Abstract
Objective
We examined cortical auditory development and behavioral outcomes in children with ANSD fitted with cochlear implants (CI).
Design
Cortical maturation, measured by P1 cortical auditory evoked potential (CAEP) latency, was regressed against scores on the Infant Toddler Meaningful Auditory Integration Scale (IT-MAIS). Implantation age was also considered in relation to CAEP findings.
Study Sample
Cross-sectional and longitudinal samples of 24 and 11 children, respectively, with ANSD fitted with CIs.
Result
P1 CAEP responses were present in all children after implantation, though previous findings suggest that only 50-75% of ANSD children with hearing aids show CAEP responses. P1 CAEP latency was significantly correlated with participants' IT-MAIS scores. Furthermore, more children implanted before age two years showed normal P1 latencies, while those implanted later mainly showed delayed latencies. Longitudinal analysis revealed that most children showed normal or improved cortical maturation after implantation.
Conclusion
Cochlear implantation resulted in measureable cortical auditory development for all children with ANSD. Children fitted with CIs under age two years were more likely to show age-appropriate CAEP responses within 6 months after implantation, suggesting a possible sensitive period for cortical auditory development in ANSD. That CAEP responses were correlated with behavioral outcome highlights their clinical decision-making utility.
Keywords: P1 cortical auditory evoked potential, Central auditory maturation, Auditory neuropathy spectrum disorder (ANSD), Auditory neuropathy/dys-synchrony (AN/AD), cochlear implant, neuroplasticity, development
Introduction
Auditory Neuropathy Spectrum Disorder (ANSD) is a relatively recently described disorder (Starr et al, 1991, 1996), which has been shown to affect approximately 10-15% of all children with sensorineural hearing loss (Uus & Bamford, 2006; Kirkim et al, 2008; Talaat et al, 2009; Berlin et al, 2010; Maris et al, 2011; Roush et al, 2011; Bielecki, Horbuleweicz, & Wolan, 2012; Mittal et al, 2012). Diagnosis of ANSD has become all but routine in many audiology clinics throughout the world. ANSD is diagnosed by examining a combination of results across several traditional audiologic measures, such as present otoacoustic emissions (OAEs), absent or grossly abnormal auditory brainstem response (ABR), which exhibits a robust cochlear microphonic that is inverted with reversal of the polarity of the stimulus, and abnormal acoustic reflexes (AR) (Starr et al, 1996; Berlin et al, 1998; Sininger, 2001; Berlin et al, 2003, 2005). In addition, ANSD patients typically perform worse on measures of speech perception than their behavioral audiograms would predict. In fact, many reports in the literature suggest that behavioral audiometry provides little useful information in ANSD patients (Zeng et al, 1999; Rance et al, 2002). Speech understanding in ANSD patients is severely degraded in the presence of competing signals, such as background noise (Kraus et al, 2000; Sininger, 2001; Rance et al, 2012).
A major physiologic deficit in ANSD is dys-synchronous firing of the auditory nerve and auditory brainstem neural pathways (Berlin et al, 1998; Kraus et al, 2000; Starr, Picton, & Kim, 2001). Such a neural dys-synchrony apparently leads to significant perceptual deficits, particularly in the area of temporal processing (i.e., temporal resolution) (e.g., Zeng et al, 1999, 2004, 2005; Rance et al, 2004, 2005; Michaelewski et al, 2005; Zeng et al, 2006; Rance et al, 2008; Hassan, 2011), which in turn leads to poor speech perception, especially in noisy situations. In addition, the abnormal pattern of neural firing in the subcortical auditory pathways can disrupt normal central auditory maturation (Kraus et al, 2000; Rance et al, 2002; Sharma et al, 2011; Cardon et al, 2012).
Given these physiologic and perceptual vulnerabilities, it is imperative that interventions that ameliorate the negative effects of ANSD be designed and validated. Some investigators have advocated the use of CIs in children with ANSD (Miyamoto et al, 1999; Trautwein, Sinninger, & Nelson, 2000; Shallop et al, 2001; Mason et al, 2003; Peterson et al, 2003; Buss et al, 2002; Rance & Barker, 2008; 2009; Berlin et al, 2010; Teagle et al, 2010; Carvalho et al, 2011, Breneman, Gifford, & Dejong, 2012). However, cochlear implantation may not be an optimal treatment for all children with ANSD given that there are several reports of children with ANSD exhibiting poor outcomes after cochlear implantation (Miyamoto et al, 1999; Rance & Barker, 2008; 2009; Neary & Lightfoot, 2012).
Recent reports of the use of cortical auditory evoked potentials (CAEP) in children with ANSD have yielded promising results, in terms of providing prognoses and validating treatment options in children with ANSD (Rance et al, 2002; Campbell et al, 2011; Cardon & Sharma, 2011; Sharma et al, 2011; Alvarenga et al, 2012; Cardon et al, 2012). The obligatory CAEP is mainly comprised of the P1 component—a large positive-going peak occurring between approximately 90-300 ms—in infancy and early childhood. Because the P1 decreases in latency with age in the normal system, it has proven a useful biomarker of central auditory maturation in children with normal hearing, sensorineural hearing loss (SNHL), and ANSD (Eggermont, 1988; Ponton et al, 1996, 2000; Ceponiene et al, 2002; Sharma et al, 1997, 2002a, 2002b; 2005; 2011; Rance et al, 2002). Previous studies have shown that CAEPs can be recorded in approximately 50-85% of children with ANSD despite the inability to record useful ABRs in the same subjects (Starr et al, 1996; Kraus et al, 2000; Rance et al, 2005; Sharma et al., 2011; Alvarenga et al, 2012), because the dys-synchronos neural activity that completely degrades the rapidly occurring, high frequency ABR waveform peaks in ANSD doesn't have the same effect on slower, broader CAEP peaks (Kraus, 2000). In addition, there is growing evidence that CAEP results are strongly correlated with behavioral auditory skill development and speech perception outcomes in children with ANSD who use hearing aids (Rance et al, 2002; Campbell et al, 2011; Cardon & Sharma, 2011; Sharma et al, 2011; Cardon et al, 2012). For instance, Rance and colleagues (2002) showed that CAEPs could be recorded in approximately 50% of their ANSD pediatric patients fitted with hearing aids and that those children who exhibited CAEP responses showed better speech perception performance than those who had absent CAEP responses. Similarly, Sharma et al., (2011) reported that replicable P1 CAEPs could be recorded in approximately 71% of children with ANSD who wore hearing aids and the developmental status of the P1 response was strongly correlated with behavioral outcome in the ANSD patients. The results of these studies suggest that CAEPs may be useful in evaluating central auditory maturation in children with ANSD and in predicting behavioral outcome. Additionally, these reports suggest that hearing aids may be a viable treatment for a small subset of, but certainly not for all, children with ANSD. Therefore, the clinical trend to provide pediatric ANSD patients with cochlear implants is growing rapidly (e.g., Breneman, Gifford, & Dejong, 2012). The current study aims to explore central auditory maturation and behavioral outcome in children with ANSD who use CIs, and thereby comment on the effectiveness of this intervention for this population.
Materials & Methods
Subjects
Retrospective review of the audiological records of 24 children clinically diagnosed with ANSD was carried out. Participants included 15 females and 9 males that ranged in age from 1.4 to 12.6 years at the time of P1 testing (mean = 3.8 years of age). All participants were fitted with cochlear implants. On average, participants were fitted with their devices at 3.1 years of age (SD = 2.6 years) and had 0.8 (SD = 0.57) years of experience with their CIs at the time of P1 testing. Additionally, the children had a mean unaided pure tone average (PTA) of 81 dB HL (SD = 18.76 dB HL). None of the participants had a diagnosis of cochlear nerve deficiency. Pertinent patient history information can be found in Table 1. All procedures were approved by the Institutional Review Boards of the University of Colorado at Boulder and the University of Texas at Dallas.
Table 1.
Pertinent case history information for each participant (Au – bilaterally; a dash indicates that the data were unavailable).
| Subject | Etiology | ABR | OAE | PTA Unaided R (dBHL) | PTA Unaided L (dBHL) | CI Fit Age (years) | CI Exp. at Test (years) | P1 Status with HA |
|---|---|---|---|---|---|---|---|---|
| 1 | No known risk factors | - | - | - | - | 1.07 | 1.65 | - |
| 2 | No known risk factors | CM Present Au | Present Au | 115 | 115 | 1.27 | 0.61 | Delayed |
| 3 | No known risk factors | - | - | - | - | 1.69 | 0.46 | - |
| 4 | Premature, mechanical ventilation, diaphragmatic hernia, ototoxic medication | CM Present Au | Present Au | 72 | 75 | 1.21 | 0.99 | Normal |
| 5 | No known risk factors | CM Present Au | Present Au | 85 (SAT) | 75 (SAT) | 1.27 | 0.97 | Delayed |
| 6 | No known risk factors | - | - | - | - | 1.61 | 0.78 | Abnormal |
| 7 | Premature, NICU stay, jaundice (blood transfusion), mechanical ventilation | CM Present Au | Present Au | 38 | 70 | 1.76 | 0.67 | Normal |
| 8 | No known risk factors | - | Present Au | 85 (SRT) | 80 (SRT) | 1.42 | 1.25 | - |
| 9 | Jaundiced | CM Present Au | Absent Au | 118 | 108 | 1.33 | 1.53 | Normal |
| 10 | Congenital diaphramic hernia, prolonged ventilator support, Klinefelter's Syndrome | - | - | - | - | 4.44 | 0.25 | Delayed |
| 11 | No known risk factors | CM Present Au | - | 95 | 95 | 3.61 | 1.34 | - |
| 12 | Premature, jaundiced, mechanical ventilation, chronic lung disease, hypothyroidism | CM Present R | Absent Au | 80 | 82 | 1.62 | 0.34 | Delayed |
| 13 | No known risk factors | CM Present Au | - | 50 | 50 | 1.31 | 0.34 | - |
| 14 | Hyperbilirubinemia, metabolic and seizure disorder (G6PD deficiency) | CM Present Au | Present Au | 68 | 68 | 2.02 | 0.39 | Delayed |
| 15 | Premature, low birth weight, CMV, hyperbilirubinemia, liver hemigioma | CM Present Au | Absent Au | - | 93 | 2.96 | 0.0 | Abnormal |
| 16 | Premature, NICU stay, mechanical ventilation, blood transfusions, ototoxic medication | CM Present Au | Absent Au | 62 | 93 | 2.24 | 0.83 | Abnormal |
| 17 | No known risk factors | CM Present Au | Absent Au | 103 | 108 | 2.34 | 1.02 | - |
| 18 | Premature, Beckwith-Wiedemann | CM Present Au | - | 78 | 78 | 3.82 | 0.04 | Delayed |
| 19 | No known risk factors | CM Present Au | - | 90 | 90 | 1.61 | 2.55 | Delayed |
| 20 | No known risk factors | CM Present Au | Absent Au | NR | 100 | 3.54 | 0.84 | - |
| 21 | Epilepsy, other developmental delays | CM Present Au | Absent Au | 111 | 80 | 5.41 | 0.63 | Abnormal |
| 22 | Maternal tuberculosis, radiation exposure (X-Ray in utero), hernia on umbilical cord | - | - | 68 | 68 | 7.05 | 0.75 | - |
| 23 | Seizures, family Hx of hearing loss | CM Present Au | DPOAE Present TEOAE Absent | 93 | 98 | 6.62 | 1.55 | Abnormal |
| 24 | Hydrotropic cardiomyopathy | CM Present Au | Present Au | 60 | 60 | 12.43 | 0.13 | Normal |
Measures
A variety of measures were considered for each participant in the present investigation. Tests included standard clinical audiological tests such as pure tone average (PTA; 500 Hz, 1kHz, 2kHz), otoacoustic emissions (TEOAE, DPOAE, or both), and click evoked auditory brainstem response (ABR). See Table 1 for audiological test results. Additionally, the Infant Toddler Meaningful Auditory Integration Scale (IT-MAIS) (Zimmerman-Phillips, Robbins, & Osberger, 2000) was administered to each subject. Finally, the P1 CAEP was used to assess central auditory maturation in all participants. Details concerning the IT-MAIS and P1 CAEP procedures are provided below.
IT-MAIS
The Infant Toddler Meaningful Auditory Integration Scale (IT-MAIS; 2000) is an adaptation of the Meaningful Auditory Integration Scale (MAIS) (Robbins, Renshaw, & Berry, 1991) designed to focus on infants and young children. The administering clinician measures auditory skill development through the IT-MAIS by completing a structured interview with the patient's parent(s). The IT-MAIS targets three main areas of auditory development: 1) vocalization behavior; 2) alerting to sounds; 3) deriving meaning from sounds (Zimmerman-Phillips, Robbins, & Osberger, 2000). These areas of development are assessed in ten open-ended questions. After listening to the parents' response to each test item, the clinician-interviewer assigns a score, from one (lowest) to four (highest), to each question, for a total of 40 points. The IT-MAIS has been used for studies concerning auditory skill development in children with normal hearing (Kishon-Rabin et al., 2001) and hearing loss (e.g., McConkey Robbins et al., 2004), including ANSD (Peterson et al., 2003; Sharma et al, 2011). While somewhat general and not a true measure of speech perception, the IT-MAIS has proven clinically effective, for several reasons. For example, behavioral results can be obtained even for infants and young children who, due to developmental delay or other physical limitations, could not otherwise perform behavioral tests, which is often the case in working with a group of children with ANSD. Furthermore, the IT-MAIS can be completed in any language.
P1 CAEP Recordings
Stimuli
CAEPs were recorded in response to a synthesized speech syllable /ba/. The duration of the speech sound was 90 ms. The stimulus was identical to the one used in Sharma et al. (1997, 2002a, 2002b). The five-formant CV stimulus was generated using the Klatt speech synthesizer (Klatt, 1980). The starting frequencies of F1 and F2 were 234 and 616 Hz, respectively. The center frequencies for the formants of the vowel /a/ were 769, 1232, 2862, 3600 and 4500 Hz for F1, F2, F3, F4, and F5, respectively. F3, F4, and F5 were steady-state formants. The amplitude of voicing was constant for 80 ms and fell linearly to 0 in the last 10 ms of the stimuli. The fundamental frequency began at 103 Hz, increased linearly to 125 Hz over 35 ms and then decreased to 80 Hz over 55 ms.
The stimulus was presented at an offset-to-onset interstimulus interval of 610 ms. Stimuli were presented to the participants via two loudspeakers, each placed at 45° azimuth approximately three feet from the participants' heads, in a sound-attenuating booth. Typically, stimuli were presented at 75dB SPL and all participants were tested with their CIs on and set to their usual settings. Audibility was verified by reviewing audiological records and by patient observation during testing.
Electrophysiologic Recording Process
Subjects were seated comfortably in a reclining chair or on their parent's lap during testing. They watched a movie on a flatscreen monitor placed approximately four feet in front of them in the sound booth. The audio levels of the movie were muted during recording of the CAEPs. This has been shown to be an effective way of engaging youngsters (Kraus et al., 1995). Evoked potentials were collected using a Compumedics Neuroscan evoked potentials system. Silver/silver chloride cup electrodes were used for the recordings. P1 responses have been collected using an identical setup and stimuli in studies examining central auditory development in children with normal hearing and children with sensorineural hearing loss who are fitted with cochlear implants (e.g., Sharma et al., 2002a, 2002b). In order to minimize the electrical artifact produced by CIs, we recorded responses along the isopotential contour and minimized the artifact using common mode rejection cancellation techniques. An active electrode was placed at Cz and several reference electrodes were placed at locations around the forehead, nasion, orbits and mastoids. A ground electrode was placed on the forehead. Details of this procedure are discussed in detail in a previous publication by our group (Gilley et al., 2006)
Averaging of the CAEP waveforms was automatically suspended by the recording computer when eye blinks were detected. The recording window included a 100 ms pre-stimulus and 600 ms post-stimulus time. Responses were sampled at 1.0 kHz. Incoming evoked responses were analog filtered from 0.1 to 100 Hz. At least two runs of approximately 300 response sweeps were collected for each subject. The typical test session including electrode application and evoked response recordings lasted about 60 minutes.
Data Analysis for Electrophysiological Recordings
Sweeps greater than ±100μV were rejected offline, after which the remaining sweeps were averaged to compute an averaged waveform. Individual subjects had at least two averaged CAEP waveforms of 300 sweeps each. If the waveforms were judged replicable based on visual inspection, then the waveforms were averaged together to create a grand average waveform for individual subjects. The P1 peak was defined as the first robust positivity in the waveform. Peak latencies were measured in milliseconds (ms). The participants' P1 latencies were compared to the 95% confidence intervals for normal P1 latency development (Sharma et al, 2002a, 2002b). Absolute peak amplitudes were also computed. These were defined as the magnitude of positive deflection from zero, measured in microvolts (μV).
Results
P1 Morphology and Latency
All children in the current study presented with replicable CAEP waveforms with identifiable P1 peaks. These waveform characteristics were determined by waveform evaluation performed by two independent and experienced clinicians (authors AS & GC). To be considered typical, a P1 response must be replicable between two or more CAEP recording runs and must have a characteristic positive-going CAEP component during the characteristic P1 time window for children according to their age (e.g., within the approximate 100-300 ms range; e.g., Sharma et al. 1997; Ponton et al, 1996; Ceponeine et al, 2002). Previous studies in children with ANSD have shown that P1 CAEP responses were only present in 50-70% of children with hearing aids (Rance et al 2002, Sharma et al, 2011). Sharma et al. (2011) described children who did not show age-appropriate or replicable P1's as having ‘abnormal’ P1 responses. However, in the current study, P1 peak latency and amplitude could be reliably computed in 100% of children with ANSD. P1 latencies were compared against the 95% confidence intervals for normal P1 latency development for each child's age (Sharma et al, 2002b). Based on this analysis, the children in the current study fell into two groups: 1) Children with normal P1 latency (n = 11); and 2) Children with delayed P1 latency (n = 13). Differences in P1 CAEP status between these two groups were not explained by factors such as experience with the CI and unaided PTA. For experience with the CI the means were 0.84 and 0.77; SD = 0.42 and 0.69 years for children with normal and delayed P1 CAEP responses, respectively (F=0.08, p=0.78). For unaided PTA the means were 85 and 79 dB HL; SD = 23 and 16 dBHL for children with normal and delayed P1 CAEP responses, respectively, (F=0.48, p=0.49)
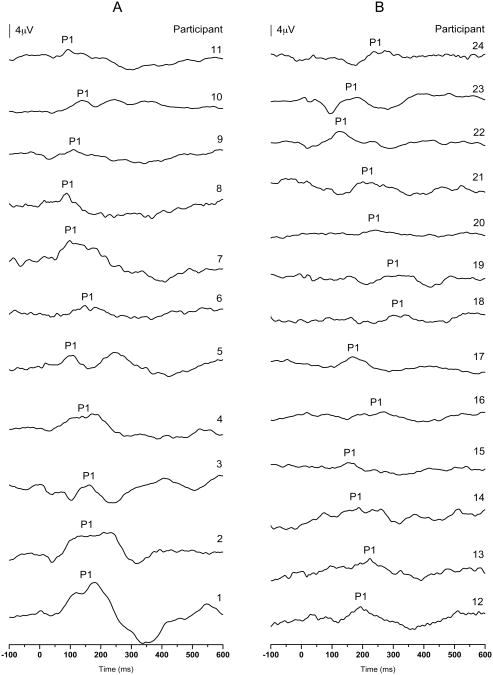
Each subject's grand average waveform can be seen in Figure 1. Waveforms are arranged from oldest at time of testing (top) to youngest (bottom) in each group—children with normal P1 responses (Figure 1A) and children with delayed P1 responses (Figure 1B).
Figure 1.
P1 CAEP waveforms for each participant in the group of children with normal P1 CAEP responses (A) and the group of children with delayed P1 CAEP responses (B). Waveforms are listed from oldest (top) to youngest (bottom).
P1 CAEP Amplitude
Absolute peak amplitude, or the difference between the baseline and the P1 peak, was calculated for each child's grand average waveform. When the groups' amplitude measurements were compared using a one-way ANOVA, we found no statistically significant result (p = 0.11; F = 2.74). However, the groups exhibited a trend in their mean amplitudes, such that the group of children with normal P1 responses showed greater P1 peak amplitudes overall than the group of children with delayed P1 responses. The mean amplitude for the children with normal P1 CAEP responses was 3.54 μV (SD = 1.75 μV), while those who presented with delayed P1 CAEP responses had a mean peak amplitude of 2.50 μV (SD = 1.34 μV).
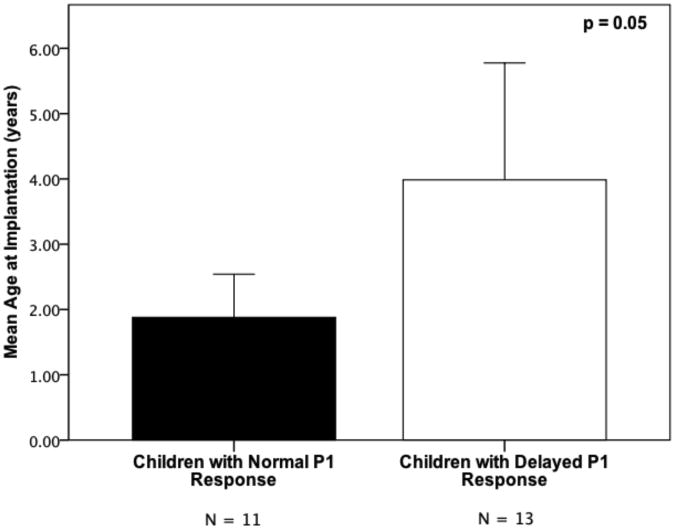
Age at Implantation
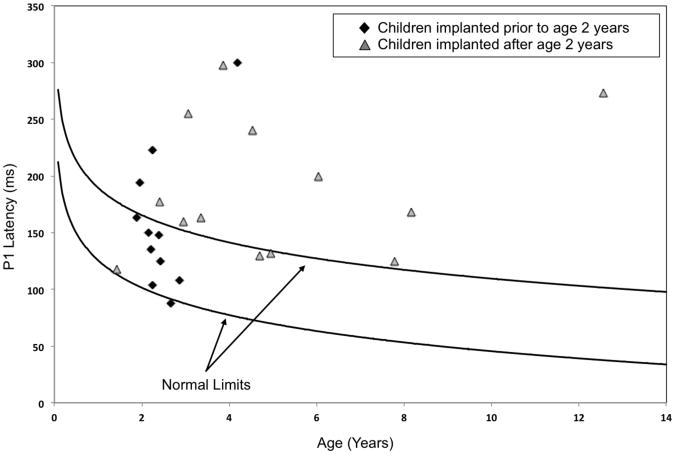
The age at which each child received his or her CI was compared using a one-way ANOVA. Results revealed a significant difference regarding age at implantation between the group of children with normal P1 responses and the group of children with delayed P1 responses (p = 0.05). The mean age at implantation was 1.88 years (SD = 1.09 years) and 4.07 years (SD = 3.15 years) respectively, for the children with normal and delayed P1 responses. This suggests that, in general, the children who showed normal P1 responses were fitted with a CI at an earlier age than the children with delayed P1 responses (see Figure 2A). Figure 2B shows P1 latencies for all participants plotted in relation to the 95% confidence intervals for normal P1 latency development. While 72% (8/11) of participants that received their CI before the age of 2 years fell within the 95% confidence intervals for P1 latency development, only 23% (3/13) of those fitted with a CI at or after the age of 2 years had normal P1 latencies. A Difference in Proportions test indicated that these percentages were significantly different from each other (p < 0.05). Again, experience with the CI use was not significantly different between these two groups.
Figure 2.
Panel A – Comparison of mean age at implantation for the groups of children with normal and delayed P1 CAEP responses, respectively. Error bars indicate the standard error of the mean.
Panel B – P1 latencies for each participant in the current study plotted against the 95% confidence intervals for normal P1 latency development (solid lines; adapted from Sharma et al, 2002a). Solid black diamonds represent children who were fitted with a CI before 2 years of age, while solid grey triangles mark children who received their CI at or after the age of 2 years.
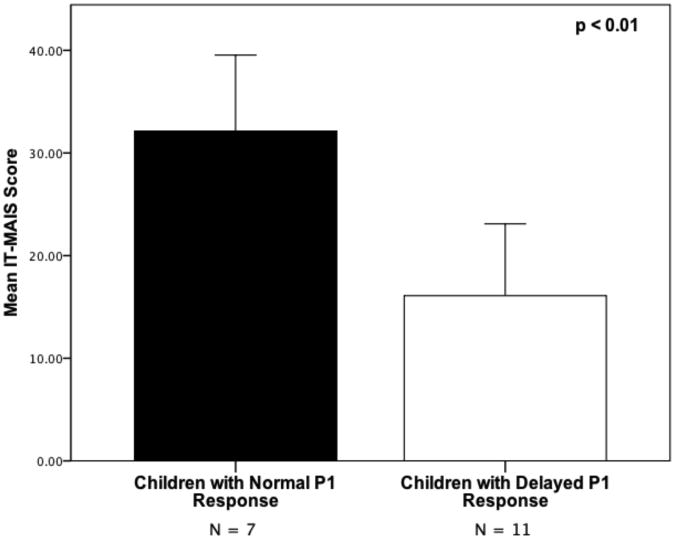
IT-MAIS Score
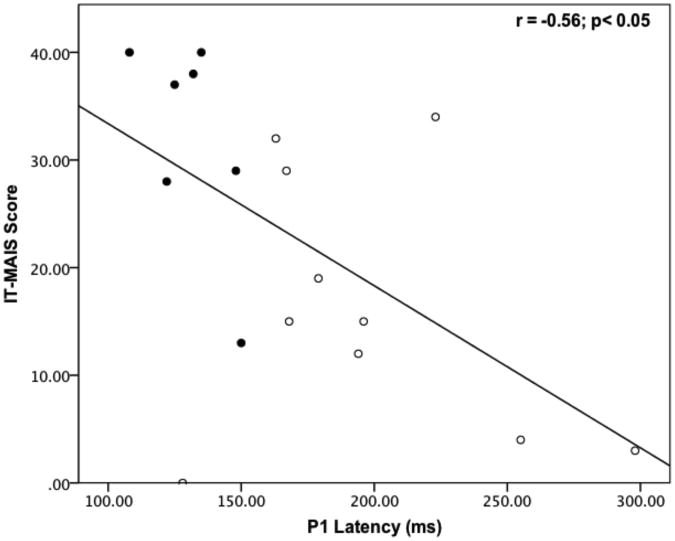
In order to explore the relationship between central auditory maturation and behavioral outcome, we compared the IT-MAIS scores between children with normal P1 responses (mean IT-MAIS = 32; SD = 9.79) and delayed P1 responses (mean IT-MAIS = 16; SD = 11.61). A one-way ANOVA showed a statistically significant difference between the groups for IT-MAIS scores (p < 0.01; see Figure 3A). Regression analysis demonstrated a significant negative relationship between IT-MAIS score and P1 latency (r = -0.55; p < 0.05; see Figure 3B). Overall, children with earlier P1 latencies tended to have higher IT-MAIS scores. This result is consistent with our previous finding of a negative correlation between IT-MAIS scores and P1 latencies in children with ANSD who are fitted with hearing aids (Sharma et al., 2011).
Figure 3.
Mean IT-MAIS scores for children with normal and delayed P1 CAEP responses (A). P1 latency plotted against IT-MAIS score for children with normal (filled circles) and delayed (open circles) P1 CAEP responses (B).
Longitudinal Results
In a subset of 11 children, on whom we had longitudinal data, we were able to examine CAEP responses before and after cochlear implantation, to allow us to evaluate the effect of implantation on the progression of central auditory maturation. All children in this portion of the study had been fitted with hearing aids prior to implantation and, following implantation, had been using their CI for at least 6 months at the time of P1 testing (mean CI experience = 1.08 years; SD = 0.48 years).
We examined data comparing the P1 responses from both pre- and post-implant testing sessions. Pre- and post-implant data are categorized according to their status relative to the 95% confidence intervals for typical P1 latency development (Sharma et al, 2002b). That is, ‘normal’ refers to P1 latencies that fell within the normal limits for P1 latency development at the age at which the P1 CAEP was recorded. Following this pattern, ‘delayed’ P1 CAEPs were those that were identifiable and replicable, but happened to fall outside the normal limits. Finally, ‘absent or ‘abnormal’ P1 CAEP findings were those waveforms which didn't exhibit an identifiable or replicable P1 CAEP, or in which the P1 was of grossly abnormal morphology, respectively (Sharma et al, 2011). In 10 out of the 11 children, the status of P1 latencies were normal or showed improvement after implantation. Three out of 11 children (27%), exhibited normal P1 status when using hearing aids, which stayed the same following implantation. In 7 out of 11 children (63%), the status of the pre-implantation P1 (delayed or abnormal) improved after implantation by at least one status category (e.g., delayed to normal; abnormal to normal; abnormal to delayed). In one case, a child who exhibited a normal P1 response with hearing aids actually progressed to have a delayed P1 response with a CI. This child also showed poor behavioral outcomes with his cochlear implant. Overall, these data suggest that when examined longitudinally, all (but one) children with ANSD show normal and/or improved central auditory maturation after cochlear implantation when they had been given sufficient experience (at least 6 months) with their devices. However, even though all in the current study sample presented with identifiable P1 CAEP responses, there could be implanted children who would not show CAEPs when a larger data set was considered.
Discussion
The current study examined central auditory maturation, behavioral outcomes, and their relationship, in children with ANSD who were fitted with CIs. Our investigation yielded several noteworthy results. First, we found replicable P1 CAEP responses in all pediatric ANSD patients fitted with CI's. This is in contrast to previous studies by our group (Sharma et al., 2011) and others (Starr et al, 1996; Rance et al, 2002; Alvarenga et al, 2012) where P1 CAEP response have been documented for only 50-70% of children with ANSD fitted with hearing aids and 85% of children with CIs. Furthermore, in our study, ANSD participants fitted with implants fell into 2 distinct categories based on their P1 latencies: Children who showed age-appropriate cortical maturation and children with delayed cortical maturation. Again, this result is in contrast to our previous study (Sharma et al., 2011) where we reported that children with ANSD who were fitted with hearing aids were divided into 3 distinct categories of P1 responses, i.e., normally developing, delayed and abnormal cortical maturation. Sharma et al. (2011) suggested that the cortical maturational status described by the P1 CAEP (i.e., normal, delayed or abnormal) underscored the severity of underlying neural-dys-synchrony. Taken together, results from the current study and the Sharma et al., (2011) study, suggest that abnormal cortical responses (and by inference the most severe disruptions in neural synchrony) are seen in ANSD participants who were fitted with hearing aids, but not with cochlear implants. Thus, it would appear that CIs are more effective at providing the auditory stimulation needed for central auditory maturation in children with severe disruptions in neural synchrony, while hearing aids may only benefit children with milder cases of dys-synchrony. This notion is in keeping with previous studies, which interpret positive post-implantation outcomes as improvements or even restoration of neural synchrony by electrical stimulation in a large portion of children with ANSD (Buss et al, 2002; Sinninger & Trautwein, 2002).
Overall, our findings are consistent with the literature, which suggests that many children with ANSD tend to have good outcomes with CI's. For instance, an early study by Shallop et al (2001) demonstrated that CIs were successful in improving communicative ability in 5 children with ANSD. Similarly, a recent study by Breneman, Gifford, & Dejong (2012) showed that the expectations for children with ANSD, in terms of cochlear implant outcomes, should be no different than for children with SNHL. Several other studies have presented similarly positive results for cochlear implantation of children with ANSD (e.g., Buss et al, 2002; Mason et al, 2003; Peterson et al, 2003; Teagle et al, 2010).
The longitudinal findings of the current study illustrate that most children (10/11) who had worn their CI for at least 6 months exhibited normal or improved central auditory maturation, as compared to the status of their auditory cortical development with hearing aids. These findings echo a recent behavioral longitudinal investigation of cochlear implantation in children with ANSD by Teagle et al (2010), in which a majority of the participants analyzed, who had worn their CIs for more than six months, showed significant improvement on either measures of auditory skill development (IT-MAIS) or speech perception (Phonetically Balanced Kindergarten; PBK; Haskins, 1949). On the other hand, several children in the cross-sectional portion of the current study persisted with delayed central auditory development even after cochlear implantation. Though we are unable to unequivocally indicate the reason for these continual delays, it is reasonable to conjecture that they are due to factors such as very severe forms of dys-synchrony that might result from factors such as demyelination, limiting outcomes with a cochlear implant. These findings are consistent with studies which assert that expectations may need to be lower for children with ANSD who use CIs, as opposed to their peers with SNHL (Miyamoto et al, 1999; Rance & Barker, 2008; 2009; Neary & Lightfoot, 2012). In fact, a recent review of the ANSD literature pointed out that up to 27% of children with ANSD who had received CIs presented with open-set speech perception scores of less than 30% (Roush et al, 2011).
A possible sensitive period for cortical maturation in ANSD
A second important result of our study was that children with ANSD who had normal central auditory maturation (as evidenced by normal P1 responses) were fitted with their CIs significantly earlier than those who had delayed cortical maturation (See Figure 3). This finding is in keeping with reports that have focused on early cochlear implantation in children with SNHL. For instance, previous investigations performed by our group (Sharma et al, 2002a, Sharma et al, 2002b, Sharma et al, 2005, Sharma et al, 2007, Sharma et al, 2009; Sharma & Dorman, 2006) have shown that the vast majority of children fitted with a CI before the age of 3.5 years, showed age-appropriate P1 responses within 3-6 months after implant experience. On the other hand, those who were fitted after the age of seven years of age rarely developed a normal P1 response even after years of implant usage. Combined with PET brain imaging studies, which reveal similar age cut-offs (Lee et al, 2001; Oh et al, 2003; Lee et al, 2007), our studies point to the existence of a sensitive period for central auditory maturation after cochlear implantation in deaf children. Restoration of appropriate and sufficient auditory stimulation during the sensitive period seems to lead to improved development of synaptic connections throughout the central auditory system, since neuroplasticity is at its maximum during that time (Hammes et al, 2002; Harrison et al, 2005; Kral et al, 2005; Tomblin et al, 2007).
Given that there is ample evidence for sensitive periods for auditory cortical development in the case of complete sensory deprivation such as deafness, its reasonable to assume that sensory degradation (as result of neural dys-synchrony) of input to the cortex may also be ameliorated within a sensitive period with appropriate treatment. Animal studies have revealed that introduction of abnormal sound conditions or stimulation during early life has the ability to change the development of the cortex. For instance, rodents that were reared in constant noise exhibited delayed auditory cortical maturation and altered sensitive periods for this type of development (Chang and Merzenich, 2003; Villers-Sidani et al., 2007). Additionally, Sur and colleagues (1988) showed that an altered pattern of stimulation of the cortex by subcortical neural pathways changed the structure and function of the auditory cortex during development. These findings could have parallels to ANSD, in that peripheral abnormalities causing neural dys-synchrony have the potential to lead to irregularities in higher brain regions. It is possible that these cortical defects could, in turn, result in delayed or abnormal CAEPs. Additionally, ongoing research has elucidated some of the important factors in sensitive period regulation. For example, several studies have indicated that inhibitory neural activity in the cortex (i.e., specifically in auditory cortex) is vital in the mediation of sensitive periods (Fagiolini et al, 2000; Kral et al, 2001; Hensch, 2005; Takesian et al, 2009). Furthermore, it seems that appropriate and consistent sensory experience is needed to induce cortical inhibitory activity (Foeller & Feldman, 2004). These and other studies make it readily evident that extrinsic factors applied during important developmental periods have the ability to greatly influence the maturation of the auditory cortex. Because abnormalities in the input to the cortex via subcortical elements has been shown to alter, not only the structure and function of the cortex, but also sensitive periods that mediate neuroplasticity, it is reasonable to believe that the atypical stimulation of the cortex, caused by neural dys-synchrony, in children with ANSD could lead to variation in the age range for maximal neural plasticity during cortical maturation. Furthermore, the high degree of prematurity in the ANSD population could potentially contribute to the alteration of the timing of developmental sensitive periods, possibly including their earlier initiation. Though the details regarding the affects of neural dys-synchrony on central auditory maturation are not fully known at this time, future research efforts should be devoted to this issue. What is clear, from the current findings, is that early intervention (i.e., < 2 years of age) appears to be more beneficial for children with ANSD than later intervention (i.e., > 2 years of age), which may suggest a sensitive period for treatment in this population.
Central auditory maturation as a predictor of outcome in children with ANSD
Another relevant finding from our study is that there was a significant correlation (r = -0.55; p < 0.05) between central auditory maturation (as measured by P1 latencies) and behavioral outcome (as evidenced by IT-MAIS scores). These results are consistent with our previous study Sharma et al (2011), in which we reported a strong correlation between P1 latency and IT-MAIS score (r = -0.86; p < 0.01) in children with ANSD who used hearing aids. Our finding is also consistent with other studies, which demonstrate a meaningful relationship between CAEPs and behavioral speech perception performance (Rance et al, 2002; Alvarenga et al, 2012). Taken together, these studies suggest that the P1 CAEP is an effective predictor of behavioral outcome in children with ANSD. This is significant in that, although invaluable in the diagnosis of ANSD, many of the conventional audiologic assessments that are performed clinically don't provide information relevant for determining cochlear implant candidacy in children with ANSD. For instance, many reports, including the current investigation, have shown that behaviorally elicited auditory thresholds do not predict speech understanding in people with ANSD, especially in the presence of background noise (e.g., Kraus, 2001; Sininger et al, 2001) and speech perception tests are often difficult to administer or unreliable in the population at young ages. Overall, we have found that P1 CAEP measurements may have an important role in clinical management of children with ANSD. Sharma et al., (2005), Cardon et al., (2012), and Cardon and Sharma (2012) have reported on longitudinal P1 recordings and behavioral assessments in multiple case studies of children with ANSD. Some ANSD patients benefitted adequately with cochlear implants and these children had normally developing P1 responses and good behavioral outcomes. On the other hand, many cases showed abnormal P1 responses even after prolonged hearing aid use (or with no intervention). These children were considered good CI candidates based on both P1 results and standard audiological assessment. Thus, longitudinal P1 recordings can be a useful addition to a comprehensive audiological test battery used for assessing CI candidacy. Similarly, the P1 CAEP can be used as an objective measure to help evaluate the success of cochlear implantation for ANSD children in a timely fashion. If central auditory maturation is persistently delayed after implantation, then it may be an early indication that the child is at-risk for acquiring oral speech and language and alternative approaches should be evaluated. Overall, the current results and recent studies (Rance et al, 2002; Campbell et al, 2011; Cardon et al, 2011, 2012; Sharma et al, 2011; Alvarenga et al, 2012) suggest that the P1 CAEP may have a useful role as part of a comprehensive audiologic test battery for assessing CI candidacy in ANSD patients. Ongoing studies from our laboratory are focused on exploring the clinical utility of CAEPs in the evaluation of children with ANSD (see Campbell et al, 2011; Cardon & Sharma, 2011; Cardon et al, 2012).
Conclusion
We examined central auditory development and behavioral outcomes in pediatric ANSD patients fitted with cochlear implants. Our results show that all (but one) children showed progress in cortical maturation after being fit with cochlear implants. Although many children showed age-appropriate central auditory maturation after implantation, several others continued to show delayed cortical responses. However, there were no children who showed abnormal cortical responses after implantation, a finding that was reported in approximately a third of children after treatment with hearing aids (Sharma et al., 2011). Our results also suggest that age at implantation may play an important role in determining the effectiveness of implants to allow normal central auditory development for children with ANSD. Children implanted under 2 years of age were more likely to show normal cortical responses compared to children implanted after 2 years of age. This finding points to a possible sensitive period for treatment in pediatric patients with ANSD, though further research is needed to fully understand the effects of neural dys-synchrony on sensitive period regulation. Finally, we report a significant correlation between cortical maturation (as measured by P1 CAEP latencies) and behavioral outcomes (as measured by IT-MAIS scores) similar to a previous finding in children with ANSD who were fitted with hearing aids (Sharma et al., 2011). P1 responses are a good predictor of behavioral outcomes and therefore may be considered a clinically useful biomarker of cortical development. As such, P1 responses may be a useful addition to the comprehensive battery of audiological tests used in the clinical management of pediatric ANSD patients.
Acknowledgments
Research supported by a grant from the National Institutes of Health (NIH) to A.S. (R01DC0625). We would also like to acknowledge the assistance of Kathryn Henion, M.A in data collection.
Abbreviations
- CAEP
cortical auditory evoked potential
- ANSD
auditory neuropathy spectrum disorder
- AR
acoustic reflexes
- IT-MAIS
Infant Toddler Meaningful Auditory Integration Scale
- ABR
auditory brainstem response
- DPOAE
distortion product otoacoustic emission
- TEOAE
transient evoked otoacoustic emission
- PTA
pure tone average
- SNHL
sensorineural hearing loss
- MAIS
Meaningful Auditory Integration Scale
- CV
consonant-vowel
- CI
cochlear implant
References
- Alvarenga KF, Amorim R, Agostinho-Pesse RS, Costa OA, Nascimento LT, Bevilacqua MC. Speech perception and cortical auditory evoked potentials in cochlear implant users with auditory neuropathy spectrum disorders. Int J Pediatr Otolaryngol. 2012;76(9):1332–1338. doi: 10.1016/j.ijporl.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Berlin C, Bordelon J, St John P, Wilenski D, Annette K, Hood L. Reversing click polarity may uncover auditory neuropathy in infants. Ear Hear. 1998;19(1):37–47. doi: 10.1097/00003446-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Berlin C, Li L, Hood L, Morlet T, Rose K, Brashears S. Auditory neuropathy/dys-synchrony: diagnosis and management. Ment Retard Dev Disabil Res Rev. 2003;9:225–231. doi: 10.1002/mrdd.10084. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, Wilensky D, St John P, Montgomery E, Thibodeaux M. Absent or elevated middle ear muscle reflexes in the presence of normal otoacoustic emissions: a universal finding in 136 cases of auditory neuropathy/dys-synchrony. J Am Acad Audiol. 2005;16(8):546–553. doi: 10.3766/jaaa.16.8.3. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Morlet T, Wilensky D, Li L, Mattingly KR, Taylor-Jeanfreau J, Keats BJ, John PS, Montgomery E, Shallop JK, Russell BA, Frisch SA. Multi-site diagnosis and management of 260 patients with Auditory Neuropathy/Dys-synchrony (Auditory Neuropathy Spectrum Disorder) Int J Audiol. 2010;49(1):30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- Bielecki I, Horbulewicz A, Wolan T. Prevalence and risk factors for Auditory Neuropathy Spectrum Disorder in a screened newborn population at risk for hearing loss. Int J Ped Otorhinolaryngol. 2012;76(11):1668–1670. doi: 10.1016/j.ijporl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Breneman AI, Gifford RH, Dejong MD. Cochlear implantation in children with auditory neuropathy spectrum disorder: long-term outcomes. J Am Acad Audiol. 2012;23(1):5–17. doi: 10.3766/jaaa.23.1.2. [DOI] [PubMed] [Google Scholar]
- Buss E, Labadie RF, Brown CJ, Gross AJ, Grose JH, Pillsbury HC. Outcome of Cochlear Implantation in Pediatric Auditory Neuropathy. Otol & neurotol. 2002;23(3):328. doi: 10.1097/00129492-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Campbell J, Cardon G, Sharma A. Clinical application of the P1 cortical auditory evoked potential biomarker in children with sensorineural hearing loss and auditory neuropathy spectrum disorder. Semin Hear. 2011;32(2):117–122. doi: 10.1055/s-0031-1277236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon G, Sharma A. Cortical auditory evoked potentials in auditory neuropathy spectrum disorder: clinical implications. Perspectives on Hearing and Hearing Disorders in Children. 2011;21:31–37. [Google Scholar]
- Cardon G, Campbell J, Sharma A. Plasticity in the developing auditory cortex: evidence from children with sensorineural hearing loss and auditory neuropathy spectrum disorder. J Am Acad Audiol. 2012;23(6):396–411. doi: 10.3766/jaaa.23.6.3. quiz 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho ACM, Bevilacqua MC, Sameshima K, Costa Filho OA. Auditory neuropathy/Auditory dyssynchrony in children with Cochlear Implants. Braz J Otorhinolaryngol. 2011;77(4):481–487. doi: 10.1590/S1808-86942011000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Näätänen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113(6):870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich M. Environmental noise retards auditory cortical development. Science. 2003;300(5618):498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. On the rate of maturation of sensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1988;70(4):293–305. doi: 10.1016/0013-4694(88)90048-x. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14(1):89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Geers AE. Factors influencing spoken language outcomes in children following early cochlear implantation. Adv Oto-rhino-laryngol. 2006;64:50–65. doi: 10.1159/000094644. [DOI] [PubMed] [Google Scholar]
- Geers AE, Moog JS, Biedenstein J, Brenner C, Hayes H. Spoken Language Scores of Children Using Cochlear Implants Compared to Hearing Age-Mates at School Entry. J Deaf Stud Deaf Educ. 2009;14(3):371–385. doi: 10.1093/deafed/enn046. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Finley CC, Panch AS, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin Neurophysiol. 2006;117(8):1772–1782. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Hammes DM, Novak MA, Rotz LA, Willis M, Edmondson DM, Thomas JF. Early identification and cochlear implantation: Critical factors for spoken language development. Ann Otol Rhinol Laryngol Suppl. 2002;189:74–78. doi: 10.1177/00034894021110s516. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–261. doi: 10.1002/dev.20052. [DOI] [PubMed] [Google Scholar]
- Haskins H. Unpublished master's thesis. Northwestern University; 1949. A phonetically balanced test of speech discrimination for children. [Google Scholar]
- Hassan D. Perception of temporally modified speech in auditory neuropathy. Int J Audiol. 2011;50(1):41–49. doi: 10.3109/14992027.2010.520035. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Kirkim G, Serbetcioglu B, Erdag T, Ceryan K. The frequency of auditory neuropathy detected by universal newborn hearing screening program. Int J Pediatr Otorhinolaryngol. 2008;72(10):1461–1469. doi: 10.1016/j.ijporl.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Klatt D. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–995. [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Delayed maturation and sensitive periods in the auditory cortex. Audiol Neurootol. 2001;6(6):346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- Kral A, Tillein J, Heid S, Hartmann R, Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb Cortex. 2005;15(5):552–562. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Kishon-Rabin L, Taitelbaum R, Elichai O, Maimon D, Debyiat D, Chazan N. Developmental aspects of the IT-MAIS in normal-hearing babies. Isr J Speech Hear. 2001;23:12–22. [Google Scholar]
- Kraus N, McGee T, Carrell TD, King C, Tremblay K, Nicol T. Central Auditory System Plasticity Associated with Speech Discrimination Training. J Cogn Neurosci. 1995;7(1):25–32. doi: 10.1162/jocn.1995.7.1.25. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunningham J, King CD, Koch DB, Nicol TG, McGee TJ, Stein LK, Wright BA. Consequences of a neural asynchrony: a case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001;409(6817):149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Giraud AL, Kang E, Oh SH, Kang H, Kim CS, Lee DS. Cortical activity at rest predicts cochlear implantation outcome. Cereb Cortex. 2007;17(4):909–917. doi: 10.1093/cercor/bhl001. [DOI] [PubMed] [Google Scholar]
- Maris M, Venstermans C, Boudewyns AN. Auditory neuropathy/dyssynchrony as a cause of failed neonatal hearing screening. Int J Pediatr Otorhinolaryngol. 2011;75(7):973–975. doi: 10.1016/j.ijporl.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Mason JC, De Michele A, Stevens C, Ruth RA, Hashisaki GT. Cochlear Implantation in Patients With Auditory Neuropathy of Varied Etiologies. Laryngoscope. 2003;113(1):45–49. doi: 10.1097/00005537-200301000-00009. [DOI] [PubMed] [Google Scholar]
- McConkey Robbins A, Burton Koch D, Osberger MJ, Zimmerman-Phillips S, Kishon-Rabin L. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol - Head Neck Surg. 2004;130(5):570–574. doi: 10.1001/archotol.130.5.570. [DOI] [PubMed] [Google Scholar]
- Michalewski H, Starr A, Nguyen T, Kong Y, Zeng F. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005;116(3):669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Zeng FG, Dimitrijevic A. N100 cortical potentials accompanying disrupted auditory nerve activity in auditory neuropathy (AN): Effects of signal intensity and continuous noise. Clin Neurophysiol. 2009;120(7):1352–1363. doi: 10.1016/j.clinph.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R, Ramesh AV, Panwar SS, Nilkanthan A, Nair S, Mehra PR. Auditory neuropathy spectrum disorder: Its prevalence and audiological characteristics in an Indian tertiary care hospital. Int J Pediatr Otorhinolaryngol. 2012;76(9):1351–1354. doi: 10.1016/j.ijporl.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Miyamoto RT, Kirk KI, Renshaw J, Hussain D. Cochlear implantation in auditory neuropathy. Laryngoscope. 1999;109(2 Pt 1):181–185. doi: 10.1097/00005537-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Nash A, Gilley P, Sharma A. Cortical organization and variability in unilateral auditory neuropathy spectrum disorder: a case study in preparation. [Google Scholar]
- Neary W, Lightfoot G. Auditory neuropathy spectrum disorder: Examples of poor progress following cochlear implantation. Audiological Medicine. 2012;10(3):142–149. [Google Scholar]
- Oh SH, Kim CS, Kang EJ, Lee DS, Lee HJ, Chang SO, Ahn SH, Hwang CH, Park HJ, Koo JW. Speech perception after cochlear implantation over a 4-year time period. Acta Otolaryngol. 2003;123(2):148–153. doi: 10.1080/0036554021000028111. [DOI] [PubMed] [Google Scholar]
- Pearce W, Golding M, Dillon H. Cortical auditory evoked potentials in the assessment of auditory neuropathy: two cases. J Am Acad Audiol. 2007;18:380–390. doi: 10.3766/jaaa.18.5.3. [DOI] [PubMed] [Google Scholar]
- Peterson A, Shallop J, Driscoll C, Breneman A, Babb J, Stoeckel R, Fabry L. Outcomes of cochlear implantation in children with auditory neuropathy. J Am Acad Audiol. 2003;14(4):188–201. [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Waring MJ, Masuda A. Matuation of human cortical auditory function: Differences between normal-hearing children and children with cochlear implants. Ear Hear. 1996;17(5):430–437. doi: 10.1097/00003446-199610000-00009. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111(2):220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9(1):1–43. doi: 10.1177/108471380500900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech perception in children with auditory neuropathy/dyssynchrony managed with either hearing aids or cochlear implants. Otol Neurotol. 2008;29(2):179–182. doi: 10.1097/mao.0b013e31815e92fd. [DOI] [PubMed] [Google Scholar]
- Rance G, Barker EJ. Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with either cochlear implants or hearing aids. Int J Audiol. 2009;48(6):313–320. doi: 10.1080/14992020802665959. [DOI] [PubMed] [Google Scholar]
- Rance G, Beer D, Cone-Wesson B, Shepherd R, Dowell R, King A, et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear. 1999;20(3):238. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear. 2002;23(3):239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Rance G, McKay C, Grayden D. Perceptual characterization of children with auditory neuropathy. Ear Hear. 2004;25(1):34–46. doi: 10.1097/01.AUD.0000111259.59690.B8. [DOI] [PubMed] [Google Scholar]
- Rance G, Ryan MM, Carew P, Corben LA, Yiu E, Tan J, Delatycki MB. Binaural speech processing in individuals with auditory neuropathy. J Neurosci. 2012;226:227–235. doi: 10.1016/j.neuroscience.2012.08.054. [DOI] [PubMed] [Google Scholar]
- Sarant JZ, Blamey PJ, Dowell RC, Clark GM, Gibson WPR. Variation In Speech Perception Scores Among Children with Cochlear Implants. Ear Hear. 2001;22(1):18. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Shallop J, Peterson A, Facer G, Fabry L, Driscoll C. Cochlear implants in five cases of auditory neuropathy: postoperative findings and progress. Laryngoscope. 2001;111:555–562. doi: 10.1097/00005537-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF. Central auditory development in children with cochlear implants: Clinical implications. Adv Otorhinolaryngol. 2006;64:66–88. doi: 10.1159/000094646. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002a;13(10):1365–1368. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002b;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Cardon G, Henion K, Roland P. Cortical maturation and behavioral outcomes in children with auditory neuropathy spectrum disorder. Int J Audiol. 2011;50(2):98–106. doi: 10.3109/14992027.2010.542492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42(4):272–279. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Gilley PM, Dorman MF, Baldwin R. Deprivation induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46(9):494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr Clin Neurophysiol. 1997;104(6):540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Sharma A, Martin K, Roland P, Bauer P, Sweeney MH, Gilley P, Dorman M. P1 latency as a biomarker for central auditory development in children with hearing impairment. J Am Acad Audiol. 2005a;16(8):564–573. doi: 10.3766/jaaa.16.8.5. [DOI] [PubMed] [Google Scholar]
- Sininger Y, Oba S. Patients with auditory neuropathy: Who are they and what can they hear? In: Sininger Y, Starr A, editors. Auditory neuropathy: A new perspective on hearing disorders. San Diego: Singular; 2001. pp. 67–82. [Google Scholar]
- Starr A, McPherson D, Patterson J, Don M, Luxford W, Shannon R, Sininger Y, Tonakawa L, Waring M. Absence of both auditory evoked potentials and auditory percepts dependent on time cues. Brain. 1991;114(3):1157–1180. doi: 10.1093/brain/114.3.1157. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Kim R. Pathophysiology of auditory neuropathy. In: Sininger Y, Starr A, editors. Auditory neuropathy: A new perspective on hearing disorders. San Diego: Singular; 2001. pp. 67–82. [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory Neuropathy. Brain. 1996;119(3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988;242(4884):1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Robbins AM, Kirk KI, Pisoni DB, Miyamoto RT. Language Development in Profoundly Deaf Children with Cochlear Implants. Psych Sci. 2000;11(2):153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol. 2009;4(3):331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat HS, Kabel A, Samy H, Elbadry M. Prevalence of auditory neuropathy (AN) among infants and young children with severe o profound hearing loss. Int J Pediatr Otorhinolaryngol. 2009;73(7):937–939. doi: 10.1016/j.ijporl.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Teagle HF, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear. 2010;31(3):325–335. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- Tobey EA, Geers AE, Brenner C, Altuna D, Gabbert G. Factors Associated with Development of Speech Production Skills in Children Implanted by Age Five. Ear Hear. 2003;24(1):36S. doi: 10.1097/01.AUD.0000051688.48224.A6. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Barker BA, Hubbs S. Developmental constraints on language development in children with cochlear implants. Int J Audiol. 2007;46(9):512–523. doi: 10.1080/14992020701383043. [DOI] [PubMed] [Google Scholar]
- Trautwein PG, Sininger YS, Nelson R. Cochlear implantation of auditory neuropathy. J Am Acad Audiol. 2000;11(6):309–315. [PubMed] [Google Scholar]
- Trautwein P, Shallop J, Fabry L. Cochlear implantation of children with auditory neuropathy. In: Sininger Y, Starr A, editors. Auditory neuropathy: A new perspective on hearing disorders. San Diego: Singular; 2001. pp. 203–232. [Google Scholar]
- Uus K, Bamford J. Effectiveness of population-based newborn hearing screening in England: ages if interventions and profile of cases. Pediatrics. 2006;117(5):e887–e893. doi: 10.1542/peds.2005-1064. [DOI] [PubMed] [Google Scholar]
- Villers-Sidani E, Chang EF, Bao S, Merzenich M. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27(1):180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J, Gibson WPR, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol & Neurotol. 2008;29(3):302–309. doi: 10.1097/MAO.0b013e318164d0f6. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93(6):3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Liu S, Stickney G, Del Rio E, Kong YY, Chen H. On the dichotomy in auditory perception between temporal envelope and fine structure cues. J Acoust Soc Am. 2004;116(3):1351–1354. doi: 10.1121/1.1777938. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49(2):367–380. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]
- Zeng F, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuro Report. 1999;10(16):3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zimmerman-Phillips S, Robbins AM, Osberger MJ. Assessing cochlear implant benefit in very young children. Ann Otol Rhinol Laryngol Suppl. 2000;185:42–43. doi: 10.1177/0003489400109s1217. [DOI] [PubMed] [Google Scholar]