Abstract
Introduction
Over half of all gallbladder carcinoma (GBC) is discovered incidentally after cholecystectomy for benign disease. There are scant data comparing presentation and outcome for patients with incidental versus suspected GBC. The goal of this study is to determine the clinical differences between these two entities.
Study Design
Patients with GBC were identified retrospectively from records at academic healthcare institutions in Temuco, Chile; Atlanta, GA; and Rochester, MN between 1984 and 2008. Overall survival was compared for patients with and without preoperative suspicion using Kaplan–Meier curves and a multivariate Cox proportional hazards model.
Results
Of 571 patients, 128 (22.4 %) had preoperative suspicion of malignancy, and 443 (77.6 %) were discovered incidentally. Incidental tumors were of lower stage, better differentiated, and with lower rates of metastases. Median survival for incidentally discovered GBC was 32.3 versus 5.8 months for suspected GBC (p<0.0001). In a Cox proportional hazards model controlling for operation extent, T stage, differentiation, and other factors, preoperative suspicion remains a strong risk factor (odds ratio, 2.0; confidence interval, 1.5–2.9; p<0.0001).
Conclusions
Tumor characteristics differed significantly between patients with incidentally discovered versus preoperatively suspected GBC. Incidental GBC has a significantly better median survival.
Keywords: Gallbladder, Cancer, Carcinoma, Survival, Imaging, Incidental, Suspected
Introduction
Gallbladder carcinoma (GBC) is the fifth most common gastrointestinal malignancy in Western countries, with approximately 5,000 new cases diagnosed annually in the USA.1 Symptomatic GBC generally presents at an advanced stage and carries a very poor prognosis. However, up to 61 % of GBC are diagnosed incidentally during surgery for benign disease or on postoperative histology.2,3 Depending on regional prevalence, between 0.1 and 6 % of all patients undergoing cholecystectomy will have an incidental discovery of GBC.1,4,5
Several retrospective studies have examined prognostic factors for patients with GBC. Tumor stage, according to the American Joint Committee on Cancer (AJCC) staging system, is the strongest overall predictor of patient survival.2,6 Other prognostic factors include the extent of surgical re-section, lymph node status, age, and sex.6,7 The presentation and management of incidental GBC have been described previously.8 There are scant data, however, on the importance of incidental discovery of GBC as a prognostic indicator of patient survival. Incidental GBC may represent early-stage cancer that would eventually progress to symptomatic disease, or it may represent a unique histological entity with a different prognosis.
The current study examines multicenter data from Chile and the USA in an effort to understand the clinical differences between incidental and suspected GBC. We hope to explore the impact of time of diagnosis on survival in patients with GBC and the interaction between preoperative suspicion and other known prognostic factors, including tumor stage.
Methods
Patient Population and Data Collection
Patients were identified from the prospectively maintained surgical records of three healthcare centers with academic affiliation in Atlanta, GA; Rochester, MN; and Temuco, Chile. Records were reviewed for 571 patients with histologically confirmed GBC who underwent surgical resection between 1984 and 2008.
A manual retrospective chart review was performed to record age at operation, gender, presence of associated cholelithiasis, stage at presentation, and histologic characteristics. Operative characteristics were also recorded, including the extent of surgical resection. For the purpose of the analysis, this was dichotomized to cholecystectomy alone or cholecystectomy with liver resection. Suspicion of malignancy prior to operation was ascertained based on preoperative records. Patients were considered to have pre-operative suspicion of cancer if either the surgeon's notes or the radiologist's interpretation of a preoperative imaging study contained any suspicion of nonbenign gallbladder disease. The types of imaging studies obtained prior to the operation leading to the diagnosis were also recorded. The amount of time between the operation and the date of last follow-up was recorded along with status (alive or deceased) at last follow-up.
Stage at presentation is recorded in accordance with the AJCC Staging Manual, sixth edition, as data collection was performed prior to the release of the AJCC seventh edition. Although the recent edition includes changes to staging of GBC, the definition of primary T stage is identical between the two editions.9,10
To validate the multicenter data, pathologists at the Temuco and Atlanta centers randomly reviewed histologic features of a selected portion of the dataset. The sample group demonstrated over 90 % agreement between centers.
Statistical Analysis
Patient characteristics were compared using either chi-square test of independence or Fisher's exact test for categorical variables and two-sample t test for continuous variables. Survival analysis was performed using the Kaplan–Meier method.11 Differences in observed survival distributions among patient groups were compared by two-sided log-rank test. Survival time was measured from the date of operation until the date of last follow-up or the date of death from any cause. For multivariate survival analysis, a Cox proportional hazards model was carried out.12 Potential covariates were examined in univariate analyses. All variables found on univariate analysis to be significant predictors of mortality were included in the final proportional hazards model, unless more than 10 % of data was missing for a particular covariate.
The predictive value of the selected variables on mortality from all causes was analyzed using a multivariate logistic regression model. The same candidate covariates used in the Cox model were considered in a stepwise fashion for the final logistic regression (p=0.10 for entry and p=0.05 to remain in the model). Forward and backward selection procedures were used to validate the stability of the model; the same covariates were selected regardless of selection procedure. All statistical tests were performed using the SAS 9.2 statistical software package for Windows (SAS Institute Inc, Cary, NC, USA).
Results
Study Population
Of 571 patients identified on chart review following surgery for adenocarcinoma of the gallbladder, 443 were discovered incidentally and 128 were suspected on preoperative imaging. Patient characteristics are compared in Table 1. The two cohorts were similar in age and gender, but tumor characteristics differed significantly. Only 14 % of suspected tumors were well differentiated, versus 28 % of incidentally discovered tumors (p=0.0013). Rates of nodal metastases (p=0.0001) and perineural (p<0.0001), perilymphatic (p< 0.0001), and perivascular invasion (p<0.0001) were also significantly higher in tumors suspected preoperatively. T stage also differed, with significantly more T0 cancers in the incidental group (17 versus 3 %, p=0.0002; see Table 1).
Table 1.
Patient and tumor characteristics for patients with and without preoperative suspicion of gallbladder cancer on imaging, presented as N (percentage) or mean±SD
| Variable | Preop suspicion (n=128) | No preop suspicion (n=443) | p valueb |
|---|---|---|---|
| Female gender | 97 (76 %) | 365 (82 %) | 0.0936 |
| Age | 63±12 | 63±13 | 0.8373 |
| Cholelithiasis | 86 (76 %) | 432 (98 %) | <0.0001 |
| AJCCa T0 | 3 (3 %) | 65 (17 %) | 0.0002 |
| AJCC T1 | 8 (7 %) | 1 (<1 %) | <0.0001c |
| AJCC T2–T5 | 101 (90 %) | 327 (83 %) | 0.0712 |
| Well differentiated | 17 (14 %) | 116 (28 %) | 0.0013 |
| Nodal metastasis | 35 (40 %) | 62 (16 %) | 0.0001 |
| Vascular invasion | 28 (30 %) | 44 (10 %) | <0.0001 |
| Perineural invasion | 25 (29 %) | 26 (6 %) | <0.0001 |
| Lymphatic invasion | 44 (44 %) | 38 (9 %) | <0.0001 |
| Chile | 50 (39 %) | 410 (93 %) | <0.0001 |
Percentages reflect the percentage of patients in each cohort with complete data available for the given variable
American Joint Committee on Cancer Staging system
P values obtained from Chi-square test of independence for categorical variables and t test for continuous variables
P value obtained from two-sided Fisher's exact test
Tumor characteristics also differed significantly depending on geography, and are compared in Table 2. The three centers had similar distributions of gender (83 % female in Chile versus 71 % in Atlanta and 75 % in Rochester, p=0.0922) and age (mean age 62, 64, and 65 years, p=0.1752). The types of GBC seen at each center, however, differed significantly. In Chile, 11 % of cancers are suspected preoperatively, compared with 50 % in the Atlanta cohort and 76 % in the Rochester cohort. In Chile, 98 % of patients were diagnosed with cholelithiasis, compared with 78 % in Atlanta and 77 % in Rochester (p<0.0001). No patients in Rochester or Atlanta had T0 cancer, but 16 % of tumors in Chile were stage T0 (p< 0.0001). Indications of aggressive disease, including nodal metastasis and perineural, perivascular, and perilymphatic invasion, are all significantly more common in the American centers (p<0.0001, see Table 2).
Table 2.
Patient and tumor characteristics between gallbladder cancer comparing patients from three centers, presented as N (percentage) or mean±SD
| Variable | Temuco, Chile (n=460) | Atlanta, GA (n=24) | Rochester, MN (n=87) | p valueb |
|---|---|---|---|---|
| Preoperative suspicion | 50 (11 %) | 12 (50 %) | 66 (76 %) | <0.001 |
| Female gender | 380 (83 %) | 17 (71 %) | 65 (75 %) | 0.0922 |
| Age | 62±13 | 64±11 | 65±12 | 0.1752 |
| Cholelithiasis | 448 (98 %) | 18 (78 %) | 52 (74 %) | <0.0001 |
| AJCCa T0 | 68 (16 %) | 0 | 0 | <0.0001 |
| AJCC T1 | 0 | 6 (25 %) | 3 (4 %) | <0.0001 |
| AJCC T2–T4 | 335 (78 %) | 18 (75 %) | 75 (96 %) | <0.0001 |
| AJCC T5 | 28 (7 %) | 0 | 0 | 0.0130 |
| Well differentiated | 129 (29 %) | 3 (14 %) | 1 (1 %) | <0.0001 |
| Nodal metastasis | 57 (15 %) | 10 (45 %) | 30 (50 %) | <0.0001 |
| Vascular invasion | 51 (11 %) | 13 (57 %) | 8 (16 %) | <0.0001 |
| Perineural invasion | 32 (7 %) | 15 (79 %) | 4 (9 %) | <0.0001 |
| Lymphatic invasion | 43 (10 %) | 13 (57 %) | 26 (46 %) | <0.0001 |
Percentages reflect the percentage of patients in each cohort with complete data available for the given variable
American Joint Committee on Cancer Staging system
P values obtained from Chi-square test of independence for categorical variables and ANOVA for continuous variables
Treatment plan is compared in Table 3. Patients with preoperative suspicion of disease had a much higher likelihood of not undergoing resection (46.1 versus 3.7 %, p<0.0001). Only 29.7 % of preoperatively suspected cancers were treated with isolated cholecystectomy; 8.6 % had concomitant liver resection and 15.6 % had a two-stage operation with deferred liver resection. In patients without preoperative suspicion, 89 % of patients are treated with cholecystectomy alone.
Table 3.
Extent of surgery for patients with and without preoperative suspicion, N (percentage)
| Type of surgery | Preop suspicion (n=128) | No preop suspicion (n=443) | p valuea |
|---|---|---|---|
| No resection | 59 (46.09 %) | 18 (3.65 %) | <0.0001 |
| Cholecystectomy only | 38 (29.69 %) | 394 (88.94 %) | <0.0001 |
| With liver resection | 11 (8.59 %) | 12 (2.71 %) | 0.0029 |
| Deferred liver resection | 20 (15.63 %) | 18 (4.06 %) | <0.0001 |
P values obtained from Chi-square test of independence
The type of preoperative imaging also differed between the different patient groups. The majority of patients with incidentally discovered cancer were evaluated with ultrasound alone (408, 89 %). For the remaining patients, 6 (1.4 %) had no preoperative imaging, 16 (4 %) were evaluated with MRI, and 8 (2 %) received more than one imaging study prior to operation. In comparison, 52 patients (43 %) with preoperative suspicion of cancer were evaluated with ultrasound alone, while 35 (29 %) received MRI studies and 33 (27 %) were evaluated with more than one imaging modality.
Survival Data
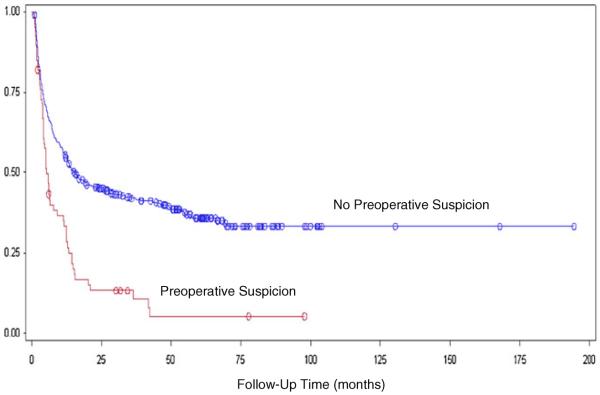
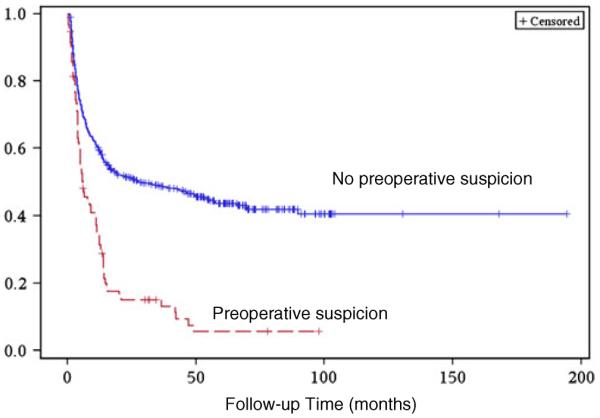
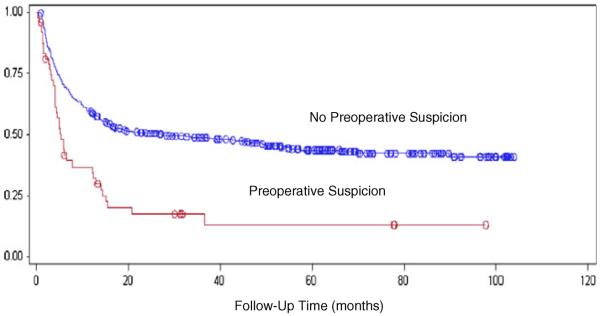
The median survival time for incidentally discovered GBC was 32.3 months. Suspected GBC had a median survival time of 5.8 months. The difference in survival was statistically significant (log-rank test p<0.0001; Fig. 1). The patients were then stratified based on AJCC T stage. Separate Kaplan–Meier curves were created to assess the effect of preoperative suspicion in patients with stage T0–T1 and patients with stage T2-T4 cancers. In both cohorts, the survival difference remained statistically significant (p= 0.0097, Fig. 2 and p<0.0001, Fig. 3).
Fig. 1.
Kaplan–Meier analysis of postoperative survival time in patients with GBC discovered incidentally (median survival, 32.3 months) versus GBC suspected on preoperative imaging (median survival, 5.8 months), log-rank test p<0.0001
Fig. 2.
Postoperative survival time in patients with stage T0–T1 GBC comparing incidental versus suspected disease, log-rank test p=0.0097
Fig. 3.
Postoperative survival time in patients with stage T2–T4 GBC comparing incidental versus suspected disease, log-rank test p<0.0001
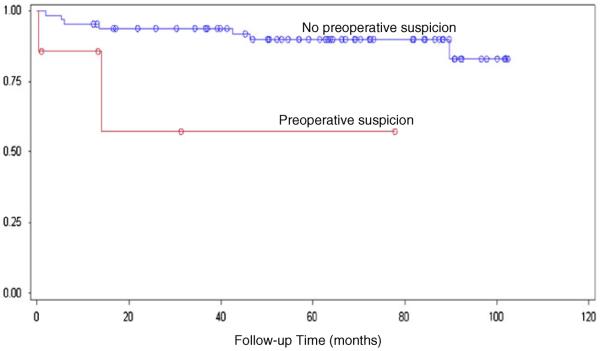
Patients were also stratified based on country of origin to address the different tumor characteristics seen in each population (Table 2). In both cohorts, preoperative suspicion remained a strong predictor of mortality (p<0.0001; Figs. 4 and 5).
Fig. 4.
Postoperative survival time in patients from Temuco, Chile comparing incidental versus suspected disease, log-rank test p<0.0001
Fig. 5.
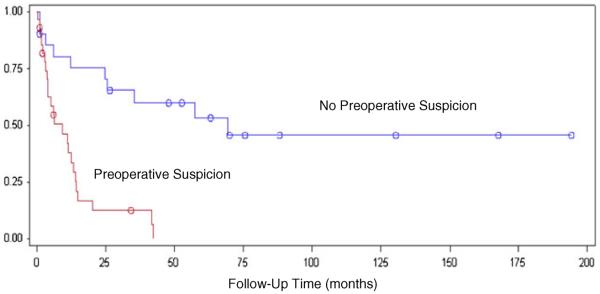
Postoperative survival time in patients from American centers (Rochester, MN and Atlanta, GA) comparing incidental versus suspected disease, log-rank test p<0.0001
The patient and operative characteristics were analyzed in a univariate logistic regression model with mortality at 1 year as the outcome. The variables with a significant (defined as p<0.05) correlation in a univariate analysis were: preoperative suspicion of cancer, age, T stage (dichotomized as T0/T1 versus T2–T4), degree of differentiation, extent of resection (cholecystectomy alone versus with liver resection), gender, presence of cholelithiasis, and country of origin. These variables were included in a multivariate Cox model (Table 4). Preoperative suspicion remained a significant predictor of survival when controlling for other risk factors (p<0.0001; HR, 2.042). In a multivariate logistic regression analysis, the same variables were considered in a stepwise fashion with p<0.10 with 1-year mortality as the dependent variable. Age at surgery, T stage, degree of differentiation, and preoperative suspicion were found to be significant predictors of mortality. In this final model, preoperative suspicion remained a strong predictor (OR, 4.86; 95 % CI, 2.34–10.08, p<0.0001; Table 5).
Table 4.
Multivariate Cox proportional hazards model for prognostic variables with univariate significance for survival
| Variablea | p value | Hazards ratio (95 % CI) |
|---|---|---|
| Preoperative suspicion | <0.0001 | 2.042 (1.458–2.860) |
| Age | 0.3652 | 1.005 (0.995–1.015) |
| Cholecystectomy with liver resectionb | 0.0376 | 0.581 (0.349–0.969) |
| Lithiasis | 0.8009 | 0.930 (0.527–1.640) |
| Chilec | 0.3531 | 0.780 (0.462–1.317) |
| T staged | <0.0001 | 7.682 (3.778–15.619) |
| Degree of differentiation | <0.0001 | 1.756 (1.469–2.099) |
Variables represent all possible covariates with significant univariate association with survival, excluding those missing for >10 % of the dataset
Versus cholecystectomy only
Versus Atlanta or Rochester clinic
AJCC stage T0 or T1 versus T2–T4
Table 5.
Multivanate logistic regression model for mortality
| Variablea | p value | Odds ratio (95 % CI) |
|---|---|---|
| Preoperative suspicion | <0.0001 | 4.857 (2.339–10.084) |
| Age | 0.0180 | 1.021 (1.004–1.039) |
| T stageb | <0.0001 | 10.894 (5.213–22.768) |
| Degree of differentiation | <0.0001 | 2.179 (1.598–2.972) |
Variables represent all possible covariates with significant univariate association with survival, excluding those missing for >10 % of the dataset
AJCC stage T0 or T1 versus T2–T4
Discussion
Over 50 % of GBC are detected incidentally after surgery for benign disease. Approximately 0.2 % of patients undergoing cholecystectomy will have an incidental discovery of GBC.13,14 As the number of cholecystectomy operations increase, it is likely that the number of incidentally discovered GBC will also increase. An incidentally discovered GBC forces a rapid decision on the surgeon during the initial operation and presents an unexpected challenge to patients postoperatively. In order to effectively manage the disease and counsel patients regarding the diagnosis, it is necessary to understand the prognosis of incidental GBC.
Many studies designed to analyze prognostic factors for survival with GBC do not consider preoperative suspicion of cancer.6,7 In one of the few studies attempting to stratify patients on the basis of preoperative suspicion, Lohe et al. performed a retrospective analysis of 152 patients with GBC to assess the effect of time of diagnosis on patient survival. Although time of diagnosis was strongly predictive of survival in a univariate analysis, the association disappeared when patients were stratified by stage. They conclude that only tumor stage and extension of resection are significant predictors of patient survival.2
Drawing from multiple centers, the current study is able to address this question in a much larger sample. Our results indicate that incidental GBC carries a significantly improved prognosis in both univariate and multivariate analyses. When patients are stratified by T stage, early T0–T1 cancer (Fig. 2) and late-stage T2–T4 disease (Fig. 3) still differ significantly on the basis of preoperative suspicion (p<0.01 in both groups). It has been theorized that the survival difference seen with incidental GBC is an example of lead-time bias, and that the incidentally discovered tumors are simply of earlier stage than those that present clinically. To assess this explanation, we performed multivariate analyses controlling for T stage and degree of differentiation. In these models, the association between preoperative suspicion and survival remains (Tables 2 and 3, p<0.0001). Although incidentally discovered cancer does tend to be earlier in stage with better differentiation (Table 1), these factors do not sufficiently explain the survival benefit conferred by an incidental diagnosis.
The current study also presents data from three separate institutions with large hepatobiliary surgery services. This is both a strength and a potential weakness of our data. The frequency of GBC differs significantly in different geographic regions.15 The disease is endemic in Chile when compared to the rates in American centers. Additionally, the frequency and type of preoperative imaging in Chile differ when compared to Atlanta or Rochester. A significantly larger proportion of GBC is detected incidentally in Chile, likely reflecting a difference both in prevalence and hospital protocol or access to care. Even within American centers, 50 % of GBC is detected incidentally in Atlanta versus 24 % in Rochester. This difference likely represents the different specializations of the two centers and the likelihood of a patient being referred to each center after receiving a diagnosis of GBC on imaging elsewhere.
The multicenter patient population also allows for a larger sample size than would be possible in a single center. In a Kaplan–Meier plot looking at survival as a function of preoperative suspicion in the Chilean cohort separate from the American cohort (Figs. 4 and 5), preoperatively suspected cancers continue to have a significantly shorter mean survival. The multiple centers remain as a potential confounder, however, as patient, tumor, and institutional characteristics in each country are significantly different. More extensive data are needed to fully examine the potential effect of geographic, genetic, and procedural factors.
We also examine the differences in definitive operations for these groups. Patients with suspicion of cancer prior to surgery are more likely to be found inoperable at time of diagnosis (46 % of these patients undergo no resection; Table 3). In our dataset, a greater percentage of preoperatively suspected cancers are offered a two-step operation, with a deferred resection of the gallbladder fossa (15.6 versus 4 %, p<0.0001). It is likely that this reflects the earlier stage of cancers found without preoperative suspicion. It is also possible that our dataset misses some two-stage operations for those patients who obtain further surgery at a different institution. A further study to specifically examine the impact of planned cholecystectomy with liver resection, versus patients with a deferred liver resection, would be valuable.
Early-stage diagnosis and treatment, as well as varying pre- and intraoperative techniques, certainly impact survival with GBC, but they do not adequately explain the significance of incidental diagnosis. Controlling for age, extent of surgery, the presence of gallstone disease, country of origin, and stage of tumor are unable to explain the association (Tables 4 and 5). We hypothesize that these data may suggest a previously unrecognized histological difference between cancer that becomes symptomatic and that which is detected only incidentally. It is possible that the growth patterns in these two situations differ biologically and carry different prognoses for the patient.
Further research is warranted to fully understand the implications of the current study. In univariate and multivariate analyses, controlling for known risk factors and stratifying by presumed confounders, preoperative suspicion remains one of the strongest predictors of survival for patients with gallbladder cancer. Whether this represents an unknown biologic difference between incidental and suspected cancer is not yet clear. The current implications of our data lie in allowing surgeons to better counsel patients regarding an unexpected diagnosis of gallbladder cancer. This disease continues to carry a very poor prognosis, and the current study may help in defining expectations for this patient population.
Acknowledgments
Grant Support This study was supported in part by a TL1 grant RR025010 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources.
Footnotes
Presentation The abstract and preliminary analysis for the manuscript were presented at the Scientific Papers Session of the American College of Surgeons Clinical Congress on October 6, 2010.
References
- 1.Oddsdottir M PT, Hunter JG. In: Chapter 32. Gallbladder and the Extrahepatic Biliary System (Chapter), in Schwartz's Principles of Surgery. Brunicardi FC AD, Billiar TR, Dunn DL, Hunter JG, Matthews JB, Pollock RE, editors. 2009. [Google Scholar]
- 2.Lohe F MG, Schauer C, Angele M, Jauch KW, Schauer RJ. The time of diagnosis impacts surgical management but not the outcome of patients with gallbladder carcinoma. European Journal of Medical Research. 2009;14:345–351. doi: 10.1186/2047-783X-14-8-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frauenschuh D GR, Kraas E. How to proceed in patients with carcinoma detected after laparoscopic cholecystectomy. Langenbeck's Archives of Surgery. 2000;385:495–500. doi: 10.1007/s004230000177. [DOI] [PubMed] [Google Scholar]
- 4.Bazoua G HN, Lazim T. Do we need histology for a normal-looking gallbladder? Journal of Hepatobiliary and Pancreatic Surgery. 2007;14:564–568. doi: 10.1007/s00534-007-1225-6. [DOI] [PubMed] [Google Scholar]
- 5.Goldin RD RJ. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218–229. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 6.Yildirim E CO, Gulben K, Berberoglu U. The surgical management of incidental gallbladder carcinoma. Journal of Cancer Surgery. 2005;31:45–52. doi: 10.1016/j.ejso.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Kayahara M NT, Nakagawara H, Kitagawa H, Ohta T. Prognostic factors for gallbladder cancer in Japan. Annals of Surgery. 2008;248(5):807–814. doi: 10.1097/SLA.0b013e31818a1561. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik TM, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11(11):1478–86. doi: 10.1007/s11605-007-0309-6. discussion 1486–7. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL TA, Fritz AG, Compton CC, Byrd DR, Edge SB, editors. Chapter 20. Gallbladder, in AJCC Cancer Staging Manual. 7th Ed American Joint Committee on Cancer; Chicago, IL: 2010. [Google Scholar]
- 10.Greene FL PD, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. Chapter 15. Gallbladder., in AJCC Cancer Staging Manual. 6th Ed American Joint Committee on Cancer; Chicago, IL: 2002. [Google Scholar]
- 11.Kaplan EL MP. Nonparametric estimation for incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 12.Cox D. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 13.Braghetto I BJ, Csendes A, Chiong H, Compan A, Valladeres H, Rojas J. Gallbladder carcinoma during laparoscopic cholecystectomy: is it associated with bad prognosis? International Surgery. 1999;84(4):344–349. [PubMed] [Google Scholar]
- 14.Antonakis P AN, Mylonaki D, Leandros E, M Konstadoulakis M, Zografos G, Androulakis G. Incidental finding of gallbladder carcinoma detected during or after laparoscopic cholecystectomy. European Journal of Surgical Oncology. 2003;29(4):358–360. doi: 10.1053/ejso.2002.1402. [DOI] [PubMed] [Google Scholar]
- 15.Randi G FS, Vecchia CL. Gallbladder cancer worldwide: Geographical distribution and risk factors. International Journal of Cancer. 2005;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]