, trachea or oesophagus [3].
Microscopically, the tumour is surrounded by a capsule, and fibrous septa extend into the tumour creating a lobular appearance. Tumour cells are large and mainly consist of chief cells, with sparse oxyphil and transitional oxyphil cells between the chief cells [65]. Tumour cells are arranged in trabecular, solid or acinar structural patterns [66]. Mitotic figures are usually present. Focal calcification, cystic changes and coagulative necrosis can be seen, and capsular invasion is commonly seen. Vascular invasion is also seen but is less common than capsular invasion [10].
Clinical Presentation
The overwhelming majority of parathyroid cancers are functioning tumours. Therefore, patients most often present with symptoms and signs of hypercalcaemia [4,25,67–69]. Manifestations of hyperparathyroidism appear well before local invasion of tumour produces any symptoms. Symptoms of hypercalcaemia, such as fatigue, malaise, weakness, weight loss and anorexia, are common. Many organs are affected by elevated calcium levels. Psychiatric manifestations (i.e. depression) and digestive symptoms (e.g. nausea, vomiting, abdominal pain, peptic ulcer, pancreatitis and constipation) are all described. More prominent are symptoms of renal and skeletal involvement. Patients quite often complain of polyuria, renal colic and nephrolithiasis. Bone pain and pathological fractures are also reported. Most patients manifest evidence of both renal and skeletal involvement at the time of presentation, contrary to benign adenomas. On physical examination, up to 70% of patients present with a palpable neck mass; this is quite rare in benign disease [67].
Similar to benign primary hyperparathyroidism, levels of calcium and parathyroid hormone (PTH) are elevated and usually to an exaggerated degree. In a subset of patients with carcinoma, a different moiety of PTH is produced. The significance of this form is not yet clear [70]. Serum levels of alkaline phosphatase and α and β subunits of human chorionic gonadotrophin are also raised [71]. On imaging studies, evidence of bony involvement (e.g. salt and pepper skull, osteitis fibrosa cystica, subperiosteal bone resorption) can be seen [67].
Non-functioning carcinomas are exceedingly rare (19 cases since 1929), and usually present with signs and symptoms of local growth and invasion (e.g. neck mass, hoarseness, dysphagia) [72].
Differential Diagnosis
The primary goal is to differentiate between malignant and benign disease before any surgical attempts. This task can be quite difficult as there are no specific differentiating characteristics for parathyroid cancer. Usually, the effects of hypercalcaemia are more pronounced in carcinoma than adenoma, and a few effects occur much more frequently in patients with carcinoma. These characteristics are summarised in Table 1. In general, severe hypercalcaemia, younger age, male gender, hypercalcaemic crisis, concurrent bone and renal involvement, and palpable mass should raise the suspicion of malignant disease [25,67,73]. In cases of palpable disease or obvious mass, fine needle aspiration is not usually recommended. This is due to the extreme difficulty of differentiating between benign and malignant disease on cytology [74,75], in addition to the potential for seeding of tumour cells in the tract [76]. Differentiating between malignant and benign lesions based on tissue specimens can be quite difficult. The only unequivocal proof of malignancy is the presence of metastases. However, this feature is not commonly seen at presentation [77]. Many pathological criteria have been proposed to distinguish between benign and malignant lesions. Early criteria, such as those proposed by Schantz and Castleman in the 1970s [78], are valuable but not absolute. They describe using a combination of mitotic figures, fibrous trabeculae, and capsular and vascular invasion to determine malignancy. However, later investigators showed that none of these criteria are sufficiently sensitive or specific to confirm or discard the diagnosis reliably. Mitotic activity and trabecular patterns can be seen in benign lesions, as reported by McKeown et al. [79], Bondeson et al. [65] and others [80–82]. On the other hand, capsular invasion can only be seen in a portion of specimens, and vascular invasion in even fewer cases [83,84]. These difficulties, naturally, have intensified interest in developing other methods such as immunohistochemistry and DNA analysis [85]. No single marker thus far has shown to be perfectly sensitive and specific [67]. Staining for parafibromin [86], Rb expression [29,30,32], Ki-67 [87] and galectin-3 [88] have all been used. Most recent studies suggest using a combination of different markers. A recent report by Frenandez-Ranvier et al. [89] proposed using a combination of loss of parafibromin plus Rb expression and overexpression of galectin-3 as a reliable differentiating profile of parathyroid carcinoma from atypical adenoma and other non-malignant lesions.
Table 1.
Frequency of clinical features in benign vs malignant parathyroid tumours
| Benign disease |
Malignant disease |
|
|---|---|---|
| Women:men ratio | 3–4:1 | 1:1 |
| Average age at presentation (years) |
55 | 48 |
| Renal involvement | <20% | 56–84% |
| Serum calcium | ≤11.2 mg/dl | >14 mg/dl |
| Serum parathyroid hormone | Mild elevation |
3–10-fold elevation (up to 75-fold) |
| Palpable mass | Rare | 50–70% |
| Radiological skeletal features (osteitis fibrosa cystica etc.) |
<5% | 44–91% |
| Hypercalcaemic crisis | Very rare | More common |
Pathological diagnosis of parathyroid carcinoma continues to be a challenge. In the absence of a gold standard test, a multidisciplinary approach, considering all clinical, biochemical and structural aspects of the disease, offers the best chance for accurate diagnosis.
Work-up
Levels of PTH and calcium are measured routinely in all patients. Neck ultrasound and 99mTc-sestamibi scan are the most common imaging studies in benign disease [90]. These are also of value in malignant cases [75]. Ultrasound provides general information about the location of the lesion and its structure [91]. Sestamibi scan can reveal abnormal and ectopic parathyroid tissue in the neck and elsewhere [92,93]. In cases where malignancy is suspected, higher-resolution anatomical studies are of considerable value [94]. Computed tomography (CT) with contrast will provide excellent details on the location of the lesion and its relation with other structures [95], and can also reveal invasion of surrounding structures and enlarged lymph nodes. Magnetic resonance imaging (MRI) with gadolinium and fat suppression will give the best detail on soft tissues of the neck, and can supplement further information, specifically in the setting of preoperative assessment. MRI is also superior to CT in assessing recurrent cases where surgical clips in the field can cause significant artefacts in CT studies [96]. Fluorodeoxyglucose-positron emission tomography (FDG-PET) has mainly been used in adenoma cases, and very few reports exist on its use in parathyroid carcinoma [97].A practical point to remember is that the lytic bone lesions (Brown tumours) appear FDG avid on PET. In cases of parathyroid carcinoma, they can be mistaken as bone metastases [97]. Interest in fusion of Single Photon Emission Computed Tomography (SPECT) and CT has increased recently. SPECT/CT has been used in localising parathyroid adenomas [98], but the results are not consistent. SPECT/CT is more sensitive than sestamibi and as specific. However, in localisation of multifocal disease, SPECT/CT is not superior to sestamibi. Use of SPECT/CT has not been reported in parathyroid carcinoma to date. Lastly, selective venous catheterisation and PTH measurement can be used if non-invasive tests have failed to reveal the location of the lesion or when results are ambiguous [91].
Management
The management of parathyroid cancer can be divided into two categories: attempts aimed at potential cure, and therapeutic interventions to control incurable cases.
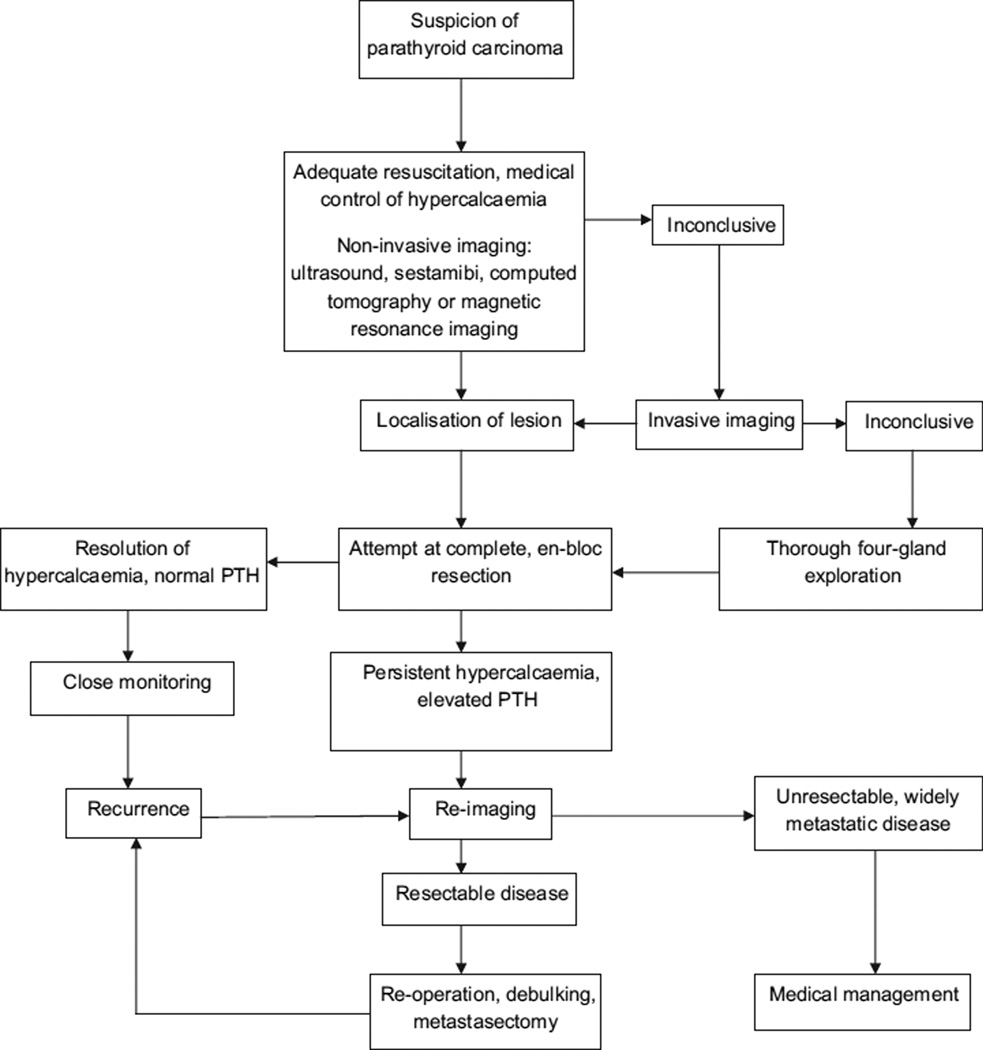
Complete en-bloc surgical excision is the only technique that can potentially offer cure [67]. Neither radiation nor chemotherapy has shown evidence of tumour eradication [3]. Surgical excision and removal of hormone-producing tissue does offer meaningful alleviation of symptoms and is indicated. In cases of widespread metastatic disease or surgically unresectable disease, different types of medication have been tried to reduce serum calcium levels and alleviate the effects of rampant hypercalcaemia. The general approach to management of parathyroid carcinoma is described in Fig. 1.
Fig. 1.
Approach to management of parathyroid carcinoma. PTH, parathyroid hormone.
Surgical Management
Surgical excision can be carried out in two clinical scenarios: initial surgery at the time of diagnosis of hyperparathyroidism, and surgical excision of recurrent or metastatic disease.
Complete en-bloc resection of the tumour offers the best chance for cure. In order to achieve this, the surgeon should approach the operation with adequate preparation. This starts well before the operative procedure itself. In cases of suspected carcinoma, it is prudent to proceed with adequate anatomical imaging, including CT and MRI in addition to ultrasound and sestamibi scan that are usually obtained in benign cases. Attention should be paid to detailed examination and interpretation of these studies to gather as much information as possible before embarking on tumour excision. Knowledge of the status of vocal cord mobility is helpful to determine the possibility of recurrent laryngeal nerve involvement, as this can significantly change the operative plan and extent. During surgery, it is imperative to adhere to the principles of head and neck surgery, and oncological resection. These include adequate exposure, maintaining a bloodless field and attention to surrounding structures [99]. As it is possible to encounter both adenoma and carcinoma synchronously, all four glands should be found and examined [20]. The need for thorough exploration rules out minimally invasive approaches that have limited exposure. After adequate exploration, the lesion should be removed with minimal manipulation in an en-bloc fashion. This requires, at minimum, removal of the ipsilateral lobe of the thyroid [100–102]. If there is any evidence of invasion of the strap muscles or other structures, the tissues should be removed in continuity with the tumour. Every effort should be made not to rupture the capsule of the tumour and spill tumour cells in the field. If there is evidence of nodal involvement, regional lymph node dissection of that compartment should be undertaken. In most cases, the recurrent laryngeal nerve can be preserved without jeopardising its integrity. However, if the nerve is involved, it should be sacrificed [91]. More extensive operations have been advocated by a few investigators. Holmes et al. [69] recommend isthmusectomy, skeletonisation of the trachea, removal of the ipsilateral strap muscles and resection of the recurrent laryngeal nerve in addition to removal of the tumour and the ipsilateral lobe of the thyroid. This approach has not gained popularity and is not usually undertaken. Therapeutic neck dissection is recommended if there is evidence of disease [100]. Prophylactic lateral neck dissection, on the other hand, has been tried previously and did not improve survival but increased morbidity [12]. Therefore, prophylactic neck dissection is not currently recommended. Use of a rapid PTH test has been reported in parathyroid cancer. If this tool is available, its use can be helpful. If the PTH level falls significantly and specifically within normal limits, the surgeon has high assurance that most of the disease, if not all, has been removed. If the values remain elevated, the decision-making can be complicated. This can be due to residual tumour tissue in the neck or undetected metastatic foci. At that time, the surgeon should make a decision whether or not to pursue further exploration, considering that unguided exploration may not improve outcome but will probably increase morbidity. After surgery, close monitoring of calcium levels and adequate replacement is necessary to avoid severe hypocalcaemia due to ‘hungry bone syndrome’.
If the diagnosis of parathyroid cancer is made after initial excision, decision-making is more complicated. If the patient is normocalcaemic, PTH levels are within normal limits and the diagnosis is solely based on suspicious pathology without evidence of extensive vascular or capsular invasion, most tend to follow the patient closely without an attempt at immediate re-excision [103]. Patients need to be followed closely with serial PTH measurements and cervical ultrasound. If the patient continues to be symptomatic, PTH levels remain elevated or there is extensive evidence of vascular and capsular invasion, re-operation may be beneficial [67]. In these cases, adequate pre-operative imaging is of importance to localise the disease as accurately as possible. This will guide the surgeon in the conduct of the operation and can potentially improve the success rate and reduce complications.
The importance of en-bloc resection on the future course of the disease has been well described. Patients who were diagnosed before or during surgery, and therefore had an en-bloc resection, had a recurrence rate of 33%. On the other hand, patients who were diagnosed after initial surgery had a local recurrence rate of more than 50% [3,12,100].
All patients need to be followed closely after surgery. In the immediate postoperative period, close monitoring of serum calcium levels and adequate replacement is mandatory. Intravenous calcium in addition to oral supplementation and calcitriol is usually necessary. The goal is to keep calcium levels at the lower limit of normal [67]. After bone replenishment and recovery of the remaining gland, most patients can be maintained on a normal diet with minimum oral supplementation of calcium. Calcium levels and PTH have to be monitored every 3 months after this initial period to detect recurrence.
Surgical management of recurrent disease
In spite of all technological and technical advances, recurrence is very common in parathyroid carcinoma [100]. Different case series report recurrence rates as high as 100% [104] and as low as 40%. Most series put the recurrence rate above 50%. As mentioned before, patients who had a complete resection at the time of initial surgery have a lower recurrence rate. Unfortunately, a significant proportion of patients are not diagnosed at initial surgery and do not undergo complete resection [105]. On average, recurrence occurs 2–3 years after initial surgery [100]. Much longer time lapses, up to 23 years, have been reported previously [67]. Patients present with gradually increasing PTH and serum calcium levels. Severe hypercalcaemic crisis is rare. The treatment strategy consists of controlling hypercalcaemia, localising studies and surgical excision of resectable disease.
Parathyroid carcinoma metastasises through both lymphatic and haematogenous routes. The most common sites of dissemination are the regional lymph nodes followed by the lungs, bones and liver in descending order. After medical control of hypercalcaemia, adequate imaging studies should be obtained to localise and assess the extent of disease. Ultrasound is helpful in studying the lymphatics of the neck. Sestamibi scan will show local and metastatic disease. If further operations are planned, anatomical studies such as CT and MRI are indicated. In cases where non-invasive studies are inconclusive, selective venous catheterisation and PTH measurement may be necessary.
Resectable disease should be managed surgically. Removal of all functioning tumour tissue gives the most effective relief of symptoms [67,91,106]. Regional disease in the neck or mediastinum should be resected with margins as wide as possible. Distant metastatic foci that are amenable to excision should also be resected. Many patients may require multiple operations and this approach, in fact, is justified as it offers the best palliative option [67,91,106]. Most patients will require two to three operations in the course of disease. The reported morbidity of operations can be as low as 6.2% if one excludes intentional sacrifice of the recurrent laryngeal nerve [91]. The frequency of intentional sacrifice of the recurrent laryngeal nerve is higher in repeat operations than in initial operations. Metastatectomy [107] is carried out routinely if the disease is limited and technically resectable. Mediastinal lymphadenectomy, and open or thoracoscopic lung resections are the most common procedures in the chest.
Most patients will benefit from excision of recurrent disease and a reduction in the levels of PTH and serum calcium after surgery, and most patients will have symptom relief. Pre-operative calcium level is a good predictor of operative success. Patients with levels of 13.5 ± 2.0 mg/dl had a significantly higher rate of normalisation in comparison with patients whose levels were 16.3 ± 1.9 mg/dl before surgery, according to Kebebew et al. [91]. Unfortunately, surgical resection, as effective as it is in controlling recurrent disease, rarely results in cure. Most patients will have further recurrences.
Radiotherapy
Traditionally, radiotherapy was not deemed effective in the treatment of parathyroid carcinoma, either as a single technique or in addition to surgery [3]. In the past decade, reports from three different institutions have challenged this notion. Chow et al. [108] from Princess Margaret Hospital in Toronto, Munson et al. [109] from the Mayo Clinic, and Busaidy et al. [83] from MD Anderson Cancer Center reported lower recurrence and longer disease-free survival with use of adjuvant radiotherapy. All three series were very small. The total number of patients treated with adjuvant radiotherapy was 16. Of these 16 patients, only one developed recurrence, which was much lower than in the non-treated group. These limited data are provocative but should be examined carefully. Thus, there may be a role for adjuvant radiotherapy in control of the disease. Currently, the best practice seems to be approaching each patient individually in a multidisciplinary fashion. Due to very low incidence of this disease, the feasibility of a randomised study seems remote.
Chemotherapy
Most data on the use of chemotherapy in parathyroid cancer come from case reports [25,110,111]. No structured trials have ever been carried out. Considering the rarity of this disease, this is not surprising. Reports of response in both non-functioning and functioning carcinomas do exist [110, 112]. However, these remain limited to single case reports. Wynne et al. [25] reported the use of mithramycin, 5-fluorouracil and doxorubin alone or in combination in five patients, and a combination of cyclophosphamide, vincristine and dacarbazine in another patient. The first five patients did not see any benefit from chemotherapy, and the course of disease in the sixth patient was not reported at the time of publication. In general, chemotherapy has not been promising in parathyroid cancer. The advent of new agents and better understanding of the pathogenesis of this disease may change this in future. However, rare occurrence will always be an obstacle to carrying out large-scale studies.
Medical Management of Hypercalcaemia
Parathyroid carcinoma becomes a chronic disease for most patients. As mentioned previously, most of the morbidity and mortality from this disease come from uncontrolled hypercalcaemia. Medical management of hypercalcaemia becomes necessary in patients with widespread metastatic or unresectable local disease. Medical management is also indicated in hypercalcaemic crisis and for patients awaiting surgical intervention.
Urgent management of hypercalcaemia requires volume expansion with saline infusion and use of loop diuretics to promote renal excretion of calcium [113]. Eventually, preservation of bone mass becomes necessary. Bisphophonates, such as clodronate, etidronate, pamidronate and zoledronate, act through interference with osteoclasts. Potent agents such as pamidronate and zoledronate can be used intravenously [67]. These agents can control hypercalcaemia for a while, but unfortunately most patients will become resistant eventually. Use of other agents is more limited due to their toxicity. Mithramycin is toxic and not very effective, but can be used in life-threatening scenarios unresponsive to bisphosphonates [67]. Plicamycin and gallium nitrate [114] can be used, but the former is only effective transiently and is toxic, and the latter is nephrotoxic.
Calcitonin does reduce serum calcium levels but again only transiently. The long-acting somatostatin analogue, octreotide, has been used in two patients with good results in lowering the PTH level [115,116]. Immunotherapy directed against PTH [117,118] and dendritic cells [119] has also been reported.
Calcimimetics are allosteric modulators of parathyroid calcium-sensing receptor. Calcimimetic agents bind to these receptors and increase their sensitivity to extracellular calcium. This action results in a reduction of PTH secretion by parathyroid cells [120]. R-568, a first-generation calcimimetic, controlled calcium levels effectively in one patient with metastatic parathyroid cancer for 2 years. Cinacalcet, a second-generation calcimimetic, was used in a multicentre study of inoperable metastatic parathyroid carcinoma [121]. In this study, 29 patients were treated with cinacalcet. Two-thirds of patients (18/29) responded to the treatment with an average reduction in serum calcium level from 15 mg/dl to 11.2 mg/dl. Patients with the highest calcium levels at study entry had the most pronounced response. The dose of cinacalcet titrated from 30 mg Bd up to 90 QID. The mean duration of treatment was 328 ± 306 days. Most patients tolerated the drug quite well, with nausea and vomiting being the most common side-effect. Interestingly, in spite of a reduction in serum calcium levels, PTH levels did not decrease significantly. Currently, there is no clear explanation for this observation. There is no evidence that cinacalcet will alter the course of parathyroid cancer. Therefore, it cannot replace surgical excision in cases of resectable disease. However, in patients with widely metastatic disease, this agent can potentially alleviate the effects of hypercalcaemia. This agent can also be used safely in patients with renal insufficiency, which is common in patients with longstanding parathyroid cancer.
Prognosis
Parathyroid carcinoma usually has a slow but progressive course. The tumour invades surrounding structures and spreads to regional lymph nodes. Tumour cells also disseminate haematogenously, and distant metastases occur in lungs, liver and bones. The most important factor affecting prognosis is the completeness of tumour resection. Patients who undergo complete en-bloc tumour resection can have survival rates as high as 90% at 5 years and 67% at 10 years [122]. Negative prognostic factors include lymph node metastases at the time of diagnosis, distant metastases and non-functioning carcinomas [3]. In spite of best efforts, a significant proportion of patients develop recurrence. Recurrence rates from 33 to 78% have been reported in published studies. Most recurrences will manifest in the first 3 years, but recurrences as late as 20 years have been reported [67,123]. Once the disease has recurred, the chances of cure are remote. However, aggressive resection of residual disease and meta-stasectomy does improve survival.
The largest series of patients with parathyroid carcinoma based on the Surveillance, Epidemiology and End Results (SEER) database [2] put 5-year survival at 86% and 10-year survival at 49% for all patients. Other series reported 5-year survival from 40 to 90% [122]. The most common causes of death are complications of hypercalcaemia (renal failure, cardiac arrhythmias, pancreatitis) rather than tumour burden [25,75,103,106].
Summary
Parathyroid carcinoma is a rare disease. Men and women are equally affected, usually in the fourth or fifth decade of life. The most common presentation is complications of hypercalcaemia. The cause is not known, but mutations in the HRPT2 gene seem to play a crucial role in pathogenesis. Differentiation between benign and malignant disease is difficult on tissue specimens. The best chance of cure is complete surgical resection at the time of first operation. Recurrence is common and many patients require multiple resections to reduce the tumour burden. Other treatments such as chemotherapy and radiation have not been reported to be effective, although the data are not comprehensive. In cases of unresectable disease, medical management with diuretics, bisphosphonates and, more recently, calcimimetics is helpful in alleviating symptoms. Other experimental treatments, such as immunotherapy or newer chemotherapy agents, have been reported with sporadic success. More research is needed to elucidate the genetic causes of parathyroid carcinoma. Designing clinical trials for new treatments is quite difficult considering the rarity of this disease. The best chance for success relies on multi-institutional, multidisciplinary designs to accrue an adequate number of patients. Short of a major biological or molecular breakthrough, surgery remains the most effective therapeutic and palliative option.
Footnotes
Conflict of Interest
None declared.
References
- 1.Beus KS, Stack BC., Jr Parathyroid carcinoma. Otolaryngol Clin N Am. 2004;37:845–854. doi: 10.1016/j.otc.2004.02.014. x. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985–1995: a National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 3.Koea JB, Shaw JH. Parathyroid cancer: biology and management. Surg Oncol. 1999;8:155–165. doi: 10.1016/s0960-7404(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 4.Rao SR, Shaha AR, Singh B, Rinaldo A, Ferlito A. Management of cancer of the parathyroid. Acta Otolaryngol. 2002;122:448–452. doi: 10.1080/00016480260000184. [DOI] [PubMed] [Google Scholar]
- 5.Rawat N, Khetan N, Williams DW, Baxter JN. Parathyroid carcinoma. Br J Surg. 2005;92:1345–1353. doi: 10.1002/bjs.5182. [DOI] [PubMed] [Google Scholar]
- 6.De Quervain F. Parastruma maligna aberrata. Deutsche Zeitschr Chir. 1904;100:334–352. [Google Scholar]
- 7.Marcocci C, Cetani F, Rubin MR, Silverberg SJ, Pinchera A, Bilezikian JP. Parathyroid carcinoma. J Bone Miner Res. 2008;23:1869–1880. doi: 10.1359/jbmr.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn K, Silverman M, Corrado J, Sedgewick C. Parathyroid carcinoma: the Lahey Clinic experience. Surgery. 1985;98:1095–1100. [PubMed] [Google Scholar]
- 9.Hakaim AG, Esselstyn CB., Jr Parathyroid carcinoma: 50-year experience at the Cleveland Clinic Foundation. Cleve Clin J Med. 1993;60:331–335. doi: 10.3949/ccjm.60.4.331. [DOI] [PubMed] [Google Scholar]
- 10.Wang CA, Gaz RD. Natural history of parathyroid carcinoma. Diagnosis, treatment, and results. Am J Surg. 1985;149:522–527. doi: 10.1016/s0002-9610(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 11.Shane E, Bilezikian JP. Parathyroid carcinoma: a review of 62 patients. Endocr Rev. 1982;3:218–226. doi: 10.1210/edrv-3-2-218. [DOI] [PubMed] [Google Scholar]
- 12.Sandelin K, Auer G, Bondeson L, Grimelius L, Farnebo LO. Prognostic factors in parathyroid cancer: a review of 95 cases. World J Surg. 1992;16:724–731. doi: 10.1007/BF02067369. [DOI] [PubMed] [Google Scholar]
- 13.Kodama T, Ito Y, Obara T, et al. [Recently experienced ten cases of insulinoma – preoperative diagnosis of localization and intraoperative simultaneous monitoring of glucose and insulin] Nippon Geka Gakkai Zasshi. 1988;89:398–407. [PubMed] [Google Scholar]
- 14.Obara T, Okamoto T, Kanbe M, Iihara M. Functioning parathyroid carcinoma: clinicopathologic features and rational treatment. Semin Surg Oncol. 1997;13:134–141. doi: 10.1002/(sici)1098-2388(199703/04)13:2<134::aid-ssu9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Hamill J, Maoate K, Beasley SW, Corbett R, Evans J. Familial parathyroid carcinoma in a child. J Paediatr Child Health. 2002;38:314–317. doi: 10.1046/j.1440-1754.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- 16.Ireland JP, Fleming SJ, Levison DA, Cattell WR, Baker LR. Parathyroid carcinoma associated with chronic renal failure and previous radiotherapy to the neck. J Clin Pathol. 1985;38:1114–1118. doi: 10.1136/jcp.38.10.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christmas TJ, Chapple CR, Noble JG, Milroy EJ, Cowie AG. Hyperparathyroidism after neck irradiation. Br J Surg. 1988;75:873–874. doi: 10.1002/bjs.1800750914. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J, Gierlowski TC, Schneider AB. A prospective study of hyperparathyroidism in individuals exposed to radiation in childhood. J Am Med Assoc. 1990;264:581–584. [PubMed] [Google Scholar]
- 19.Rasmuson T, Damber L, Johansson L, Johansson R, Larsson LG. Increased incidence of parathyroid adenomas following X-ray treatment of benign diseases in the cervical spine in adult patients. Clin Endocrinol (Oxf) 2002;57:731–734. doi: 10.1046/j.1365-2265.2002.01616.x. [DOI] [PubMed] [Google Scholar]
- 20.Dionisi S, Minisola S, Pepe J, et al. Concurrent parathyroid adenomas and carcinoma in the setting of multiple endocrine neoplasia type 1: presentation as hypercalcemic crisis. Mayo Clin Proc. 2002;77:866–869. doi: 10.4065/77.8.866. [DOI] [PubMed] [Google Scholar]
- 21.Agha A, Carpenter R, Bhattacharya S, Edmonson SJ, Carlsen E, Monson JP. Parathyroid carcinoma in multiple endocrine neoplasia type 1 (MEN1) syndrome: two case reports of an unrecognised entity. J Endocrinol Invest. 2007;30:145–149. doi: 10.1007/BF03347413. [DOI] [PubMed] [Google Scholar]
- 22.Haven CJ, van Puijenbroek M, Tan MH, et al. Identification of MEN1 and HRPT2 somatic mutations in paraffin-embedded (sporadic) parathyroid carcinomas. Clin Endocrinol (Oxf) 2007;67:370–376. doi: 10.1111/j.1365-2265.2007.02894.x. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins PJ, Satta MA, Simmgen M, et al. Metastatic parathyroid carcinoma in the MEN2A syndrome. Clin Endocrinol (Oxf) 1997;47:747–751. doi: 10.1046/j.1365-2265.1997.3421147.x. [DOI] [PubMed] [Google Scholar]
- 24.Dinnen JS, Greenwoood RH, Jones JH, Walker DA, Williams ED. Parathyroid carcinoma in familial hyperparathyroidism. J Clin Pathol. 1977;30:966–975. doi: 10.1136/jcp.30.10.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Parathyroid carcinoma: clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. [PubMed] [Google Scholar]
- 26.Wassif WS, Moniz CF, Friedman E, et al. Familial isolated hyperparathyroidism: a distinct genetic entity with an increased risk of parathyroid cancer. J Clin Endocrinol Metab. 1993;77:1485–1489. doi: 10.1210/jcem.77.6.7903311. [DOI] [PubMed] [Google Scholar]
- 27.Simonds WF, James-Newton LA, Agarwal SK, et al. Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Kytola S, Farnebo F, Obara T, et al. Patterns of chromosomal imbalances in parathyroid carcinomas. Am J Pathol. 2000;157:579–586. doi: 10.1016/S0002-9440(10)64568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cryns VL, Thor A, Xu HJ, et al. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. N Engl J Med. 1994;330:757–761. doi: 10.1056/NEJM199403173301105. [DOI] [PubMed] [Google Scholar]
- 30.Cetani F, Pardi E, Viacava P, et al. A reappraisal of the Rb1 gene abnormalities in the diagnosis of parathyroid cancer. Clin Endocrinol (Oxf) 2004;60:99–106. doi: 10.1111/j.1365-2265.2004.01954.x. [DOI] [PubMed] [Google Scholar]
- 31.Cryns VL, Rubio MP, Thor AD, Louis DN, Arnold A. p53 abnormalities in human parathyroid carcinoma. J Clin Endocrinol Metab. 1994;78:1320–1324. doi: 10.1210/jcem.78.6.8200932. [DOI] [PubMed] [Google Scholar]
- 32.Pearce SH, Trump D, Wooding C, Sheppard MN, Clayton RN, Thakker RV. Loss of heterozygosity studies at the retinoblastoma and breast cancer susceptibility (BRCA2) loci in pituitary, parathyroid, pancreatic and carcinoid tumours. Clin Endocrinol (Oxf) 1996;45:195–200. doi: 10.1046/j.1365-2265.1996.d01-1561.x. [DOI] [PubMed] [Google Scholar]
- 33.Shattuck TM, Kim TS, Costa J, et al. Mutational analyses of RB and BRCA2 as candidate tumour suppressor genes in parathyroid carcinoma. Clin Endocrinol (Oxf) 2003;59:180–189. doi: 10.1046/j.1365-2265.2003.01814.x. [DOI] [PubMed] [Google Scholar]
- 34.Arnold A, Shattuck TM, Mallya SM, et al. Molecular pathogenesis of primary hyperparathyroidism. J Bone Miner Res. 2002;17(Suppl. 2):N30–N36. [PubMed] [Google Scholar]
- 35.Cetani F, Pardi E, Ambrogini E, et al. Genetic analyses in familial isolated hyperparathyroidism: implication for clinical assessment and surgical management. Clin Endocrinol (Oxf) 2006;64:146–152. doi: 10.1111/j.1365-2265.2006.02438.x. [DOI] [PubMed] [Google Scholar]
- 36.Kennett S, Pollick H. Jaw lesions in familial hyperparathyroidism. Oral Surg Oral Med Oral Pathol. 1971;31:502–510. doi: 10.1016/0030-4220(71)90347-1. [DOI] [PubMed] [Google Scholar]
- 37.Jackson CE, Norum RA, Boyd SB, et al. Hereditary hyperparathyroidism and multiple ossifying jaw fibromas: a clinically and genetically distinct syndrome. Surgery. 1990;108:1006–1012. discussion 12–13. [PubMed] [Google Scholar]
- 38.Cavaco BM, Barros L, Pannett AA, et al. The hyperparathyroidism-jaw tumour syndrome in a Portuguese kindred. QJM. 2001;94:213–222. doi: 10.1093/qjmed/94.4.213. [DOI] [PubMed] [Google Scholar]
- 39.Howell VM, Haven CJ, Kahnoski K, et al. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657–663. doi: 10.1136/jmg.40.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shattuck TM, Valimaki S, Obara T, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 41.Cetani F, Pardi E, Borsari S, et al. Genetic analyses of the HRPT2 gene in primary hyperparathyroidism: germline and somatic mutations in familial and sporadic parathyroid tumors. J Clin Endocrinol Metab. 2004;89:5583–5591. doi: 10.1210/jc.2004-0294. [DOI] [PubMed] [Google Scholar]
- 42.Simonds WF, Robbins CM, Agarwal SK, Hendy GN, Carpten JD, Marx SJ. Familial isolated hyperparathyroidism is rarely caused by germline mutation in HRPT2, the gene for the hyperparathyroidism-jaw tumor syndrome. J Clin Endocrinol Metab. 2004;89:96–102. doi: 10.1210/jc.2003-030675. [DOI] [PubMed] [Google Scholar]
- 43.Warner J, Epstein M, Sweet A, et al. Genetic testing in familial isolated hyperparathyroidism: unexpected results and their implications. J Med Genet. 2004;41:155–160. doi: 10.1136/jmg.2003.016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villablanca A, Calender A, Forsberg L, et al. Germline and de novo mutations in the HRPT2 tumour suppressor gene in familial isolated hyperparathyroidism (FIHP) J Med Genet. 2004;41:e32. doi: 10.1136/jmg.2003.012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley KJ, Cavaco BM, Bowl MR, et al. Parafibromin mutations in hereditary hyperparathyroidism syndromes and parathyroid tumours. Clin Endocrinol (Oxf) 2006;64:299–306. doi: 10.1111/j.1365-2265.2006.02460.x. [DOI] [PubMed] [Google Scholar]
- 46.Bradley KJ, Cavaco BM, Bowl MR, Harding B, Young A, Thakker RV. Utilisation of a cryptic non-canonical donor splice site of the gene encoding parafibromin is associated with familial isolated primary hyperparathyroidism. J Med Genet. 2005;42:e51. doi: 10.1136/jmg.2005.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guarnieri V, Scillitani A, Muscarella LA, et al. Diagnosis of parathyroid tumors in familial isolated hyperparathyroidism with HRPT2 mutation: implications for cancer surveillance. J Clin Endocrinol Metab. 2006;91:2827–2832. doi: 10.1210/jc.2005-1239. [DOI] [PubMed] [Google Scholar]
- 48.Kelly TG, Shattuck TM, Reyes-Mugica M, et al. Surveillance for early detection of aggressive parathyroid disease: carcinoma and atypical adenoma in familial isolated hyperparathyroidism associated with a germline HRPT2 mutation. J Bone Miner Res. 2006;21:1666–1671. doi: 10.1359/jbmr.060702. [DOI] [PubMed] [Google Scholar]
- 49.Cetani F, Ambrogini E, Viacava P, et al. Should parafibromin staining replace HRTP2 gene analysis as an additional tool for histologic diagnosis of parathyroid carcinoma? Eur J Endocrinol. 2007;156:547–554. doi: 10.1530/EJE-06-0720. [DOI] [PubMed] [Google Scholar]
- 50.Hewitt KM, Sharma PK, Samowitz W, Hobbs M. Aberrant methylation of the HRPT2 gene in parathyroid carcinoma. Ann Otol Rhinol Laryngol. 2007;116:928–933. doi: 10.1177/000348940711601210. [DOI] [PubMed] [Google Scholar]
- 51.Krebs LJ, Shattuck TM, Arnold A. HRPT2 mutational analysis of typical sporadic parathyroid adenomas. J Clin Endocrinol Metab. 2005;90:5015–5017. doi: 10.1210/jc.2005-0717. [DOI] [PubMed] [Google Scholar]
- 52.Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, et al. The parafibromin tumor suppressor protein is part of a human PAF1 complex. Mol Cell Biol. 2005;25:612–620. doi: 10.1128/MCB.25.2.612-620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yart A, Gstaiger M, Wirbelauer C, et al. The HRPT2 tumor suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol Cell Biol. 2005;25:5052–5060. doi: 10.1128/MCB.25.12.5052-5060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hahn MA, Marsh DJ. Identification of a functional bipartite nuclear localization signal in the tumor suppressor parafibromin. Oncogene. 2005;24:6241–6248. doi: 10.1038/sj.onc.1208778. [DOI] [PubMed] [Google Scholar]
- 55.Lin L, Czapiga M, Nini L, Zhang JH, Simonds WF. Nuclear localization of the parafibromin tumor suppressor protein implicated in the hyperparathyroidism-jaw tumor syndrome enhances its proapoptotic function. Mol Cancer Res. 2007;5:183–193. doi: 10.1158/1541-7786.MCR-06-0129. [DOI] [PubMed] [Google Scholar]
- 56.Bradley KJ, Bowl MR, Williams SE, et al. Parafibromin is a nuclear protein with a functional monopartite nuclear localization signal. Oncogene. 2007;26:1213–1221. doi: 10.1038/sj.onc.1209893. [DOI] [PubMed] [Google Scholar]
- 57.Hahn MA, Marsh DJ. Nucleolar localization of parafibromin is mediated by three nucleolar localization signals. FEBS Lett. 2007;581:5070–5074. doi: 10.1016/j.febslet.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 58.Woodard GE, Lin L, Zhang JH, Agarwal SK, Marx SJ, Simonds WF. Parafibromin, product of the hyperparathyroidism-jaw tumor syndrome gene HRPT2, regulates cyclin D1/PRAD1 expression. Oncogene. 2005;24:1272–1276. doi: 10.1038/sj.onc.1208274. [DOI] [PubMed] [Google Scholar]
- 59.Zhang C, Kong D, Tan MH, et al. Parafibromin inhibits cancer cell growth and causes G1 phase arrest. Biochem Biophys Res Commun. 2006;350:17–24. doi: 10.1016/j.bbrc.2006.08.169. [DOI] [PubMed] [Google Scholar]
- 60.Iwata T, Mizusawa N, Taketani Y, Itakura M, Yoshimoto K. Parafibromin tumor suppressor enhances cell growth in the cells expressing SV40 large T antigen. Oncogene. 2007;26:6176–6183. doi: 10.1038/sj.onc.1210445. [DOI] [PubMed] [Google Scholar]
- 61.Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 62.Brown JJ, Mohamed H, Williams-Smith L, Osborne R, Coker J, Yee B. Primary hyperparathyroidism secondary to simultaneous bilateral parathyroid carcinoma. Ear Nose Throat J. 2002;81:395–398. 400–401. [PubMed] [Google Scholar]
- 63.Flye MW, Brennan MF. Surgical resection of metastatic parathyroid carcinoma. Ann Surg. 1981;193:425–435. doi: 10.1097/00000658-198104000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Heerden JA, Weiland LH, ReMine WH, Walls JT, Purnell DC. Cancer of the parathyroid glands. Arch Surg. 1979;114:475–480. doi: 10.1001/archsurg.1979.01370280129019. [DOI] [PubMed] [Google Scholar]
- 65.Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol. 1993;17:820–829. doi: 10.1097/00000478-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 66.DeLellis RA. Parathyroid carcinoma: an overview. Adv Anat Pathol. 2005;12:53–61. doi: 10.1097/01.pap.0000151319.42376.d4. [DOI] [PubMed] [Google Scholar]
- 67.Shane E. Clinical review 122: parathyroid carcinoma. J Clin Endocrinol Metab. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 68.Fujimoto Y, Obara T. How to recognize and treat parathyroid carcinoma. Surg Clin N Am. 1987;67:343–357. doi: 10.1016/s0039-6109(16)44188-5. [DOI] [PubMed] [Google Scholar]
- 69.Holmes EC, Morton DL, Ketcham AS. Parathyroid carcinoma: a collective review. Ann Surg. 1969;169:631–640. doi: 10.1097/00000658-196904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubin MR, Silverberg SJ, D’Amour P, et al. An N-terminal molecular form of parathyroid hormone (PTH) distinct from hPTH(1 84) is overproduced in parathyroid carcinoma. Clin Chem. 2007;53:1470–1476. doi: 10.1373/clinchem.2007.085506. [DOI] [PubMed] [Google Scholar]
- 71.Rubin MR, Bilezikian JP, Birken S, Silverberg SJ. Human chorionic gonadotropin measurements in parathyroid carcinoma. Eur J Endocrinol. 2008;159:469–474. doi: 10.1530/EJE-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilkins BJ, Lewis JS. Non-functional parathyroid carcinoma: a review of the literature and report of a case requiring extensive surgery. Head Neck Pathol. 2009;3:140–149. doi: 10.1007/s12105-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levin KE, Galante M, Clark OH. Parathyroid carcinoma versus parathyroid adenoma in patients with profound hypercalcemia. Surgery. 1987;101:649–660. [PubMed] [Google Scholar]
- 74.Rubello D, Casara D, Dwamena BA, Shapiro BParathyroid carcinoma. A concise review. Minerva Endocrinol. 2001;26:59–64. [PubMed] [Google Scholar]
- 75.Thompson SD, Prichard AJ. The management of parathyroid carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:93–97. doi: 10.1097/00020840-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Spinelli C, Bonadio AG, Berti P, Materazzi G, Miccoli P. Cutaneous spreading of parathyroid carcinoma after fine needle aspiration cytology. J Endocrinol Invest. 2000;23:255–257. doi: 10.1007/BF03343718. [DOI] [PubMed] [Google Scholar]
- 77.Smith JF, Coombs RR. Histological diagnosis of carcinoma of the parathyroid gland. J Clin Pathol. 1984;37:1370–1378. doi: 10.1136/jcp.37.12.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schantz A, Castleman BParathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–605. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 79.McKeown PP, McGarity WC, Sewell CW. Carcinoma of the parathyroid gland: is it overdiagnosed? A report of three cases. Am J Surg. 1984;147:292–298. doi: 10.1016/0002-9610(84)90110-7. [DOI] [PubMed] [Google Scholar]
- 80.Snover DC, Foucar K. Mitotic activity in benign parathyroid disease. Am J Clin Pathol. 1981;75:345–347. doi: 10.1093/ajcp/75.3.345. [DOI] [PubMed] [Google Scholar]
- 81.Grimelius L, Johansson H. Pathology of parathyroid tumors. Semin Surg Oncol. 1997;13:142–154. doi: 10.1002/(sici)1098-2388(199703/04)13:2<142::aid-ssu10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 82.Juhlin C, Akerstrom G, Klareskog L, et al. Monoclonal anti-parathyroid antibodies revealing defect expression of a calcium receptor mechanism in hyperparathyroidism. World J Surg. 1988;12:552–558. doi: 10.1007/BF01655449. [DOI] [PubMed] [Google Scholar]
- 83.Busaidy NL, Jimenez C, Habra MA, et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26:716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 84.Clayman GL, Gonzalez HE, El-Naggar A, Vassilopoulou-Sellin R. Parathyroid carcinoma: evaluation and interdisciplinary management. Cancer. 2004;100:900–905. doi: 10.1002/cncr.20089. [DOI] [PubMed] [Google Scholar]
- 85.Kameyama K, Takami H, Umemura S, et al. PCNA and Ki-67 as prognostic markers in human parathyroid carcinomas. Ann Surg Oncol. 2000;7:301–304. doi: 10.1007/s10434-000-0301-9. [DOI] [PubMed] [Google Scholar]
- 86.Rubin MR, Silverberg SJ. Editorial: HRPT2 in parathyroid cancer: a piece of the puzzle. J Clin Endocrinol Metab. 2005;90:5505–5507. doi: 10.1210/jc.2005-1578. [DOI] [PubMed] [Google Scholar]
- 87.Lloyd RV, Erickson LA, Jin L, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bergero N, De Pompa R, Sacerdote C, et al. Galectin-3 expression in parathyroid carcinoma: immunohistochemical study of 26 cases. Hum Pathol. 2005;36:908–914. doi: 10.1016/j.humpath.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 89.Fernandez-Ranvier GG, Khanafshar E, Tacha D, et al. Defining a molecular phenotype for benign and malignant parathyroid tumors. Cancer. 2009;115:334–344. doi: 10.1002/cncr.24037. [DOI] [PubMed] [Google Scholar]
- 90.Kettle AG, O’Doherty MJ. Parathyroid imaging: how good is it and how should it be done? Semin Nucl Med. 2006;36:206–211. doi: 10.1053/j.semnuclmed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Kebebew E, Arici C, Duh QY, Clark OH. Localization and reoperation results for persistent and recurrent parathyroid carcinoma. Arch Surg. 2001;136:878–885. doi: 10.1001/archsurg.136.8.878. [DOI] [PubMed] [Google Scholar]
- 92.Castellani M, Reschini E, Longari V, et al. Role of Tc-99m sestamibi scintigraphy in the diagnosis and surgical decision-making process in primary hyperparathyroid disease. Clin Nucl Med. 2001;26:139–144. doi: 10.1097/00003072-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 93.James C, Starks M, MacGillivray DC, White J. The use of imaging studies in the diagnosis and management of thyroid cancer and hyperparathyroidism. Surg Oncol Clin N Am. 1999;8:145–169. [PubMed] [Google Scholar]
- 94.Fraker DL. Update on the management of parathyroid tumors. Curr Opin Oncol. 2000;12:41–48. doi: 10.1097/00001622-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Stark DD, Gooding GA, Moss AA, Clark OH, Ovenfors CO. Parathyroid imaging: comparison of high-resolution CT and high-resolution sonography. AJR Am J Roentgenol. 1983;141:633–638. doi: 10.2214/ajr.141.4.633. [DOI] [PubMed] [Google Scholar]
- 96.Weber AL, Randolph G, Aksoy FGThe thyroid, parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin N Am. 2000;38:1105–1129. doi: 10.1016/s0033-8389(05)70224-4. [DOI] [PubMed] [Google Scholar]
- 97.Kemps B, van Ufford HQ, Creyghton W, et al. Brown tumors simulating metastases on FDG PET in a patient with parathyroid carcinoma. Eur J Nucl Med Mol Imag. 2008;35:850. doi: 10.1007/s00259-007-0712-y. [DOI] [PubMed] [Google Scholar]
- 98.Buck AK, Nekolla S, Ziegler S, et al. SPECT/CT. J Nucl Med. 2008;49:1305–1319. doi: 10.2967/jnumed.107.050195. [DOI] [PubMed] [Google Scholar]
- 99.Kebebew E, Clark OH. Parathyroid adenoma, hyperplasia, and carcinoma: localization, technical details of primary neck exploration, and treatment of hypercalcemic crisis. Surg Oncol Clin N Am. 1998;7:721–748. [PubMed] [Google Scholar]
- 100.Kebebew E. Parathyroid carcinoma. Curr Treat Options Oncol. 2001;2:347–354. doi: 10.1007/s11864-001-0028-2. [DOI] [PubMed] [Google Scholar]
- 101.Shortell CK, Andrus CH, Phillips CE, Jr, Schwartz SI. Carcinoma of the parathyroid gland: a 30-year experience. Surgery. 1991;110:704–708. [PubMed] [Google Scholar]
- 102.Hoelting T, Weber T, Werner J, Herfarth C. Surgical treatment of parathyroid carcinoma. Oncol Rep. 2001;8:931–934. doi: 10.3892/or.8.4.931. [DOI] [PubMed] [Google Scholar]
- 103.Fujimoto Y, Obara T, Ito Y, Kodama T, Nobori M, Ebihara S. Localization and surgical resection of metastatic parathyroid carcinoma. World J Surg. 1986;10:539–547. doi: 10.1007/BF01655520. [DOI] [PubMed] [Google Scholar]
- 104.Iacobone M, Lumachi F, Favia G. Up-to-date on parathyroid carcinoma: analysis of an experience of 19 cases. J Surg Oncol. 2004;88:223–228. doi: 10.1002/jso.20152. [DOI] [PubMed] [Google Scholar]
- 105.Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: an update and review. World J Surg. 1991;15:738–744. doi: 10.1007/BF01665308. [DOI] [PubMed] [Google Scholar]
- 106.McCance DR, Kenny BD, Sloan JM, Russell CF, Hadden DR. Parathyroid carcinoma: a review. J R Soc Med. 1987;80:505–509. doi: 10.1177/014107688708000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mezhir JJ, Melis M, Headley RC, Pai RK, Posner MC, Kaplan EL. Successful palliation of hypercalcemia secondary to metastatic parathyroid cancer: an unusual indication for hepatic resection. J Hepatobiliary Pancreat Surg. 2007;14:410–413. doi: 10.1007/s00534-006-1173-6. [DOI] [PubMed] [Google Scholar]
- 108.Chow E, Tsang RW, Brierley JD, Filice S. Parathyroid carcinoma – the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys. 1998;41:569–572. doi: 10.1016/s0360-3016(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 109.Munson ND, Foote RL, Northcutt RC, et al. Parathyroid carcinoma: is there a role for adjuvant radiation therapy? Cancer. 2003;98:2378–2384. doi: 10.1002/cncr.11819. [DOI] [PubMed] [Google Scholar]
- 110.Calandra DB, Chejfec G, Foy BK, Lawrence AM, Paloyan E. Parathyroid carcinoma: biochemical and pathologic response to DTIC. Surgery. 1984;96:1132–1137. [PubMed] [Google Scholar]
- 111.Bukowski RM, Sheeler L, Cunningham J, Esselstyn C. Successful combination chemotherapy for metastatic parathyroid carcinoma. Arch Intern Med. 1984;144:399–400. [PubMed] [Google Scholar]
- 112.Eurelings M, Frijns CJ, Jeurissen FJ. Painful ophthalmoplegia from metastatic nonproducing parathyroid carcinoma: case study and review of the literature. Neuro Oncol. 2002;4:44–48. doi: 10.1215/15228517-4-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mulder JEBJ. Acute management of hypercalcemia. In: Bilezikian JP, Levine MA, editors. The parathyroids: basic and clinical concepts. 2nd ed. San Diego: Academic Press; 2001. pp. 729–741. [Google Scholar]
- 114.Warrell RP, Jr, Issacs M, Alcock NW, Bockman RS. Gallium nitrate for treatment of refractory hypercalcemia from parathyroid carcinoma. Ann Intern Med. 1987;107:683–686. doi: 10.7326/0003-4819-107-5-683. [DOI] [PubMed] [Google Scholar]
- 115.Koyano H, Shishiba Y, Shimizu T, et al. Successful treatment by surgical removal of bone metastasis producing PTH: new approach to the management of metastatic parathyroid carcinoma. Intern Med. 1994;33:697–702. doi: 10.2169/internalmedicine.33.697. [DOI] [PubMed] [Google Scholar]
- 116.Denney AM, Watts NB. The effect of octreotide on parathyroid carcinoma. J Clin Endocrinol Metab. 2004;89:1016. doi: 10.1210/jc.2003-031825. [DOI] [PubMed] [Google Scholar]
- 117.Bradwell AR, Harvey TC. Control of hypercalcaemia of parathyroid carcinoma by immunisation. Lancet. 1999;353:370–373. doi: 10.1016/S0140-6736(98)06469-1. [DOI] [PubMed] [Google Scholar]
- 118.Betea D, Bradwell AR, Harvey TC, et al. Hormonal and biochemical normalization and tumor shrinkage induced by anti-parathyroid hormone immunotherapy in a patient with metastatic parathyroid carcinoma. J Clin Endocrinol Metab. 2004;89:3413–3420. doi: 10.1210/jc.2003-031911. [DOI] [PubMed] [Google Scholar]
- 119.Schott M, Feldkamp J, Schattenberg D, et al. Induction of cellular immunity in a parathyroid carcinoma treated with tumor lysate-pulsed dendritic cells. Eur J Endocrinol. 2000;142:300–306. doi: 10.1530/eje.0.1420300. [DOI] [PubMed] [Google Scholar]
- 120.Nemeth EF, Steffey ME, Hammerland LG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silverberg SJ, Rubin MR, Faiman C, et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. 2007;92:3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]
- 122.Kleinpeter KP, Lovato JF, Clark PB, et al. Is parathyroid carcinoma indeed a lethal disease? Ann Surg Oncol. 2005;12:260–266. doi: 10.1245/ASO.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 123.Sandelin K, Tullgren O, Farnebo LO. Clinical course of metastatic parathyroid cancer. World J Surg. 1994;18:594–598. doi: 10.1007/BF00353773. discussion 9. [DOI] [PubMed] [Google Scholar]