Abstract
We have developed a synthetic route to the frequently utilized deoxysugar building block di-O-acetyl-D-rhamnal originating from the inexpensive starting material methyl α-D-glucopyranoside. Our approach proceeds in five steps with minimal column chromatography purification needed to afford the title compound in good overall yield. 2009 Elsevier Ltd. All rights reserved.
Keywords: carbohydrates, deoxysugars, glycosides, natural products
Many biologically-active secondary metabolites are adorned with carbohydrate residues that have been demonstrated to be crucial in modulating the biological efficacy of the parent glycoconjugate.1,2 As a result, numerous researchers have initiated programs aimed at investigating the effects of altering the oligosaccharide domain of various natural product families such as the aminocoumarin, aureolic acid, and glycopeptide antibiotics, to mention only a few, in attempts to discover architecturally-novel compounds with enhanced biological profiles.3–7 A common structural motif found within members of these secondary metabolites are 2-deoxy and 2,6-dideoxysugars.
INSERT FIGURE 1
Figure 1.
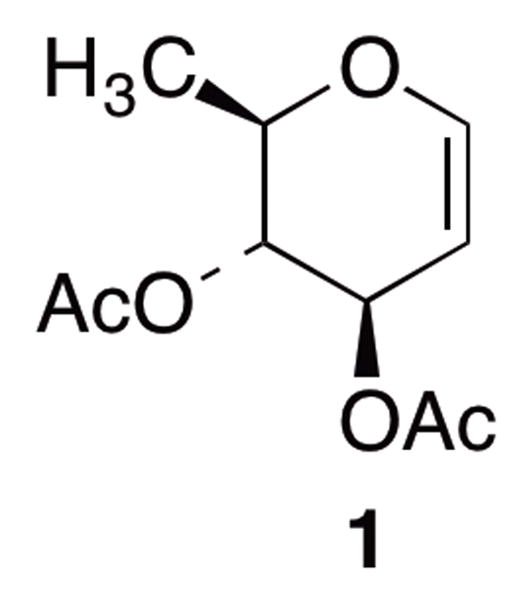
Structure of Di-O-acetyl-D-Rhamnal
A common entry point for the synthesis of various D-configured 2-deoxy and 2,6-dideoxysugars is di-O-acetyl-D-rhamnal (1) illustrated in Figure 1. While the corresponding enantiomer for 1 is readily available from L-rhamnose, at a much lower cost, the preparation of 1 requires substantially more time and effort to access.8–11 Moreover, the most commonly employed synthetic routes directed towards the production of 1 originate from the commercially-available, albeit highly expensive, tri-O-acetyl-D-glucal.12 While these approaches have become the standard means to access 1 and its subsequent derivatives, the continuing rise in cost of tri-O-acetyl-D-glucal has begun to make these approaches cost-prohibitive for many researchers. Surprisingly, few synthetic approaches to this critical carbohydrate building block, di-O-acetyl-D-rhamnal (1), have been reported in the chemical literature over the past thirty years considering its near universal role as a synthetic intermediate to access suitably derivatized D-configured 2-deoxy and 2,6-dideoxysugars.13 Moreover, an efficient and inexpensive route to 1 would add another readily accessible chiron to the pool of asymmetric building blocks for use in various synthetic applications.
During the course of our group’s work aimed at investigating various aspects related to the angucycline antitumor antibiotics, we required access to significant quantities of di-O-acetyl-D-rhamnal (1). Previously, we had employed either Torri’s or Tius’ approach to access either 1 or its deacetylated derivative D-rhamnal, respectively, depending on our specific needs.9,10 However, both of these routes required the use of tri-O-acetyl-D-glucal as the starting material, which proved to be too expensive for us to continue employing, considering the quantity of material we required. Additionally, we explored the use of Nicolaou’s apparent two-step route towards D-rhamnal as described in his group’s pursuit of the complex deoxysugar D-callipeltose.11 Unfortunately, our efforts to utilize Nicolaou’s scheme proved capricious and resulted in dramatically lower yields upon attempting larger scale reactions.
More recently, Osman described the preparation of a protected D-rhamnal derivative beginning from methyl α-D-galactopyranoside.13f However, his route was lengthy and required multiple protection/deprotection steps. As such, we deemed Osman’s synthetic approach too laborious and inefficient to pursue for our purposes. As a result, we elected to develop a simple, expedient, and cost-effective synthetic route towards di- O-acetyl-D-rhamnal (1) that would help to facilitate our group’s research goals and is presented below in Scheme 1.
Scheme 1a.
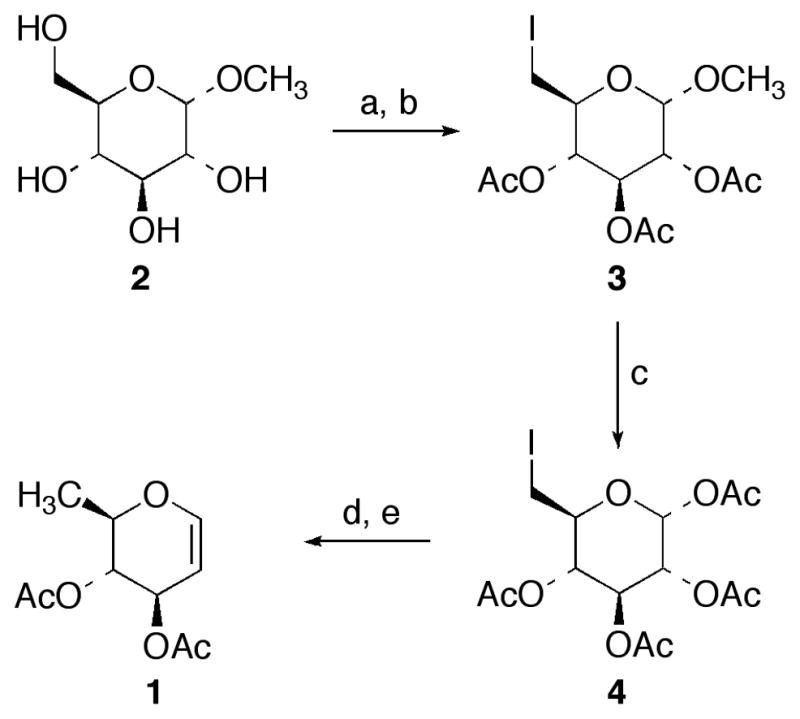
Reactions Conditionsa (a) l2, ImH, Ph3P, Ph-Me; (b) Ac2O, Pyr, DMAP (90% over two steps); (c) Ac2O, H2SO4 (88%); (d) n-Bu3SnH, Et3B, air, Ph-Me, −78 °C (78%); (e) PBr3, CH2CI2 then Zn, NaH2PO4, EtOAc (55%)
Our synthetic approach towards di-O-acetyl-D-rhamnal (1) originates from methyl α-D-glucopyranoside (2), a commercially-available and inexpensive starting material.14 Regioselective conversion of the primary C(6)-OH to an iodide utilizing iodine (I2), imidazole (ImH), and triphenylphosphine (Ph3P) in warm toluene (Ph-Me) followed by peracetylation of the remaining alcohol functional groups under standard conditions afforded methyl α-D-2,3,4-triacetoxy-6-deoxy-6-iodoglucopyranoside (3) in excellent yield for the two-step process.15 Next, exchange of the acid-labile anomeric methoxy group for an acetate was accomplished by employing sulfuric acid (H2SO4) in acetic anhydride (Ac2O) to provide iodide 4 in high yield that was isolated solely as the α-anomer as determined by 1H and 13C NMR analysis.16 From the outset, we were cognizant that the sequence of events would require careful orchestration to allow us to process large quantities of material from methyl α-D-glucopyranoside (2) to di-O-acetyl-D-rhamnal (1). In turn, we concluded that it would be critical to remove the C(6)-I functional group prior to glycal formation to avoid possible undesirable side reactions when performing our anticipated Fischer-Zach reaction to establish the endocyclic glycal olefin in 1. As such, reductive removal of the C(6)-I was realized by exposure of 4 to tributyltin hydride (n-Bu3SnH) in Ph-Me at subambient temperature (−78 °C) using triethylborane (Et3B), with a trace amount of air as the radical initiator, according to the procedure of Oshima.17 The resulting 6-deoxysugar was transformed into di-O-acetyl-D-rhamnal (1) by treatment with phosphorus tribromide (PBr3) in cold methylene chloride (CH2Cl2) to yield the corresponding anomeric bromide which immediately underwent a Fischer-Zach reaction employing zinc metal (Zn) in a sodium dihydrogen phosphate (NaH2PO4) buffer, following the recently disclosed work of Shao, to afford di-O-acetyl-D-rhamnal (1) in good overall yield.18
In summary, we have developed a simple, efficient, and inexpensive route towards the ubiquitous deoxysugar building block, and chiron, di-O-acetyl-D-rhamnal (1). Our synthetic scheme progresses in five steps (thirty-five percent overall yield) and requires only a single column chromatography purification step after reductive removal of the C(6)-I group. We anticipate that our approach will help facilitate research programs requiring the synthesis of various D-configured 2-deoxy- and 2,6-dideoxysugars in both academic and industrial research laboratories.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from Sewanee: The University of the South and the National Institute of Allergy and Infectious Diseases (R15, AI084075-02). Additionally, we thank the National Science Foundation, as part of its Major Research Instrumentation (MRI) Program (CHE-1126231), for the acquisition of a JEOL ECS-400 Nuclear Magnetic Resonance Spectrometer. Accurate mass measurements were performed by Dr. William Boggess of the Mass Spectrometry and Proteomics Facility at the University of Notre Dame.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Varki A. Glycobiology. 1993;3:97. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weymouth-Wilson AC. Nat Prod Rep. 1997;14:99. doi: 10.1039/np9971400099. [DOI] [PubMed] [Google Scholar]
- 3.Albermann C, Soriano A, Jiang J, Vollmer H, Biggins JB, Barton WA, Lesniak J, Nikolov DB, Thorson JS. Org Lett. 2003;5:933. doi: 10.1021/ol0341086. [DOI] [PubMed] [Google Scholar]
- 4.Kahne D, Leimkuhler C, Lu W, Walsh C. Chem Rev. 2005;105:425. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- 5.Ge M, Chen Z, Onishi R, Kohler J, Silver LL, Kerns R, Fukuzawa S, Thompson C, Kahne D. Science. 1999;284:507. doi: 10.1126/science.284.5413.507. [DOI] [PubMed] [Google Scholar]
- 6.Sun B, Chen Z, Eggert US, Shaw SJ, LaTour JV, Kahne D. J Am Chem Soc. 2001;123:12722. doi: 10.1021/ja0166693. [DOI] [PubMed] [Google Scholar]
- 7.Baig I, Perez M, Braña AF, Gomathinayagam R, Damodaran C, Salas JA, Méndez C, Rohr J. J Nat Prod. 2008;71:199. doi: 10.1021/np0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di-O-acetyl-L-rhamnal is readily available using commercially-available L-rhamnose monohydrate as a starting material at a cost of approximately $ 2.40 per gram (Sigma-Aldrich, catalog #: R3875–100G)
- 9.Torii S, Inokuchi T, Masatsugu Y. Bull Chem Soc Jpn. 1985;58:3629. [Google Scholar]
- 10.(a) Tius MA, Gu X-q, Gomez-Galeno J. J Am Chem Soc. 1990;112:8188. [Google Scholar]; (b) Tius MA, Gomez-Galeno J, Gu X-q, Zaidi JH. J Am Chem Soc. 1991;113:5775. [Google Scholar]
- 11.Pihko AJ, Nicolaou KC, Koskinen AMP. Tetrahedron: Asymmetry. 2001;12:937. [Google Scholar]
- 12.D-rhamnal and its derivatives are routinely synthesized from tri-O-acetyl-D-glucal which is commercially-available at an expense of approximately $ 4.70 per gram (Sigma-Aldrich, catalog #: T44407–100G)
- 13.(a) Nowacki A, Walczak D, Liberek B. Carbohydr Res. 2012;352:177. doi: 10.1016/j.carres.2012.02.008. [DOI] [PubMed] [Google Scholar]; (b) O’Keefe MB, Mans DM, Kaelin DE, Jr, Martin SF. Tetrahedron. 2011;67:6524. doi: 10.1016/j.tet.2011.05.117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tatsuta K, Tokishita S, Fukuda T, Kano T, Komiya T, Hosokawa S. Tetrahedron Lett. 2011;52:983. [Google Scholar]; (d) Iynkkaran I, Bundle DR. Carbohydr Res. 2010;345:2323. doi: 10.1016/j.carres.2010.08.017. [DOI] [PubMed] [Google Scholar]; (e) Tanaka H, Yamaguchi S, Yoshizawa A, Takagi M, Shinya K, Takahashi T. Chem Asian J. 2010;5:1407. doi: 10.1002/asia.200900640. [DOI] [PubMed] [Google Scholar]; (f) Osman H, Larsen DS, Simpson J. Tetrahedron. 2009;65:4092. [Google Scholar]; (g) Osman H, Maidin SMM, Larsen DS. ACGC Chem Res Comm. 2008;22:54. [Google Scholar]; (h) Di Bussolo V, Favero L, Romano MR, Pineschi M, Crotti P. Tetrahedron. 2008;64:8188. [Google Scholar]; (i) Lu YS, Li Q, Zhang LH, Ye XS. Org Lett. 2008;10:3445. doi: 10.1021/ol801190c. [DOI] [PubMed] [Google Scholar]; (j) Handa M, Smith WJ, III, Roush WR. J Org Chem. 2008;73:1036. doi: 10.1021/jo7022526. [DOI] [PubMed] [Google Scholar]; (k) Kumar V, Ramesh NG. Org Biomol Chem. 2007;5:3847. doi: 10.1039/b712841j. [DOI] [PubMed] [Google Scholar]; (l) Tanaka H, Yoshizawa A, Takahashi T. Angew Chem Int Ed. 2007;46:2505. doi: 10.1002/anie.200604031. [DOI] [PubMed] [Google Scholar]; (m) Kumar V, Ramesh NG. Chem Comm. 2006:4952. doi: 10.1039/b612151a. [DOI] [PubMed] [Google Scholar]; (n) Morton GE, Barrett AGM. Org Lett. 2006;8:2859. doi: 10.1021/ol061007+. [DOI] [PubMed] [Google Scholar]; (o) Kunst E, Kirschning A. Synthesis. 2006:2397. [Google Scholar]; (p) d la Figuera N, Forns P, Fernandez J-C, Fiol S, Fernandez-Forner D, Albericio F. Tetrahedron Lett. 2005;46:7271. [Google Scholar]; (q) Postema MHD, Piper JL, Komanduri V, Liu L. Angew Chem Int Ed. 2004;43:2915. doi: 10.1002/anie.200353478. [DOI] [PubMed] [Google Scholar]; (r) Lam SN, Gervay-Hague J. Org Lett. 2003;5:4219. doi: 10.1021/ol035705v. [DOI] [PubMed] [Google Scholar]; (s) Brimble MA, Davey RM, McLeod MD, Murphy M. Aust J Chem. 2003;56:787. doi: 10.1039/b301449p. [DOI] [PubMed] [Google Scholar]; (t) Durham TB, Roush WR. Org Lett. 2003;5:1875. doi: 10.1021/ol034395d. [DOI] [PubMed] [Google Scholar]; (u) Martin SF. Pure Appl Chem. 2003;75:63. [Google Scholar]; (v) Apsel B, Bender JA, Escobar M, Kaelin DE, Lopez OD, Martin SF. Tetrahedron Lett. 2003;44:1075. [Google Scholar]; (w) Crotti PD, Di Bussolo V, Favero L, Macchia F, Pineschi M. Tetrahedron. 2002;58:6069. [Google Scholar]; (x) Brimble MA, Davey RM, McLeod MD. Synlett. 2002:1318. [Google Scholar]; (y) Kirschning A, Jesberger M, Schoenberger A. Org Lett. 2001;3:3623. doi: 10.1021/ol016545v. [DOI] [PubMed] [Google Scholar]; (z) Parrish JD, Little RD. Tetrahedron Lett. 2001;42:7371. [Google Scholar]; (aa) Nicolaou KC, Rodriguez RM, Mitchell HJ, Suzuki H, Fylaktakidou KC, Baudoin O, Van Delft FL. Chem Eur J. 2000;6:3095. doi: 10.1002/1521-3765(20000901)6:17<3095::aid-chem3095>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]; (bb) Nicolaou KC, Mitchell HJ, Suzuki H, Rodriguez RM, Baudoin O, Fylaktakidou KC. Angew Chem Int Ed. 1999;38:3334. [PubMed] [Google Scholar]; (cc) Capozzi G, Falciani C, Menchetti S, Nativi C, Raffaelli B. Chem Eur J. 1999;5:1748. [Google Scholar]; (dd) Shimizu M, Nakahara Y, Yoshioka H. J Fluor Chem. 1999;97:57. [Google Scholar]; (ee) Fernandez E, Polo A, Ruiz A, Claver C, Castillon S. Chem Comm. 1998:1803. [Google Scholar]; (ff) Guo Y, Sulikowski GA. J Am Chem Soc. 1998;120:1392. [Google Scholar]; (gg) Wieczorek E, Thiem J. J Carbohydr Chem. 1998;17:785. [Google Scholar]; (hh) Mueller T, Schmidt RR. Tetrahedron Lett. 1997;38:5473. [Google Scholar]; (ii) Zegelaar-Jaarsveld K, Smits SAW, van Straten NCR, van der Marel GA, Boom JH. Tetrahedron. 1996;52:3593. [Google Scholar]; (jj) Hosoya T, Ohashi Y, Matsumoto T, Suzuki K. Tetrahedron Lett. 1996;37:663. [Google Scholar]; (kk) Grieco PA, DuBay WJ, Todd LJ. Tetrahedron Lett. 1996;37:8707. [Google Scholar]; (ll) Oscarson S, Tedebark U. Carbohydr Res. 1995;278:271. doi: 10.1016/0008-6215(95)00268-5. [DOI] [PubMed] [Google Scholar]; (mm) Sugiyama T, Murayama T, Yamashita K, Oritani T. Biosci Biotech Biochem. 1995;59:1921. doi: 10.1271/bbb.59.1921. [DOI] [PubMed] [Google Scholar]; (nn) Teichmann M, Descotes G, Lafont D. Synthesis. 1993:889. [Google Scholar]; (oo) Franck RW, Kaila N. Carbohydr Res. 239:71. doi: 10.1016/0008-6215(93)84204-j. 193. [DOI] [PubMed] [Google Scholar]; (pp) Berkowitz DB, Danishefsky SJ, Schulte GK. J Am Chem Soc. 1992;114:4518. [Google Scholar]; (qq) Berkowitz DB, Danishefsky SJ. Tetrahedron Lett. 1991;32:5497. [Google Scholar]; (rr) Petit JM, Paquet F, Beau JM. Tetrahedron Lett. 1991;32:6125. [Google Scholar]; (ss) Tatsuta K, Hidekazu M, Tanaka M, Okui T. Tetrahedron Lett. 1990;31:5495. [Google Scholar]; (tt) Sugiyama T, Murayama T, Yamashita K. Tetrahedron Lett. 1990;32:5497. [Google Scholar]; (uu) Gilleron M, Fournie JJ, Pougny JR, Puzo G. J Carbohydr Chem. 1988;7:733. [Google Scholar]; (vv) Beau JM, Jaurand G, Esnault J, Sinay P. Tetrahedron Lett. 1987;28:1105. [Google Scholar]
- 14.The cost of methyl α-D-glucopyranoside is approximately $ 0.15 per gram (Sigma-Aldrich, catalog #: M9376–1KG) compared to $ 4.70 per gram for tri-O-acetyl-D-glucal.
- 15.Garegg PJ, Samuelsson B. J Chem Soc, Perkin Trans 1. 1980:2866. [Google Scholar]
- 16.Lowary TL, Eichler E, Bundle DR. Can J Chem. 2002;80:1112. [Google Scholar]
- 17.Miura K, Ichinose Y, Nozaki K, Fugami K, Oshima K, Utimoto K. Bull Chem Soc Jpn. 1989;62:143. [Google Scholar]
- 18.Zhao J, Wei S, Ma X, Shao H. Carbohydr Res. 2010;345:168. doi: 10.1016/j.carres.2009.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


