Abstract
This overview is primarily concerned with large recent prospective cohort studies of adult populations, not patients, because the latter studies are confounded by differences in medical and surgical management for men vs. women. When early papers are uniquely informative they are also included. Because the focus is on epidemiology, details of age, sex, sample size, and source as well as study methods are provided. Usually the primary outcomes were all-cause or coronary heart disease (CHD) mortality using baseline data from midlife or older adults.
Fifty years ago few prospective cohort studies of all-cause or CHD mortality included women. Most epidemiologic studies that included community-dwelling adults did not include both sexes and still do not report men and women separately. Few studies consider both sex (biology) and gender (behavior and environment) differences. Lifespan studies describing survival after live birth are not considered here. The important effects of prenatal and early childhood biologic and behavioral factors on adult mortality are beyond the scope of this review. Clinical trials are not discussed.
Overall, presumptive evidence for causality was equivalent for psychosocial and biological exposures, and these attributes were often associated with each other. Inconsistencies or gaps were particularly obvious for studies of sex or gender differences in age and optimal measures of body size for CHD outcomes, and in the striking interface of diabetes and people with the metabolic syndrome, most of whom have unrecognized diabetes.
Introduction
The main reason for increased longevity is reduced mortality from birth to adolescence. This is still true, as reported by Wang and colleagues[1] in the largest study of sex-specific trends in mortality between 1970 and 2010. Their study of 187 countries compares mortality in children age 5 or less with mortality in adults, age 15–99 years. Since 1970, global deaths in children declined by almost 60%. During a 20-year interval male life expectancy at birth increased from 56.4 years to 67.5 years, and female life expectancy at birth increased from 61.2 years to 73.3 years. This paper is concerned with mortality and CHD in this growing number of surviving adults.
As reviewed elsewhere[2], in 1900, only 4% of the U.S. population was aged 65 and older; by 2000 that proportion had tripled (12.4%), and is expected to be 20% by 2030. Resnick[3] calculated that more than half of all people who have ever lived to age 65 are alive now. The fastest growing segment of the U.S. adult population is now age 80 and older, with almost 20 million projected for 2030. The proportion of persons who survive to age 90 years is increasing dramatically over the past century in both sexes, but women still live longer than men in every Westernized birth cohort[4].
CORONARY HEART DISEASE
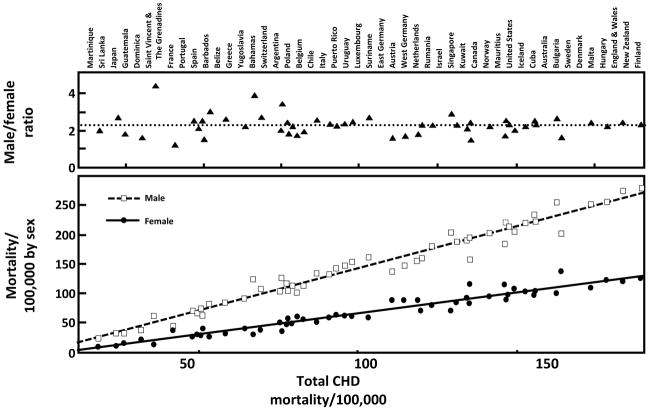
As the population ages, there is increasing interest in biologic and behavioral factors associated with survival and cardiovascular disease. The most consistent sex differences in adult mortality are attributed to coronary heart disease (CHD), which is the most common vascular condition with consistently greater mortality rates and risks in men than women. Figure 1 shows remarkably similar male to female CHD mortality ratios (2.5–4.5) in adults in 52 countries, despite very different sex specific CHD death rates[5]. These data were obtained from the World Health Organization in 1984–7, before the era when effective blood pressure medications, lipid-lowering medications, and hormone replacement therapy were widely used. This universal male excess in CHD implies an inherent male disadvantage or female advantage that operates across populations with divergent rates of heart disease, lifestyle, and risk factors, and that is unexplained by differential treatment by sex. The usual explanation is that estrogen is good for women or testosterone is bad for men, but the wide differences in CHD death rates between countries despite consistent sex ratios suggest that sex differences are also mediated by risk factors that influence both sexes, and that sex hormones may not be destiny with regard to CHD[6].
Figure 1.
Age-standardized coronary disease death rates in 1987 for adult men and women from 52 countries (Kalin MF, Zumoff B: Sex hormones and coronary disease: a review of the clinical studies. Steroids 1990; 55(8): 330–52, Copyright© 1990, by permission of Elsevier).
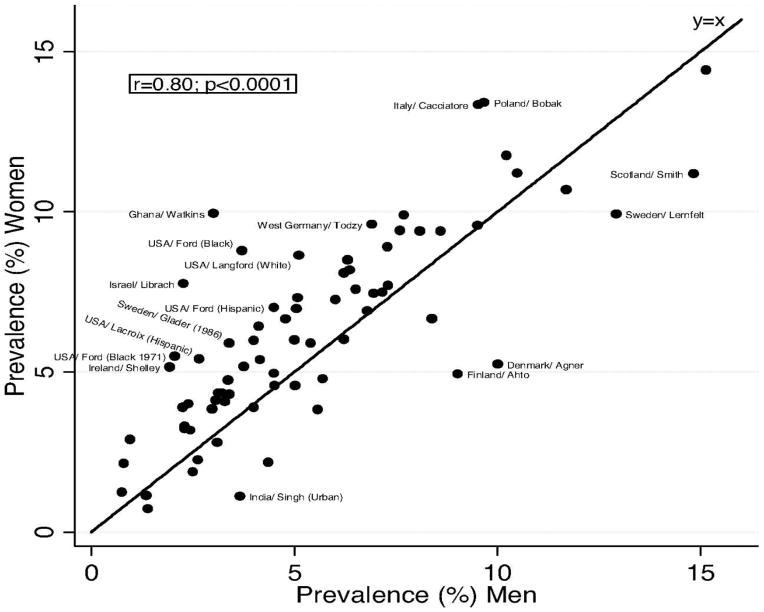
Although the incidence of acute myocardial infarction shows a universal excess among men, male sex was not associated with excess physician-defined angina pectoris in multiple studies reviewed by Hemingway and colleagues[7]. The authors used the 7-item Rose angina questionnaire, which is free of sex-specific expectations and biases of physician diagnosis, and is the only standard angina questionnaire that has been used in many countries for more than four decades. Based on almost 25,000 angina cases from 31 countries in 74 published studies of both sexes, the prevalence of Rose-angina was slightly but consistently higher in women than in men when compared with country-level sex-specific myocardial infarction mortality rates from the WHO mortality database, despite widely different myocardial infarction mortality rates in different studies (shown in Figure 2). The notable excess of female angina was observed at all ages including women before and after the age of 50, the usual age at menopause. The largest group was European with about two-thirds age ≤50.
Figure 2.
Angina prevalence in women vs. men[7]. Labels are given for populations in which the prevalence differs by at least 2.5% between women and men. (Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E: Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 2008; 117(12): 1526–36, by permission of Walters Kluwer Health).
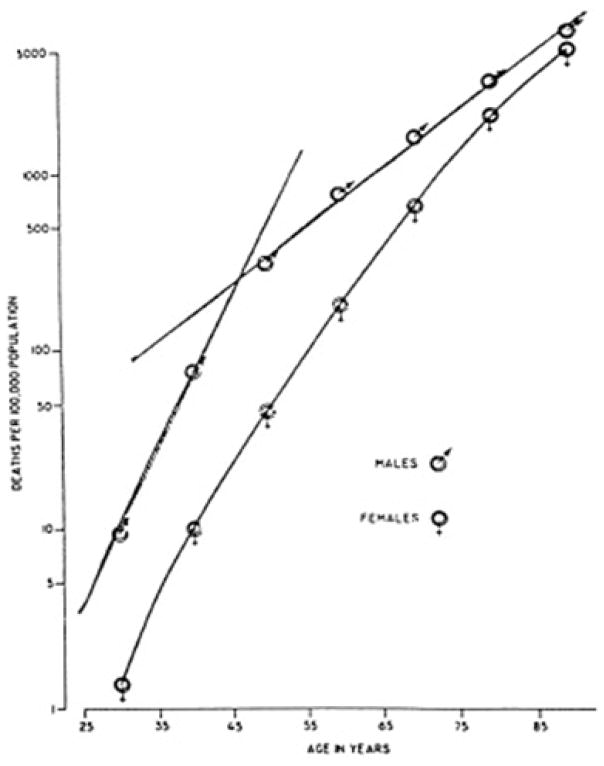
In the United States Furman and colleagues used 1955 data obtained from the National Center for Health Statistics and the National Heart Institute, and showed male mortality rates increased until about age 45, with much slower increments thereafter; in contrast, women’s lower mortality rates increased steadily with age, with no significant change at age 50 (usual age at menopause and the beginning of estrogen deficiency), as shown in Figure 3 [8]. These results suggest that sex differences in adult mortality reflect men’s decreasing mortality rates beginning in midlife, and not female menopause.
Figure 3.
Male and female mortality rates, U.S. white population, 1955, plotted as a function of age[8]. (Furman RH: Are gonadal hormones (estrogens and androgens) of significance in the development of ischemic heart disease. Ann N Y Acad Sci 1968; 149(2): 822–33. Copyright© 2006, John Wiley and Sons, reproduced by permission of the publisher).
This pattern was confirmed in a recent study of national U.S. and U.K. heart disease mortality for three birth cohorts (1916–25, 1926–35, and 1936–45)[9]. In all birth cohorts linear heart disease mortality rates peaked in men around age 45, with slower age-related increases thereafter. In women there was no accelerated increase in heart disease mortality rate at age 50 (menopause). In both sexes proportional increases fit the data better than absolute increases, presumably reflecting competing risks with aging. The authors concluded that deceleration of the age-related increase in male heart disease mortality in midlife explained sex differences in CHD mortality better than postmenopausal estrogen deficiency in women. The striking decrease in women’s breast cancer risk after age 50 indirectly confirms menopause as the usual beginning of female estrogen deficiency.
HEART DISEASE RISK FACTORS
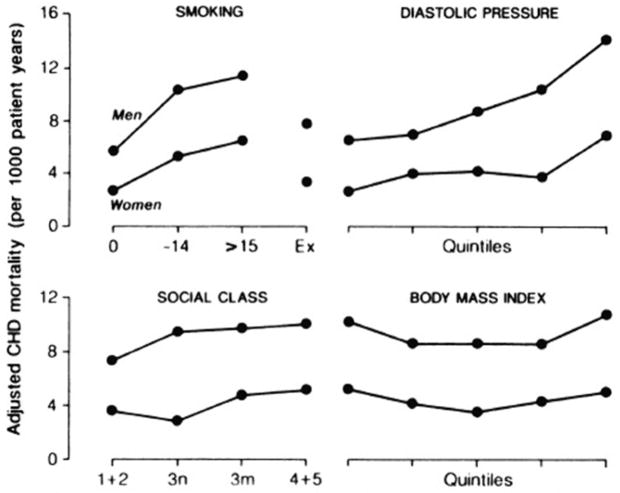
In general, men and women have the same heart disease risk factors, but men tend to have less favorable levels of these risk factors than women. For example, men in the Edinburgh Heart Study[10] had significantly (P<.001) less favorable patterns of cigarette smoking, dietary fiber, vitamin C, blood viscosity, uric acid, HDL cholesterol, and triglycerides than women. Only three cardio-protective factors were significantly more favorable in men than women: men reported more physical activity and more alcohol intake, and had lower levels of fibrinogen. Levels of common CHD risk factors did not explain the sex differences in incidence rates, which persisted in studies adjusted for risk factor differences[11] or stratified by level of risk factor[12]. The Renfrew-Paisley Study[12], also in Scotland, reported that women and men age 45–64 had similar relative risks for increasing levels of each CHD risk factor, but women’s absolute risk of CHD was lower for any given level of any risk factor (Figure 4). In this study women in the highest female quintile of cholesterol (>7.2 mmol/L) had lower CHD death rates after menopause than men in the lowest male quintile (<5.0 mmol/L). Many other studies have shown that men and women have similar cardiovascular risk factors, but different absolute risks for a given level[12].
Figure 4.
Adjusted CHD mortality rates (deaths per 1000 patient-years) for both sexes age 45–64 by cigarette smoking, diastolic blood pressure, social class, and body mass index[12]. Isles CG, Hole DJ, Hawthorne VM, Lever AF: Relation between coronary risk and coronary mortality in women of the Renfrew and Paisley survey: comparison with men. Lancet 1992; 339(8795): 702–6. Copyright© 1992, with permission from Elsevier.
Ethnicity and Race
Over the past century, both men and women experienced tremendous if uneven increases in life expectancy at birth, as extensively reviewed by Rogers and colleagues[13] using data from the NHANES III Linked Mortality File--a nationally representative sample of the U.S. population in which both non-Hispanic blacks and Mexican Americans were oversampled. NHANES III participants age 20 or older at the time of the baseline evaluation were matched for mortality using record linkage to the U.S. National Death Index through 2005. At baseline participants reported age, sex, ethnicity, education, current marital status, poverty level, visiting with friends and family, weekly religious attendance, membership in clubs and other organizations, cigarette smoking, alcohol use (frequency and amount), physical activity (more or less than 7 hours per week), and functional impairments. Measured risk factors included hypertension, cholesterol, glycosylated hemoglobin, albumin, and C - reactive protein (CRP). Some analyses used age cut- points to reflect expected life stages, for example, age <45--completing education, married, moving into the labor force, age 45–64--fully engaged in the labor force but possibly experiencing declining health, and 65 and older--retiring or higher risk of ill health and death. During the 13 year follow up, social and behavioral characteristics were the major factors associated with gender differences in mortality. After controlling for women’s less favorable baseline marital status, income, and physical exercise, the sex gap in mortality widened. After controlling for women’s more frequent social connections, attendance at religious services, and nonsmoking, this gap narrowed. Although the prevalence of biological risk factors differed by sex and by age, the impact of these risk factors on mortality was similar for both sexes. During follow up men had a 30% to 83% higher risk of death compared to women, depending on the covariates included in the model.
The REGARDS prospective cohort study[14] is one of the largest studies of fatal and nonfatal CHD incidence in community-dwelling black and white men and women in the United States. REGARDS included 24,443 black and white participants age 45 and older (mean age 63–64 years) without known CHD at baseline. The main outcome was expert-adjudicated fatal and nonfatal CHD. Race and sex were balanced by design; oversampling from the high-risk southeastern United States (the stroke belt) increased the number of expected cardiovascular events. Compared to whites, on average blacks had less education, lower income, and were more likely to smoke, report diabetes, and have higher body mass index (BMI), systolic blood pressure, and high-sensitivity CRP (hsCRP). Black men and black women had twice the age-standardized rate of fatal incident CHD compared with white men and white women, which was associated with their more unfavorable CHD risk factors at baseline. A marked sex difference was present for nonfatal CHD: black men had a lower risk of nonfatal acute myocardial infarction than white men, while black women had a higher risk than white women. The low risk in black men remained present in fully adjusted models, but the excess risk observed among black women was entirely attenuated after accounting for their greater cardiovascular risk factor burden.
More Psychosocial Factors
Traditional (Framingham) risk factors--smoking, high blood pressure, and high cholesterol--do not fully explain the excess burden of cardiovascular diseases (CVD) in the population. In 2008, Everson-Rose and Lewis[15] elegantly reviewed the extensive literature on important psychosocial domains (negative emotional states—depression or depressive symptoms, anger and hostility, anxiety; chronic psychosocial stressors, including occupation or work-related stress, acute life stress; and social ties, social support, and social conflict). The authors concluded that psychological and social characteristics explained the majority of cardiovascular events, and that the relative consistency of the findings despite the diversity of studies strengthened the hypothesis that psychosocial factors are causally related to CVD.
Nicholson and colleagues reported a meta-analysis of 21 published etiologic studies of depression or depressed mood and subsequent CHD events in 124,509 participants. They reported that the pooled relative risk of prior depressed mood for future CHD was 1.81 (1.53–2.15), but with considerable heterogeneity among the cohorts[16]. Data to adjust for conventional CHD risk factors were available in only 11 studies, and greatly reduced the risk estimates. Only half of these studies included both sexes; analyses were not stratified by sex. Missing data and other study limitations weaken the strength of evidence that depressed mood is an independent risk factor for CHD.
Case and Paxson’s [17] review of the literature showed that women had poorer self-rated health and more physician visits than men from adolescence to late middle age, but were less likely to die than men at every age. These results were based on 14 years of data from the U.S. National Health Interview Survey (self-reported health by random digit dial telephone interviews). Analysis of this survival paradox suggested that sex differences in outcomes could be explained in part by sex differences in the frequency of specific chronic conditions (Figure 5), and an equally large part could be attributed to the fact that men with the same conditions had more adverse outcomes than women. Conditions associated with excess male mortality were largely smoking-related.
Figure 5.
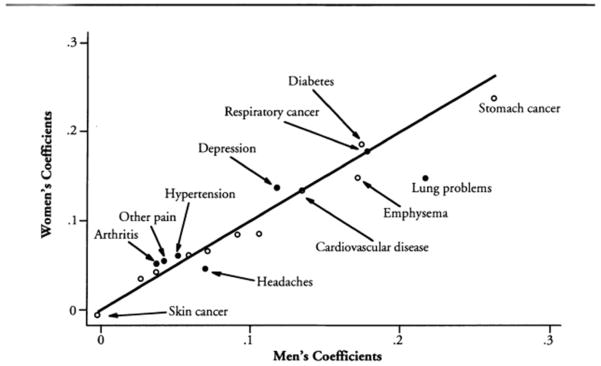
The impact of chronic conditions on the probability of reporting fair or poor health[17]. Coefficients that are significantly different for men and women are represented by solid circles. (Case A, Paxson C. Sex differences in morbidity and mortality. Demography 2005;42:189–214. Reprinted with permission of Population Association of America.)
Job Stress
Kivimaki and colleagues[18] specifically addressed the importance of high psychological work demands versus low control on CHD risk, and whether any effect differs by sex. Individual data from the Individual Participant Data Meta-Analysis for the Working Populations Consortium, established in 2008, included published and unpublished data from13 European and Scandinavian cohorts with more than 197,000 participants (49% women). Job strain was defined using standard questionnaires. Information about CHD events and death was obtained from national hospital admission and death registries. To reduce reverse causality, events that occurred in the first three years were excluded. Many more men than women had CHD events (1595 vs. 229), but a combination of high demands and low control was a greater risk factor for CHD than either alone in both sexes. There were no significant differences in the association between job strain and CHD between men and women, young and old, or at different levels of socioeconomic position.
Chandola and colleagues[19] examined the mechanisms of work stress and coronary heart disease in 10,308 London-based male and female civil servants age 35–55 (phase I of the 1985–1988 Whitehall II study). Data were collected in phase 2 through phase 7 from 1989 to 2004. Exposures included work stress (assessed at phases 1 and 2), and outcomes included behavioral risk factors, the metabolic syndrome (MetS), heart rate variability, and incident CHD (CHD death, nonfatal myyocaridal infarction, or definite angina). Chronic work stress was associated with higher risks of incident CHD, and this association was stronger among those younger than age 50 (RR 1.68, 95% CI 1.17–2.42). Gender-stratified analysis in the Whitehall II cohort (67% male) revealed similar estimates of work stress on CHD among younger men and women. Work stress was associated with low physical activity, poor diet, not drinking any alcohol, the MetS and its components, and lower heart rate variability. About 32% of the effect of work stress on CHD was attributable to its association with poor health behaviors (low physical activity and poor diet in particular) and with MetS.
PHYSICAL ACTIVITY
Large long prospective studies of physical activity are challenging to conduct and interpret because few large cohorts used noninvasive methods to validate or quantitate physical function and exercise, and most questions were about male sports. The EPIC-Norfolk cohort study designed and tested a new four-part physical activity questionnaire, asking about 1) work-related physical activity; 2) leisure physical activity including house work; 3) amount of energy expended during exercise based on sweating, rapid heart rate, and hours per week; and 4) stair climbing. Analyses based on these questions were published in 2011 based on data from 4423 men and 5711 women age 45 to 79 years from 10 European countries who were without CHD at baseline, had complete data on physical activity and CHD outcomes, and were followed for an average of 10.9 years for fatal CHD [20]. A total of 548 men and 310 women had a validated CHD event. In both sexes event rates were higher in those with MetS compared to those without: 17.4% and 9.4 % in men and 10.2% and 3.4% in women. Significant downward trends in CHD event rates with increasing physical activity were strongest in men and women with MetS (37.6 % of men and 30.2 % of women). Statistical evidence for effect modification by physical activity between MetS and CHD risk was highly significant (p = 0.006) only for both sexes combined.
OBESITY
In the 1960s there was limited evidence that overweight or obesity was a heart disease risk factor. The Men’s Pooling Project cohorts (12,381 men age 40–64 years), studied in the 1960s and1970s and followed for 4.9 to 9.6 years for CHD, showed very different patterns and standardized incidence ratios for first major coronary events as shown in Figure 6 [21]. These different patterns were not paralleled by cohort differences in the distribution of marked obesity, cigarette smoking, systolic blood pressure, or cholesterol levels. Extended follow up showed that these site-specific cardiovascular event patterns persisted for up to 32 years.
Figure 6.
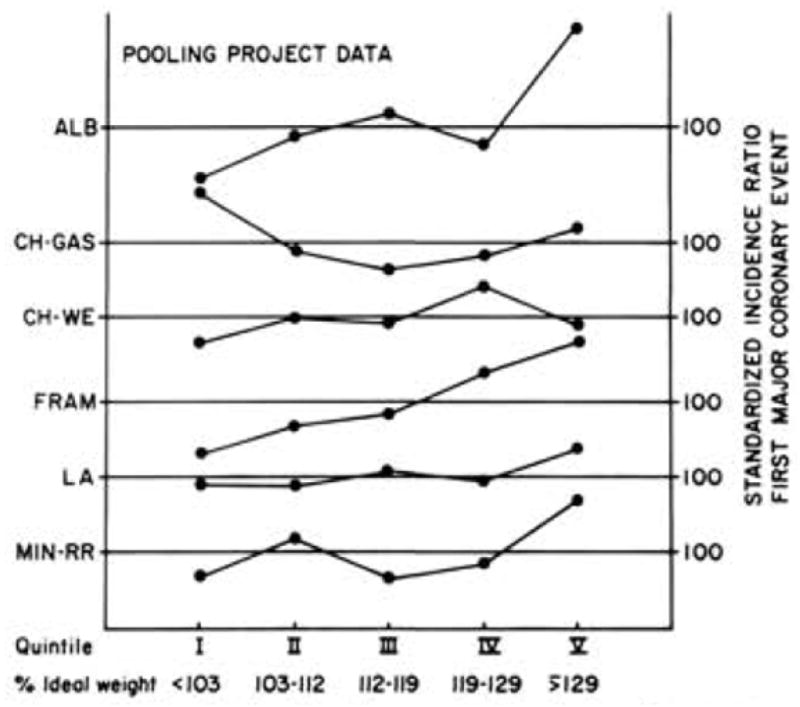
Standardized incidence ratio for first major coronary event by quintile of relative weight in six Pooling Project cohorts. ALB = Albany; Ch-GAS = Chicago People’s Gas Company; CH-WE = Chicago Western Electric; FRAM = Framingham; LA = Los Angeles Heart Study; MINN-RR = Minnesota Railroad Workers Study. Adapted from the Pooling Project Research Group[21] Barrett-Connor EL: Obesity, atherosclerosis, and coronary artery disease. Ann Intern Med 1985; 103(6 (Pt 2)): 1010–9 (reprinted with permission from American College of Physicians).
The Seven Countries Study of 15 mostly European cohorts of men age 40–59 years was started in the 1950s and used a common protocol to allow comparison of one population to another. Although known as a European study, Minnesota railroad workers were by far the largest single and the only American cohort (n = 2,315) and there were two Japanese cohorts (n = 504 and 496). The common protocol required healthy men (no known heart disease) who were asked to come to the clinic before breakfast and without smoking or exercise in the half hour before their interview. Blood pressure was measured twice in resting subjects using a standardized manometer. Dried blood was sent for cholesterol assay to a central laboratory in Minnesota. The15-year age-standardized all-cause mortality rate in seven countries was correlated with the coronary death rate (r = 0.52) indicating that 27% of deaths were attributed to CHD. There was no significant positive association of relative weight with coronary death in any population after 10 or 15 years of follow up[22]. In fact, by the 15th year, relative weight was a significant negative risk factor in southern European men. Nevertheless, in the American and European cohorts there was a similar age-adjusted increased risk of coronary disease associated with serum cholesterol, systolic blood pressure, and cigarette smoking.
Different results might be expected for more recent studies, because men in the earlier studies grew up before childhood obesity. The long-term consequences of these time trends and their mechansms have been reviewed elsewhere[23]. Prior to 1980 less than 10% of children and young adults were obese. Since that time the prevalence of childhood obesity more than doubled, such that many of today’s middle-aged overweight and obese adults have been obese for years. Those who have been obese for 15–24 years have more than double the risk of diabetes compared with those who have been obese for less than 5 years[23].
Optimal body weight remains controversial. Remarkable international information has been provided by a 2013 publication from Flegal and colleagues[24] who reviewed 97 published studies (in any language) of obesity and mortality conducted in the United States and Canada (n=41 studies), Europe (n=37), Australia (n=7), China or Taiwan (n=4), Japan (n=2), Brazil (n=2), Israel (n=2), India (n=1), and Mexico (n=1), providing a combined sample size of more than 2.88 million participants and more than 270,000 deaths. Authors selected prospective cohort studies that presented BMI categories in general populations of adults, reported hazard ratios, age or age groups, and had data for all-cause mortality Abstracted items included sex, age at baseline, length of follow up, number of deaths, and available covariates. Hazard ratios were categorized into two groups, age 65 and older, and a mixed age category, based on data available. Analyses were performed for men and women separately and combined, and by region (North America, Europe, and other). The final model used the most fully adjusted data available. The authors suggest that analyses could be over-adjusted if controlled for risk factors such as hypertension, considered to be in the causal pathway between obesity and mortality, and under-adjusted if not adjusted for age, sex, and smoking. The number of studies that included men only or women only is provided by study location with hazard ratios in multiple Forest plots. Between-study heterogeneity was less for studies with measured (vs. reported) height and weight and for cohorts older than age 65 years, and more pronounced in analyses stratified by sex. Overall there was remarkable diversity of optimal weights in different cohorts variously showing highest or lowest BMI was associated with lower or higher all-cause mortality ratios[24].
Central Obesity
Although upper body or central obesity was clinically recognized as “male pattern obesity” for years before the diabetes epidemic, it was not commonly used to evaluate sex differences in large prospective studies of mortality or cardiovascular disease. The Rotterdam Study, one of the first large prospective population-based studies, included 6296 men and women from the Netherlands (baseline age 55–102 years) who were followed for an average of 5.4 years[25]. Because smoking is a major risk factor for both central obesity and CHD, the most useful results are about never smokers. The mean BMI among never smoking men was 25.5 and 26.9 among never smoking women. Among never smoking men an increased risk of all-cause mortaity was only weakly associated with a BMI >30 (the highest BMI category in the study). In another model, high quintiles of waist girth but not of BMI or waist hip ratio (WHR) were associated with all-cause mortality among never smoking men. Among women who never smoked, no measures of body fatness predicted increased mortality. These weak or absent associations may reflect the frequent walking and biking in a flat country with good public transportation and limited urban parking.
INTERHEART was the first standardized case-control study large enough to assess whether central obesity based on waist girth or waist to hip ratio (WHR) improved the prediction of acute myocardial infarction beyond body mass index [26]. The study included 15,152 heart attack cases and 14,820 age and sex-matched controls from 52 countries representing every inhabited continent and several ethnic groups. All 52 sites had the same protocol, which (assuming protocols are followed equally well at all sites) reduced limitations of pooled analyses where different results may reflect methodological differences. INTERHEART cohorts showed striking differences in the prevalence of obesity (defined as BMI >30) by ethnicity with the lowest rates in Asians and the highest rates in Australasia and South and North America. Heart attack cases had higher WHRs than controls in all regions of the world, with remarkably similar associations in men and women. The graded association of BMI with myocardial infarction was largely explained by central obesity, shown in analyses adjusted for WHR or waist girth, which were highly correlated with each other. The observed excess risk (population attributable risk = PAR) for myocardial infarction was 24% in the top two WHR quintiles, compared to 7.7% in the top two BMI quintiles [26].
Central obesity was one of nine CHD risk factors that contributed to the INTERHEART PAR[27]; others were smoking status, raised ApoB/ApoA1 ratio, hypertension, diabetes, lack of daily fruits and vegetables, lack of regular alcohol consumption, lack of regular physical activity, and psychosocial factors. Each contributed independently to risk of myocardial infarction in both sexes and accounted for 90% of the PAR in men and 94% of the PAR in women[27].
The European Prospective Investigation into Cancer and Nutrition (EPIC) study also contributed extensively to understanding the association of BMI, waist circumference, and WHR with all-cause mortality, using data from 359,387 participants from nine countries[28]. During a mean follow up of 9.7 years 14,723 participants died. The optimal BMI for survival was 25.3 in men and 24.3 in women. Relative risks among men and women in the highest quintile of waist circumference were 2.05 (95% confidence interval [CI], 1.80 – 2.33) and 1.78 (95% CI, 1.56 – 2.04), respectively, and in the highest quintile of WHR, the relative risks were 1.68 (95% CI, 1.53 – 1.84) and 1.51 (95% CI, 1.37 – 1.66), respectively. BMI was still significantly associated with the risk of death in models that included waist circumference or WHR (P<0.001).
Diet and Lifestyle Risk Factors for Weight Gain
In order to identify specific lifestyle and dietary factors associated with long-term weight gain, and to evaluate the association between changes in lifestyle and weight change at 4-year intervals, Mozaffarian and colleagues[29] prospectively studied three separate published cohort studies that included 120,877 United States women and men free of chronic diseases and not obese at baseline. Cohort-specific and sex-specific results were similar and were pooled in their meta-analysis. Four-year weight change was positively associated with high-salt foods, sugar-sweetened beverages, and red meats and inversely associated with the intake of vegetables, whole grains, fruits, nuts, and yogurt. Also independently associated with weight change (P<0.001) were physical activity; alcohol consumption, smoking (weight gain for new quitters) and sleep (more weight gain with <6 or >8 hours of sleep). Reported television watching was associated with 0.31 lb per hour weight gain per day (across quintiles of dietary change)[29].
The Prospective Studies Collaboration (Whitlock and colleagues) obtained individual data from the authors of 57 published prospective cohort studies with baseline BMI in 894,576 participants, 92% from Europe, with a mean age of 46 years and a median recruitment year of 1979(1975–85)[30]. After deaths occurring within the first 5 years were excluded (to reduce reverse causality), there were 66,525 deaths of known cause during a mean eight (SD 6) year follow up with a mean age at death of 67 years. In both sexes mortality was lowest at a BMI about 22.5–25. The weighted average of sex- specific deaths per 1000 by BMI levels showed similar J- or U-shaped patterns in men and women, with consistently higher death rates in men than women. In cause-specific analyses starting with a BMI above 22.5–25, there was a 40% increased hazard ratio for vascular mortality, and a 60–120% hazard ratio for diabetic, renal, and hepatic mortality. Below a BMI of 22–25 kg/m2, there was a definite increase in mortality mainly due to smoking-related diseases. The authors speculate that the weakening obesity-fatal heart disease association in old age may reflect the weaker associations of blood pressure and cholesterol with cardiac events in the elderly.
Despite the continuing obesity epidemic, Peeters and Backholer[23] reported the magnitude of the age-adjusted health risk associated with increasing body weight decreased between 1980 and 2002. The age-standardized CHD mortality rate in adults age ≥35 years decreased by 52% in men and 49% in women in the United States, with similar decreases observed in most other developed countries[23]. Analysis of National Health and Nutrition Examination Surveys over time showed a decreasing relative risk of mortality associated with obesity in U.S. adults [31].
Some observational studies suggest that optimal weight is higher in old age than in middle age[25, 32]. In their systematic review and meta-analysis of more than 30 publications about men and women age ≥65 years, Janssen and Mark[32] noted that nearly all studies had short follow up and were unable to evaluate baseline confounding by morbidity or unintentional weight loss. Observational studies with data collected before old age are necessary to evaluate weight change and survival bias.
For example, the Leisure World Cohort Study (initiated in 1981–1985)[33] reported BMI and weight change were predictors of all-cause mortality in 13,451 participants followed for up to 23 years. Participants who were either overweight or obese at age 21 had increased mortality; those who reported weight loss between age 21 and study entry had increased mortality regardless of their BMI category at age 21. Moderate obesity was associated with significantly increased mortality during follow up only among persons age <75 years and never or past smokers.
A 2011 review from The Framingham Heart Study[34] provided an exceptionally long (1948–1998) follow up of relatively few (1256) participants who met eligibility criteria: without diabetes (based on diabetes medication or FPG >200 mg/dl at a given examination) and obese on at least two consecutive (of 24 biennial) examinations. The unadjusted risk of Type 2 diabetes was similar for men (1.13) and women (1.12). Duration of obesity was a diabetes risk factor independent of BMI. When analyses were stratified by both age and sex, the dose response relationship by duration of obesity was strong in men. In women dose response was significant only when obesity began before age 50.
Smoking
The prevalence of smoking in the United States peaked around 1960 for men and 1980 for women. A substantial number quit smoking since then, allowing studies of the effects of smoking cessation on mortality. Jha and colleagues [35] reported smoking and smoking cessation history between 1997 and 2004 for 113,752 women and 88,496 men aged 25 to 79 years of age, using data from a representative sample of the U.S. population who were interviewed by telephone using the National Health Interview Survey (NHIS). Women in this cohort represent the first generation of U.S. women who began smoking in youth and who could have smoked for decades. Mortality through 2006 was ascertained by periodic matching of records from the U.S National Death Index with cause of death based on the death certificate. The death rate for current smokers at baseline was three times higher than that among never smokers, and shorter by at least 10 years among current smokers compared with never smokers (70% vs. 38% among women and 61% vs. 26% among men). Those who quit before age 40 had a 90% reduced risk of dying compared to those who continued to smoke. Results were not materially changed after adjusting for education, alcohol use, and adiposity. The excess mortality among smokers was largely from vascular, neoplastic, or respiratory diseases. Dramatic decreases in male sudden death during these years may have occurred because more men than women die before reaching the hospital, compatible with men’s earlier smoking cessation compared to women’s.
Alcohol
For many years population-based studies showed that moderate alcohol intake was associated with reduced cardiovascular mortality, with little attention to social circumstances. An interesting analysis from the Male Health Professionals Study[36] reported the protective association of alcohol consumption for the risk of myocardial infarction among 38,077 men who were free of known heart disease and cancer at baseline and had completed the Willett Harvard food frequency questionnaire at baseline and every four years for a total of 12 years. Participants were asked about consumption of beer, red wine, white wine, and liquor every four years. There were 1418 documented cases of fatal or nonfatal myocardial infarction from 1968–1998. Men who drank alcohol 3 to 7 days a week had an adjusted reduced relative risk of 0.68 (0.55–0.84) compared to men who drank alcohol less than once a week. No single type of alcohol conferred additional benefit nor did the proportion of alcohol consumed with meals.
A moderate cardio-protective effect of alcohol against diabetes was reported by Koppes and colleagues who reviewed 15 prospective studies published between 1966 and mid-2004, and found 11,959 incident cases of type 2 diabetes had occurred in 369,862 individuals who were followed for an average of 12 years[37]. Moderate alcohol consumption reduced the risk of diabetes by about 30%, independent of BMI. There were no significant sex differences in risk reduction but only three cohorts included both sexes. It was not possible to control formost cultural, dietary, or lifestyle differences.
Multiple Healthy Behaviors
The first prospective population-based U.S. cohort study of multiple behaviors and sex-specific all-cause mortality was published in 2011 [38]. The cohort included 17,069 participants age 17 and older from the NHANES Survey III Mortality Study (1998 to 2006 evaluation) whose vital status was confirmed and whose underlying cause of death was ascertained using public use files. Four low- risk behaviors (never smoked, healthy diet, adequate physical activity, and moderate alcohol intake) were analyzed by sex and ethnicity. Although very few participants reported all four low-risk behaviors, these participants were 66% less likely to die of malignancy, 65% less likely to die of a major cardiovascular event, and 57% less likely to die from other causes. Gender differences were statistically significant only for all-cause mortality (p =.021), likely due to low statistical power within small disease-specific subgroups.
KIDNEY DISEASE
Although chronic renal disease affects at least 10% of the U.S. adult population and is a major cause of morbidity and mortality world-wide, few studies have reported kidney-related sex differences in mortality. A recent meta-analysis[39] from the NIH Chronic Kidney Disease Collaboration obtained individual data from 46 cohorts, including more than 2 million men and women age 18 and older from Europe, North America, South America, Asia, and Australasia. Except for participants with chronic kidney disease, eligible cohorts had to have at least 1000 subjects with baseline measurement of renal function (preferably estimated glomerular filtration rate (eGFR) and albumin creatinine ratio (ACR), and at least 50 events (all-cause or CVD death or end-stage renal disease). In sex-specific analyses, the risk of all-cause mortality and cardiovascular mortality was higher in men at all eGFR levels and ACR ratios. In sex-specific multiply adjusted analyses (adjusted for CVD, stroke, diabetes, smoking and BMI) the slope of the risk association was steeper in women than men. This large sample provides robust evidence for sex differences, not yet understood.
DIABETES
Gregg and colleagues[40] used data from four waves of National Health Interview Surveys telephone interviews (1997–8, 1999–2000, 2001–2002, 2003–2004; 50% women) to determine whether CVD mortality changed among U.S. adults age 18 and older who did or did not self-report diabetes. Between the earliest and latest waves the overall CVD death rate declined by 40% and the all-cause mortality rate declined by 23%. There was a greater decline (P = 0.02 test for interaction) among diabetic men (5.2 deaths/1000 men) than diabetic women (3.5 deaths/ 1000 women. In this study the sex differences in declining death rates were observed only among diabetics; there were no sex differences among nondiabetics. These differences may reflect sex differences in diagnostic testing, diabetes duration, or diabetes treatment.
Glycemia and heart disease
As reviewed elsewhere, three early community-based cohort studies of diabetes and CVD in the United States included both men and women[41] and examined the sex differences in cardiovascular mortality by diabetes status after controlling for other heart disease risk factors; as shown in Table 1, the adjusted relative risk was larger in women than men in Framingham (reported in 1979), Evans County (reported in 1980), and in Rancho Bernardo (reported in 1983). Smaller or absent differences in men with and without diabetes may reflect the weak or absent definition of diabetes in these early studies.
Table 1.
Sex differences in heart disease mortality among diabetics based on three early U.S. prospective population-based studies
| Study | Period | Age range | No. Diabetics | Deaths* | Risk† | Adjusted risk | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Rancho Bernardo | 1972–80 | 40–79 | 343 | 24 | CSR | 2.4 | 3.5 |
| Framingham | 1948–68 | 45–74 | ≠ | ≠ | RR | 1.7 | 3.3 |
| Evans County | 1967–74 | ≠ | 158 | 7 | SMR | 1 | 2.8 |
Evans County identified coronary heart disease, Framingham identified cardiovascular disease, and Rancho Bernardo identified ischemic heart disease[41].
CSR, Cox standardized risk ratio; RR, relative risk; SMR, standardized mortality ratio.
Not given in study
The Rancho Bernardo Study (RBS) was the first single-center United States cohort study designed specifically to describe the effect of diabetes on sex differences in heart disease in relatively healthy community-dwelling older adults. As reviewed elsewhere [42], we found the prevalence of diabetes by history or fasting plasma glucose criteria (FPG ≥ 140 mg/dl) was nearly twice as high in men as in women, men had higher levels of systolic blood pressure, body mass index, and triglycerides than women, and each of these risk factors was higher at every level of glucose in both sexes[43]. The 14-year heart disease mortality risk by baseline FPG in persons without diabetes (defined by negative history and FPG <140 mg/dl) showed a linear association in men; the higher the fasting glucose, the higher the risk for cardiovascular disease. In women, increased risk did not begin until the FPG was nearly 140 0 mg/dl, shown in Figure 7[43]. This female protection at higher levels of fasting glucose could explain some of women’s cardioprotection.
Figure 7.
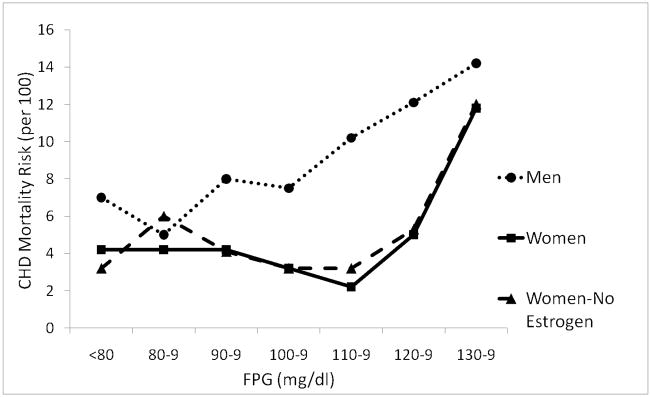
Age-adjusted ischemic heart disease mortality risk by fasting plasma glucose (FPG) levels among nondiabetics aged 40–79 years, Rancho Bernardo, CA, 1972–1987[43]. Linear association in men and threshold at 110 mg/dl in women = relatively greater importance of glucose in women than men. (Scheidt-Nave C, Barrett-Connor E, Wingard DL, Cohn BA, Edelstein SL: Sex differences in fasting glycemia as a risk factor for ischemic heart disease death. Am J Epidemiol 1991; 133(6): 565–76. By permission of Oxford University Press.)
In Rancho Bernardo, sex-specific CVD mortality differences by baseline diabetes status using 15-year follow-up data and −log (−log survival) curves (Figure 8) [44] illustrate how diabetes overrides the female cardiovascular advantage over time. Women without diabetes had a better CVD survival than any other group, and men with baseline diabetes had poorest survival. Women with diabetes had a CVD risk similar to men without diabetes for the first 12 years of follow up, after which they had a risk similar to men with diabetes.
Figure 8.
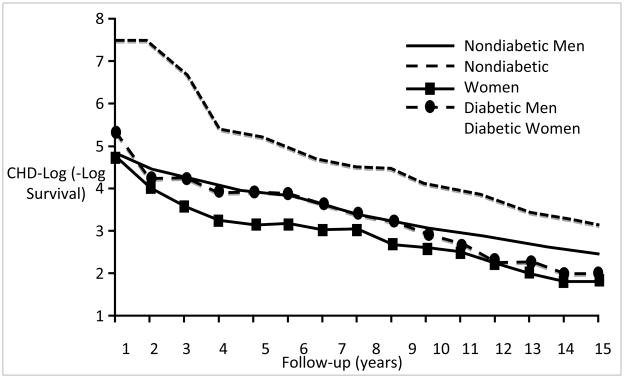
Age-adjusted ischemic heart disease −log (−log survival) by sex and diabetes, Rancho Bernardo Study, 1972–1988[44]. Curves were estimated by a Cox model blocked on both sex and diabetes status and adjusted for age. CHD (coronary heart disease). (Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL: Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991; 265(5): 627–31. Copyright ©1991, American Medical Association. All rights reserved.)
Kanaya and colleagues found Rancho Bernardo women with diabetes were more likely than men with diabetes to have clusters of classic (Framingham) CVD risk factors, differences that might explain their increased risk of heart disease[45]. A meta-analysis of 8 published cohort studies that had sex-specific data for blood glucose and other common heart disease risk factors showed sex differences in cardiovascular risk were no longer statistically significant in any cluster-adjusted analyses (pooled relative risk 2.3 in men, 2.9 in women). These results suggest the loss of female cardio-protection in the presence of diabetes is explained in large part by women’s higher prevalence of clustered classic risk factors, many of which are modifiable[45].
The importance of an oral glucose tolerance test (OGTT) for diagnosis and prognosis has been an important focus of Rancho Bernardo research. We found the prevalence of diabetes (by any criteria) was 12.7% in women and 16.5% in men, but few of these cases were diagnosed by fasting glucose, medication, or physician diagnosis. Overall 72% of women and 59% men with diabetes by any criteria were diagnosed as diabetic only by isolated 2-hour post-challenge hyperglycemia (IPH). Thus, more than half of older adults with diabetes would be missed without an OGTT[46]. This means published epidemiologic studies are often unable to exclude persons with diabetes from their CVD analyses.
Rancho Bernardo women who had post-challenge hyperglycemia without fasting hyperglycemia had a multiply adjusted hazard ratio of 2.6 for CVD death and 2.9 for CHD death[45]; the same analyses in men showed no significant association. Similar results were reported from a pooled analysis of 13 prospective European cohort studies (six included women) with baseline OGTT and a median follow up of 10 years; FPG alone did not identify individuals at increased risk of death[47].
A meta-analysis by Sawar and colleagues of the Emerging Risk Factors Collaboration [48] included individual data from 102 prospective studies of 698,782 participants (52,765 nonfatal or fatal vascular outcomes; 8.49 million person-years at risk). For those with diabetes the adjusted hazard ratio for CHD was 2.00 (95% CI, 1.83 – 2.19). Hazard ratios did not change appreciably after further adjustment for lipid, inflammatory, or renal markers. The CHD hazard ratio was higher in women than men, higher at 40 – 59 years than at ≥70 years, and higher for fatal than nonfatal disease. In people with no diabetes history, information about FPG concentration or impaired fasting glucose status did not significantly improve vascular disease prediction when added to information about conventional risk factors.
Insulin
Surprisingly little population-based evidence confirms the common hypothesis that insulin or insulin resistance is the major mechanism for the increased risk of cardiovascular disease associated with diabetes. Sawar and colleagues conducted a quantitative review of 19 population-based published studies [49] and found no significant association of fasting insulin with CHD in 14 studies involving 2649 CHD cases, and a weak association with post-challenge insulin in 8 studies of nonfasting insulin levels involving 1980 CHD cases. Three studies reported better predictive values for pro-insulin levels involving 413 CHD cases. Studies were mainly or exclusively in men. In a comparison of individuals who had circulating levels in the top third of each of these markers with those in the bottom third, the odds ratios for CHD were 1.12 (95% CI, 0.98 – 1.28) for raised fasting insulin, 1.35 (1.14 –1.60) for raised nonfasting insulin, and 2.23 (1.65 – 3.00) for raised pro-insulin.
TESTOSTERONE
The epidemiology of plasma testosterone levels in adult men is complicated by long recognized associations with age, obesity, alcohol, and health, as elegantly described in a 1981 publication by Dai and colleagues[50]. The authors conducted three pilot studies to evaluate the reliability and repeatability of a single morning sample, and concluded that a single plasma testosterone level was sufficiently consistent to be used for epidemiologic studies, although others (cited in their review) found circadian rhythms (highest levels at 6–7 AM and lowest at 6–8 PM) and seasonal variation (lowest levels in April and highest in October). The authors used plasma testosterone levels in 243 men (age 35–57) at high risk of CVD from the fourth visit of the Multiple Risk Factor Intervention Trial (MRFIT). Testosterone was measured by a highly sensitive and specific method using RIA, extraction, and column chromatography. Subjects were followed for an average of 6.9 years, with a second assay in 157 subjects who were studied again one year later. Among the multiple covariates, age and obesity were significantly associated with plasma testosterone level. Age, weight change, and blood pressure change were the only change variables significantly and independently related to testosterone change.
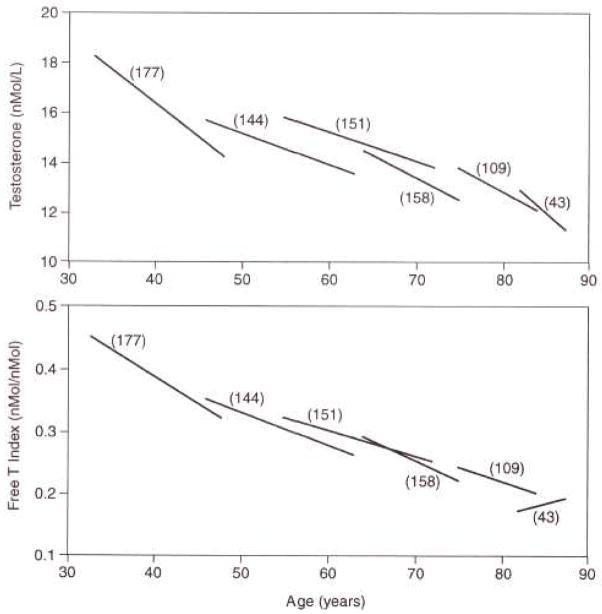
Harman and colleagues provided a unique study of the longitudinal effects of age on total testosterone (T) and free testosterone (free T) index from the Baltimore Longitudinal Study of Aging[51]. In this analysis of repeated measures (Figure 9), there was significant downward testosterone progression at every age, beginning in the 20s, with the exception of an increase in free T index in the ninth decade. These data show testosterone levels decrease at an early age before men are likely to have chronic diseases explaining these changes.
Figure 9.
Longitudinal effects of aging on date-adjusted testosterone and free testosterone index[51]. Linear segment plots for total T and free T index vs. age are shown for men with T and SHBG values on at least two visits. Each linear segment has a slope equal to the mean of the individual longitudinal slopes in each decade, and is centered on the median age, for each cohort of men from the second to the ninth decade. Numbers in parentheses represent the number of men in each cohort. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR: Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001; 86(2): 724–31. Copyright 2001, The Endocrine Society.
Araujo and colleagues[52] conducted a systematic review and meta-analysis of published observational studies reporting the association between endogenous testosterone and all-cause mortality in community-dwelling men whose mean age was 61 years. They identified 11 cohort studies for all-cause mortality and 7 for CVD mortality. The mean testosterone level was 487 ng/dl (16.90 nmol/l), and mean follow up was 9.7 years. The pooled relative risk and 95% confidence interval for all-cause mortality based on random-effects modeling was 1.35 (1.13–1.62). Their analysis for CVD mortality based on 7 studies showed similar but weaker associations. Heterogeneity in all-cause mortality risk was greater in cohorts that included older subjects, reported lower testosterone levels, had shorter follow up, and sampled blood throughout the day (which showed an unexpected stronger association with mortality than morning samples).
Ruige and colleagues[53] identified 19 published cohort studies of testosterone and CVD in apparently healthy middle-aged men. The authors used summary relative risks adjusted for the highest number of available confounding factors for each study. The presence or absence of specific confounders (alcohol, comorbidity, physical activity) or the total number of cofounding variables or combinations of classic CVD risk factors (age, smoking, obesity, lipids, hypertension, and diabetes) did not materially modify absent associations between testosterone and cardiovascular disease. This review found no association between endogenous testosterone and risk for CVD in middle-aged men; in elderly men (age >70), testosterone appeared to weakly protect against CVD. Alternately, low testosterone may reflect poor general health.
In the community-based Rancho Bernardo Study, total and bioavailable testosterone levels were measured in morning fasting blood obtained between 1984 and 1987. Hormone assays were performed in the research laboratory of SSC Yen using RIA, extraction, and column chromatography. In Rancho Bernardo, bioavailable but not total testosterone decreased with age, as shown in Figure 10. Some men in their 80s had higher testosterone levels than some men in their 60s. A wide age distribution is a common characteristic of many biologic variables in the elderly and is observable before old age, making it unlikely that age differences in testosterone levels are fully explained by comorbidity.
Figure 10.
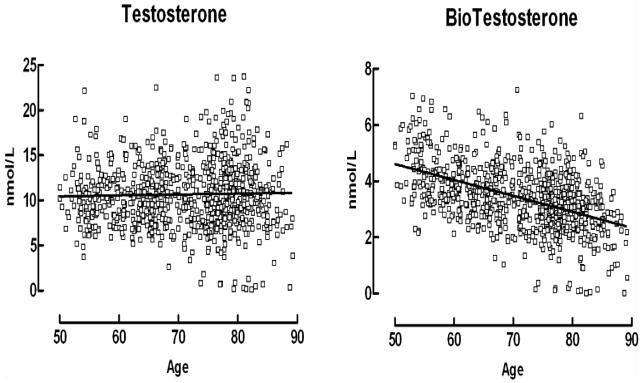
Total testosterone and bioavailable testosterone distribution in men by age in the Rancho Bernardo Study cohort. See text for hormone assay methods used in Rancho Bernardo. (G.A. Laughlin, PhD, University of California, San Diego, unpublished data.)
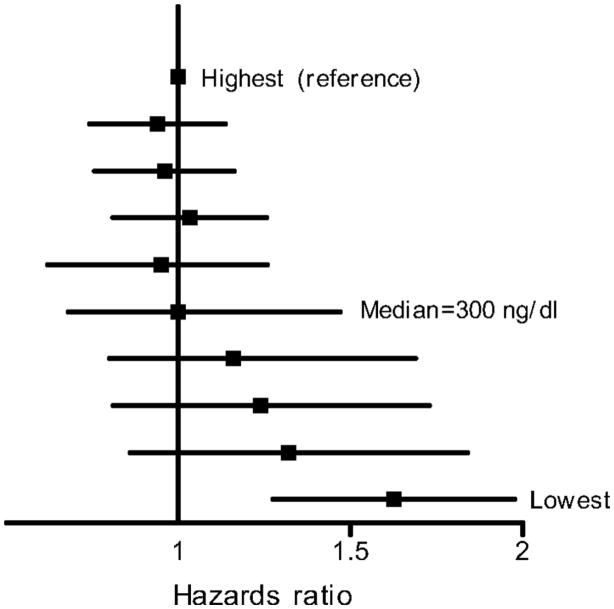
In a prospective analysis, Laughlin and colleagues[54] followed 794 Rancho Bernardo men (median age 74) for 20 years; 583 men died. Men whose total testosterone levels were in the lowest quartile (<241 ng/dl [8.36 nmol/l]) were 40% more likely to die earlier than those with higher levels, independent of age, adiposity, lifestyle, estradiol levels, and prevalent MetS, diabetes, or heart disease. When stratified by deciles of total testosterone, men with testosterone below the median (300 ng/dl [10.41 nmol/l]) were at progressively greater risk of mortality, with no added benefit for men above the median testosterone (Figure 11).
Figure 11.
All-cause mortality according to deciles of total testosterone adjusting for age, BMI, WHR, current smoking, alcohol use, and exercise. The squares represent point estimates for HRs, the lines indicate 95% CIs. Men with testosterone below the median (300 ng/dl [10.41 nmol/l]) were at progressively greater risk of mortality, with no added benefit for men above the median testosterone. Laughlin GA, Barrett-Connor E, Bergstrom J: Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008; 93(1): 68–75. Copyright 2008, The Endocrine Society.
Sex-specific comparisons in Rancho Bernardo showed endogenous testosterone was more closely associated with CHD risk factors in men, while bioavailable testosterone was more closely associated with CHD risk factors in women, and the specific risk factors differed by sex (Table 2). In sex-specific analyses, incident diabetes based on OGTT measured twice 8 years apart was associated with testosterone levels in opposing directions[55] such that men in the lowest quartile of total testosterone had a 2.7-fold increased risk of diabetes, while women in the highest quartile of bioT had a 2.9-fold increased risk of diabetes. The completely opposite direction of testosterone associations in men versus bioT in women parallels the heart disease risk factor associations shown in Table 2.
Table 2.
CHD risk factors by total and bioavailable testosterone in men and women*
| Risk Factor | Men (n= 834) | Women (n= 638) | ||
|---|---|---|---|---|
| Total T** | BioT** | Total T** | BioT** | |
| Age | -- | −0.46 | 0.13 | -- |
| BMI | −0.28 | -- | -- | 0.19 |
| Waist | −.029 | -- | -- | 0.18 |
| Total Chol | -- | -- | -- | 0.17 |
| LDL | -- | -- | -- | 0.14 |
| HDL* | 0.23 | -- | 0.20 | -- |
| Triglycerides* | −0.34 | -- | −0.15 | -- |
| SBP | −0.10 | -- | -- | -- |
| DBP | −0.13 | -- | 0.13 | 0.16 |
| HOMA IR* | −0.24 | -- | -- | -- |
Only correlations of 0.13+ are shown.
Log-transformed for analysis, age-adjusted, all P≤ 0.01
CHD (coronary heart disease); HDL (high-density lipoprotein) ; LDL (low density lipoprotein); Total Chol (cholesterol); Total T (total testosterone); bio T (bioavailable testosterone); SBP (systolic blood pressure), DBP (diastolic blood pressure); HOMA IR (homeostasis model assessment for insulin resistance)
Adapted from G.A. Laughlin, PhD, University of California, San Diego, cited in [42]
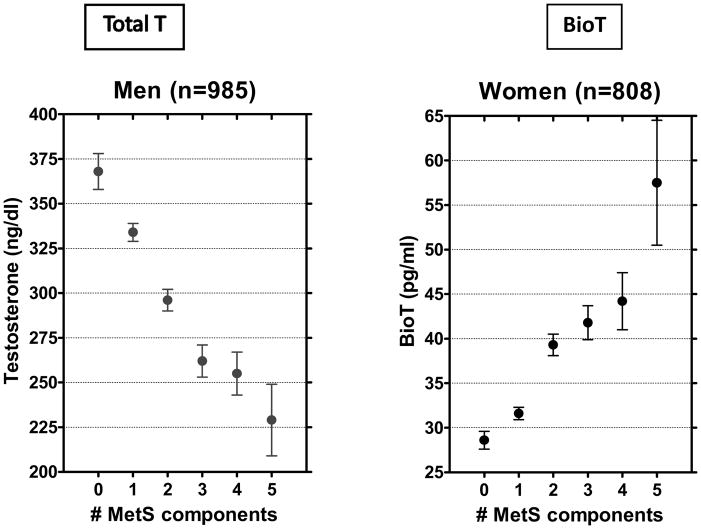
In Rancho Bernardo, similar opposite direction associations by sex were found for the MetS, a strong marker for diabetes (Figure 12)[56]. Ding and colleagues systematically reviewed endogenous sex hormones and risk of Type 2 diabetes, based on 43 published prospective and cross-sectional studies including 6974 women and 6427 men[57]. In the cross-sectional studies testosterone levels were significantly lower in men with diabetes and significantly higher in women with diabetes compared with controls (P< 0.001 for sex difference). In the nine prospective studies, men with higher testosterone levels (449.6–605.2 ng/dl [15.60–21.00 nmol/l]) had a 42% lower risk of diabetes, but there were no significant testosterone-diabetes associations in women.
Figure 12.
Mean testosterone levels by the number of metabolic syndrome (MetS) components, Rancho Bernardo Study, 1984 to 1987. Adapted from Laughlin GA, Barrett-Connor E. Sex-Specific Association of the Metabolic Syndrome with the Serum Androgen to Estrogen Ratio in Older Adults: The Rancho Bernardo Study. Presented at 87th Annual Endocrine Society Meeting, Boston, MA 2006. BioT, bioavailable testosterone; total T, total testosterone.
SUMMARY
This paper reviews mostly recent large population-based prospective cohort studies of sex and gender differences in psychosocial and environmental exposures and in established biological risk factors for all-cause and CHD mortality in adults. Although more studies now include women, surprisingly few provide analyses stratified by sex. Overall, presumptive evidence for causality was equivalent for psychosocial and biological exposures, and these attributes were often associated with each other. Inconsistencies or gaps were particularly obvious for studies of sex or gender differences in age and optimal body size and timing for CHD outcomes. The reasons why testosterone levels do not decease with age in all men, and the long-term consequences of lower levels are uncertain. In older men, low testosterone levels are associated with CHD, diabetes, and MetS, with uncertain implications for men, and with opposite associations for women.
Practice Points
Because this review is based exclusively on observational studies, it is difficult to provide practice points, which ideally are based on clinical trials. The very long follow up from midlife to old age is unlikely to be studied in a clinical trial but midlife associations with late-life outcomes have important clinical implications.
Research Agenda
Improving characteristics that might be reversible in middle age may prevent or delay CHD, diabetes, and MetS. Well-characterized midlife cohorts with extended follow up are essential to answer how midlife exposures affect the risk of late-life survival and late-life disease. Very long studies beginning in midlife can provide both discovery and training opportunities as long as relevance of existing data remains a research priority.
Acknowledgments
Elizabeth Barrett-Connor is Principle Investigator of The Rancho Bernardo Study, which was funded by research grants AG07181 and AG028507 from the National Institute on Aging, and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang H, Dwyer-Lindgren L, Lofgren KT, et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380(9859):2071–94. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 2.Liverman CT, Blazer DG, editors. IOM. Testosterone and Aging: Clinical Research Directions. Washington, D.C: National Academies Press; 2004. [PubMed] [Google Scholar]
- 3.Resnick NM. Geriatric Medicine. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 15. New York: McGraw Hill; 2001. [Google Scholar]
- 4.Newman AB, Murabito JM. The epidemiology of longevity and exceptional survival. Epidemiol Rev. 2013;35:181–197. doi: 10.1093/epirev/mxs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids. 1990;55(8):330–52. doi: 10.1016/0039-128x(90)90058-j. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95(1):252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 7.Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008;117(12):1526–36. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 8.Furman RH. Are gonadal hormones (estrogens and androgens) of significance in the development of ischemic heart disease. Ann N Y Acad Sci. 1968;149(2):822–33. doi: 10.1111/j.1749-6632.1968.tb53838.x. [DOI] [PubMed] [Google Scholar]
- 9.Vaidya D, Becker DM, Bittner V, Mathias RA, Ouyang P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ. 2011;343:d5170. doi: 10.1136/bmj.d5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowkes FG, Pell JP, Donnan PT, et al. Sex differences in susceptibility to etiologic factors for peripheral atherosclerosis. Importance of plasma fibrinogen and blood viscosity. Arterioscler Thromb. 1994;14(6):862–8. doi: 10.1161/01.atv.14.6.862. [DOI] [PubMed] [Google Scholar]
- 11.Wingard DL, Suarez L, Barrett-Connor E. The sex differential in mortality from all causes and ischemic heart disease. Am J Epidemiol. 1983;117(2):165–72. doi: 10.1093/oxfordjournals.aje.a113527. [DOI] [PubMed] [Google Scholar]
- 12.Isles CG, Hole DJ, Hawthorne VM, Lever AF. Relation between coronary risk and coronary mortality in women of the Renfrew and Paisley survey: comparison with men. Lancet. 1992;339(8795):702–6. doi: 10.1016/0140-6736(92)90599-x. [DOI] [PubMed] [Google Scholar]
- 13.Rogers RG, Everett BG, Onge JM, Krueger PM. Social, behavioral, and biological factors, and sex differences in mortality. Demography. 2010;47(3):555–78. doi: 10.1353/dem.0.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–74. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 17.Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42(2):189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- 18.Kivimaki M, Nyberg ST, Batty GD, et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380(9852):1491–7. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandola T, Britton A, Brunner E, et al. Work stress and coronary heart disease: what are the mechanisms? Eur Heart J. 2008;29(5):640–8. doi: 10.1093/eurheartj/ehm584. [DOI] [PubMed] [Google Scholar]
- 20.Broekhuizen LN, Boekholdt SM, Arsenault BJ, et al. Physical activity, metabolic syndrome, and coronary risk: the EPIC-Norfolk prospective population study. Eur J Cardiovasc Prev Rehabil. 2011;18(2):209–17. doi: 10.1177/1741826710389397. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-Connor EL. Obesity, atherosclerosis, and coronary artery disease. Ann Intern Med. 1985;103(6 Pt 2):1010–9. doi: 10.7326/0003-4819-103-6-1010. [DOI] [PubMed] [Google Scholar]
- 22.Keys A, Menotti A, Aravanis C, et al. The seven countries study: 2,289 deaths in 15 years. Prev Med. 1984;13(2):141–54. doi: 10.1016/0091-7435(84)90047-1. [DOI] [PubMed] [Google Scholar]
- 23.Peeters A, Backholer K. Is the health burden associated with obesity changing? Am J Epidemiol. 2012;176(10):840–5. doi: 10.1093/aje/kws328. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25(11):1730–5. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 28.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. [Google Scholar]
- 31.Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102(25):3137–47. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 32.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8(1):41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 33.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163(10):938–49. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdullah A, Stoelwinder J, Shortreed S, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14(1):119–26. doi: 10.1017/S1368980010001813. [DOI] [PubMed] [Google Scholar]
- 35.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 36.Mukamal KJ, Conigrave KM, Mittleman MA, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348(2):109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 37.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28(3):719–25. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 38.Ford ES, Zhao G, Tsai J, Li C. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health. 2011;101(10):1922–9. doi: 10.2105/AJPH.2011.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregg EW, Cheng YJ, Saydah S, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35(6):1252–7. doi: 10.2337/dc11-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Wingard DL. Sex differential in ischemic heart disease mortality in diabetics: a prospective population-based study. Am J Epidemiol. 1983;118(4):489–96. doi: 10.1093/oxfordjournals.aje.a113654. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E. The Rancho Bernardo Study: 40 years studying why women have less heart disease than men and how diabetes modifies women’s usual cardiac protection. Global Heart. doi: 10.1016/j.gheart.2012.12.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheidt-Nave C, Barrett-Connor E, Wingard DL, Cohn BA, Edelstein SL. Sex differences in fasting glycemia as a risk factor for ischemic heart disease death. Am J Epidemiol. 1991;133(6):565–76. doi: 10.1093/oxfordjournals.aje.a115928. [DOI] [PubMed] [Google Scholar]
- 44.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265(5):627–31. [PubMed] [Google Scholar]
- 45.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162(15):1737–45. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 46.Wingard DL, Barrett-Connor E, McPhillips JB. Community-Based Study of Prevalence of NIDDM in Older Adults. Diabetes Care. 1990;13(Suppl 2):3–8. [Google Scholar]
- 47.Glucose tolerance and mortality: comparison of WHO American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354(9179):617–21. [PubMed] [Google Scholar]
- 48.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarwar N, Sattar N, Gudnason V, Danesh J. Circulating concentrations of insulin markers and coronary heart disease: a quantitative review of 19 Western prospective studies. Eur Heart J. 2007;28(20):2491–7. doi: 10.1093/eurheartj/ehm115. [DOI] [PubMed] [Google Scholar]
- 50.Dai WS, Kuller LH, LaPorte RE, Gutai JP, Falvo-Gerard L, Caggiula A. The epidemiology of plasma testosterone levels in middle-aged men. Am J Epidemiol. 1981;114(6):804–16. doi: 10.1093/oxfordjournals.aje.a113251. [DOI] [PubMed] [Google Scholar]
- 51.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 52.Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–19. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97(11):870–5. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 54.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25(1):55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 56.Laughlin GA, Barrett-Connor E. Sex-Specific Association of the Metabolic Syndrome with the Serum Androgen to Estrogen Ratio in Older Adults: The Rancho Bernardo Study. Boston, MA. 87th Annual Endocrine Society Meeting; 2006. [Google Scholar]
- 57.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]