Abstract
Low vitamin D status is associated with an increased risk of immune-mediated diseases like inflammatory bowel disease (IBD) in humans. Experimentally vitamin D status is a factor that shapes the immune response. Animals that are either vitamin D deficient or vitamin D receptor (VDR) deficient are prone to develop IBD. Conventional T cells develop normally in VDR knockout (KO) mice but over-produce IFN-γ and IL-17. Naturally occurring FoxP3+ regulatory T cells are present in normal numbers in VDR KO mice and function as well as wildtype T regs. Vitamin D and the VDR are required for the development and function of two regulatory populations of T cells that require non-classical MHC class 1 for development. The two vitamin D dependent cell types are the iNKT cells and CD4/CD8αα intraepithelial lymphocytes (IEL). Protective immune responses that depend on iNKT cells or CD8αα IEL are therefore impaired in the vitamin D or VDR deficient host and the mice are more susceptible to immune-mediated diseases in the gut.
Keywords: Vitamin D, T cells, inflammatory bowel diseases
1.1 Introduction
Vitamin D is a fat soluble vitamin that can either be absorbed from the diet or produced in the skin following UV exposure of the skin (Holick et al., 1981). Most foods contain very little vitamin D with the exception of some oily fish (mackerel, salmon etc.). The vitamin D absorbed from the diet or produced in the skin is inactive and is processed twice to form the active form of vitamin D (1,25(OH)2D3) (Takeyama et al., 1997). The vitamin D receptor (VDR) is a nuclear receptor that in the presence of 1,25(OH)2D3 regulates transcription by binding to vitamin D response elements on the promoters of targeted genes (Haussler et al., 1998). The classical functions of vitamin D are in the regulation of calcium and phosphorous homeostasis and thus bone health. In 1983 the VDR was reported to be in cells of the immune system by two different groups (Bhalla et al., 1983; Provvedini et al., 1983). Since that time there has been increasing interest in understanding what the targets of vitamin D are in the immune system.
1.2 Vitamin D, immune mediated disease and inflammatory bowel disease (IBD)
IBD are immune-mediated diseases that result because of inappropriate T cell responses to the normal bacterial flora in the gut. The IBD are a complex and heterogenous family of diseases that involve both genetic and environmental factors. There are two classifications of IBD: Crohn’s disease affects all parts of the gastrointestinal tract from the mouth to the anus while ulcerative colitis is a disease focused mainly in the colon. Animal models of IBD have been extremely useful for understanding the factors that regulate disease development even though no one model reproduces all aspects of IBD (Saleh and Elson, 2011). Most people don’t generate T cell responses to their normal flora and it is still not clear why some people develop IBD and others do not. Relatives of patients with IBD are more likely to develop IBD and several genetic factors have been shown to predispose individuals to develop IBD (Khor et al., 2011). Not all people who are genetically predisposed to develop IBD actually develop IBD and studies that have examined identical twins show that the concordance rate for both twins developing IBD is only 14–50% for ulcerative colitis and Crohn’s disease respectively (Halme et al., 2006). Therefore there are environmental factors that alter the expression of the genes and can contribute to disease development. It has been difficult to identify the environmental factor(s) that control IBD development in genetically susceptible individuals.
There is good evidence to suggest that the composition of the normal flora is one of the environmental factors that alters IBD susceptibility. IBD patients have a less diverse normal flora compared to that of healthy individuals (Manichanh et al., 2006). Whether the dysbiosis in the normal flora of IBD is a cause of the disease or an effect of the disease is not known. Approaches that use antibiotics to suppress the normal flora or give beneficial organisms (probiotics) to patients with IBD have had some success (Chandran et al., 2003). Animal models of IBD show a critical role for the bacterial microflora in the pathogenesis of IBD (Lupp et al., 2007). Germfree mice don’t develop IBD in several different models (Chandran et al., 2003). Understanding how the normal flora is regulated and how it might be manipulated to alter the outcome of IBD is an area of intense investigation.
We propose that vitamin D is another environmental factor that affects the development of IBD. There are geographical regions where IBD is more prevalent than others (Cantorna and Mahon, 2004). In particular IBD is more prevalent in urban versus rural areas and in the Northern parts of North America and Europe (Cantorna and Mahon, 2004). These geographical areas are places where there is reduced sunlight exposure especially in the winter. In addition, vitamin D status is low in patients with IBD (Cantorna and Mahon, 2004) but whether this is a cause or effect of the disease is not known. Genome wide association studies show polymorphisms in the VDR gene and associated with increased susceptibility to CD and UC (Dresner-Pollak et al., 2004; Simmons et al., 2000). Experimentally, the active form of vitamin D (1,25(OH)2D3) has been shown to suppress several different models of experimental IBD (Cantorna and Mahon, 2004; Daniel et al., 2008). All IBD in humans and/or animals is not due to overproduction of Th1/Th17 cells and some IBD disease is due to malfunction of barrier function, regulatory T cells, innate immune cells and Th2 cells (Saleh and Elson, 2011). Vitamin D has been shown to be effective at inhibiting experimental IBD due to innate immunity, regulatory T cell dysfunction, over-production of Th1/Th17 cells, and barrier function (reviewed in (Bruce and Cantorna, 2011b)). Vitamin D deficiency accelerates the development of experimental IBD (Cantorna et al., 2000). VDR knockout (KO) models of experimental IBD show a critical role for the VDR since the mice develop a fulminating and often lethal form of the disease (Froicu and Cantorna, 2007; Froicu et al., 2003). In order to determine why VDR KO mice develop fulminate IBD; the effect of the VDR on the development and function of pathogenic and protective T cells was probed (Fig. 1).
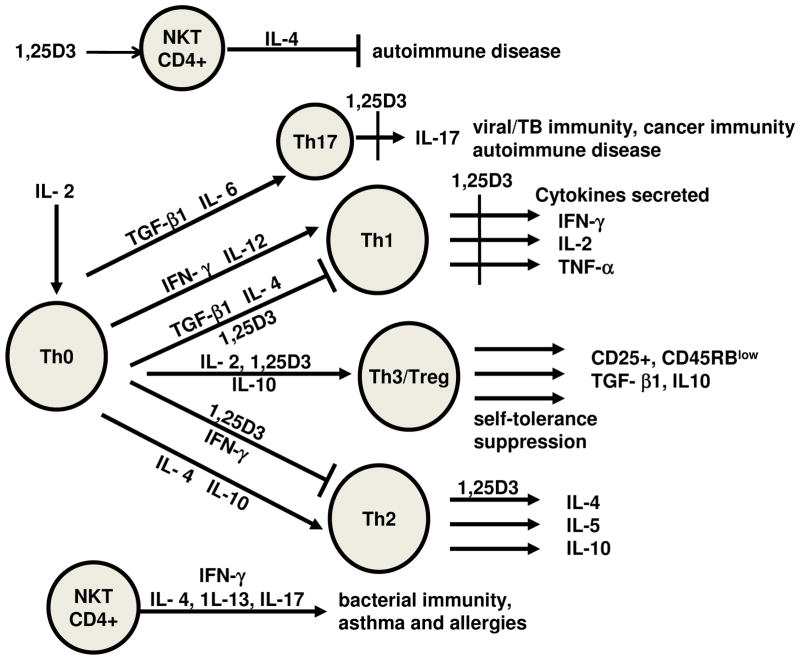
Figure 1. The effects of 1,25(OH)2D3 on CD4+ T cells.
CD4+ T cells are distinguished from one another based on combinations of cell surface markers and the cytokines they produce. 1,25(OH)2D3 (1,25D3) has been shown to inhibit proliferation of T cells, suppress Th17 and Th1 cytokine production, induce regulatory T cells, induce IL-4 production by Th2 cells and induce NKT cell functions. Together the effects of 1,25D3 are to suppress T cell responses that are pathogenic in immune mediated diseases driven by Th1 and Th17 cells and induce regulatory cells that suppress autoimmunity.
1.3 1,25(OH)2D3 T cells and disease
The early experiments treated T cells with 1,25(OH)2D3 to determine the role of vitamin D as an immune system regulator. Experiments done by several different groups of investigators established that 1,25(OH)2D3 suppressed immune responses driven by Th1 cells (Lemire, 1992; Mahon et al., 2003; Rigby et al., 1987). Th1 cells secrete several signature cytokines including IFN-γ, IL-2, and TNF-α. Th1 cells are important in the host’s ability to clear intracellular infections and tumor immunity (Fig. 1). In conjunction with Th17 cells the Th1 cells contribute to the pathogenesis of several autoimmune diseases. Transfer of pathogenic Th1 or Th17 cells induced symptoms of experimental autoimmune encephalomyelitis (EAE, multiple sclerosis model) and inflammatory bowel disease (IBD) (Bruce et al., 2011; Froicu et al., 2003; Stromnes and Goverman, 2006). 1,25(OH)2D3 inhibited Th1 and Th17 function including suppressing the production of the signature IL-17 and IFN-γ cytokines (Cantorna and Mahon, 2004; Daniel et al., 2008; Tang et al., 2009) (Fig. 1). Th2 cells are important for fighting extracellular infections and are pathogenic T cells in the development of allergy and asthma (Fig. 1). The effects of 1,25(OH)2D3 on Th2 cells is more controversial since different groups have shown that IL-4 is increased, decreased or not affected by 1,25(OH)2D3 (Boonstra et al., 2001; Imazeki et al., 2006; Matheu et al., 2003; Pichler et al., 2002; Topilski et al., 2004; Wittke et al., 2004). There are several regulatory T cells that serve to inhibit the induction and function of effector T cells. FoxP3+ T regulatory cells inhibit the generation of Th1, Th2, and Th17 cell function (Fig. 1). 1,25(OH)2D3 induced T reg cells both in vitro and in vivo (Barrat et al., 2002; Gregori et al., 2002). T reg cells suppressed effector T cells via the secretion of IL-10 and TFG-β1 (Vignali et al., 2008). Natural killer (NK)T cells have been shown to secrete high amounts of cytokines early during an immune response (Gumperz et al., 2002). Induction of NKT cells suppressed autoimmunity including EAE and IBD (Godfrey et al., 2000; Middendorp and Nieuwenhuis, 2009; Taniguchi et al., 2003) (Fig. 1) but were involved in the pathogenesis of allergic asthma (Akbari et al., 2003). 1,25(OH)2D3 has been shown to increase cytokine production of NKT cells and to potentiate their functions (Yu and Cantorna, 2008). 1,25(OH)2D3 suppressed Th1 and Th17 responses, induced cytokine production by NKT cells and induced regulatory T cells suggesting that physiologically vitamin D may be important in the induction of immune-mediated diseases like IBD.
1.4 Vitamin D, the VDR and T cell responses
Vitamin D deficiency and VDR KO mice have normal numbers of CD4+, CD8+, B cells, macrophage, and NK cells (Yu and Cantorna, 2008, 2011). Analysis of activation markers on CD4+ T cells showed that VDR KO mice have fewer naïve CD4+ T cells and more memory and activated T cells (Bruce et al., 2011). Injection of equal numbers of naïve (either CD45RBhigh or CD25−) VDR KO or wild type (WT) CD4 T cells into T and B cell deficient mice resulted in more severe colitis in mice that received the VDR KO cells (Froicu et al., 2003). The increased pathogenic potential of the naïve VDR KO CD4 cells was a result of the overproduction of IFN-γ and IL-17 (Bruce et al., 2011). CD4+ T cells from VDR KO mice produced twice as much IFN-γ and IL-17 than their WT counterparts (Bruce et al., 2011). VDR KO mice had T cells that were primed and ready to become Th17 and Th1 cells and these cells are important cells in the pathogenesis of IBD.
Experiments showed that CD4+ T cells transferred from WT mice suppressed experimental IBD while CD4+ T cells transferred from VDR KO mice did not (Yu et al., 2008). T regs, iNKT and a specialized population of CD8αα T cells have been shown to suppress Th1 cells, Th17 cells and IBD in the gut (Das et al., 2003; Saubermann et al., 2000; Vignali et al., 2008). The numbers of FoxP3+ T regs were not different in VDR KO and WT mice (Yu et al., 2008). In addition, T regs were isolated and the ability of the WT and VDR KO T regs to suppress proliferation of naïve T cells in vitro and IBD in vivo were not shown to be different from one another (Yu et al., 2008). CD1d tetramer staining of iNKT cells showed that vitamin D deficient and VDR KO mice have significantly fewer iNKT cells in the spleen, thymus, and liver than WT mice (Yu and Cantorna, 2008). In addition, VDR KO mice were hyporesponsive to the iNKT agonist α-galactoceramide (GalCer) (Yu and Cantorna, 2008). In VDR KO mice there were fewer iNKT cells that were also functionally unable to produce either IFN-γ or IL-4 when stimulated (Yu and Cantorna, 2008). VDR KO mice had normal numbers of CD4+ and CD8αβ+ T cells in the intraepithelial (IEL) cells of the small intestine (Yu et al., 2008). Conversely, there were half as many CD8αα T cells in the IEL of VDR KO mice compared to WT (Yu et al., 2008). In particular, the CD4+/CD8αα/TCRαβ+ population of T cells that in WT mice has been associated with the suppression of experimental IBD was essentially missing (Yu et al., 2008). The decreased expression of the CD8αα T cells in the VDR KO mice was associated with the reduced production of IL-10 in the gut (Yu et al., 2008). T reg cells were normal in the VDR KO mice. Conversely, expression of the VDR is critical for the development of two regulatory cells the iNKT cells and the CD8αα/TCRαβ+ T cells.
1.5 Mechanisms underlying the effects of vitamin D on iNKT cells
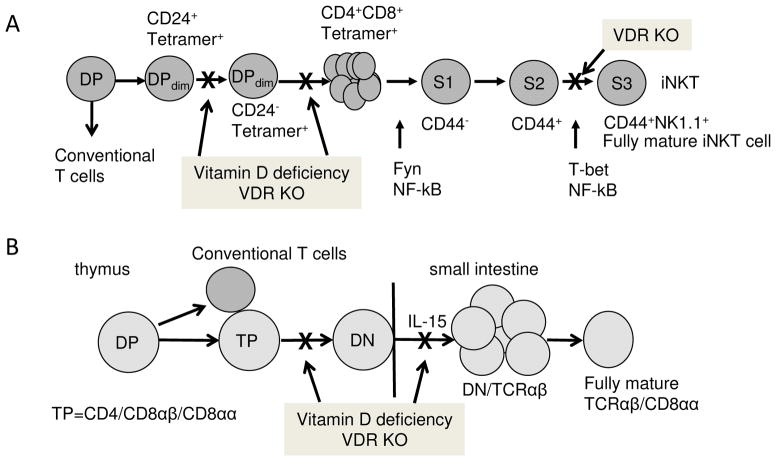
iNKT cells develop in the thymus and diverge from conventional T cells at the CD4/CD8 double positive (DP) stage (Fig. 2, (Gapin et al., 2001)). The iNKT cells can be detected once they have rearranged their TCR using CD1d loaded αGalCer tetramers (Gapin et al., 2001). The earliest TCR positive iNKT cells are DPdim and start as CD24+, the cells become more mature and undergo a rapid expansion (Bendelac et al., 2007; Benlagha et al., 2002; Benlagha et al., 2005; Gapin et al., 2001). Proliferation of vitamin D deficient (D−) and WT iNKT cells in vivo was not different (Yu and Cantorna, 2011). Instead the D− iNKT cells in the thymus were undergoing more apoptosis than the vitamin D sufficient (D+) WT counterparts (Yu and Cantorna, 2011). In D+ WT mice 91% of the DPdim iNKT cells were of the mature CD24− phenotype and most of the apoptosis in the D+ iNKT cells was occurring in the CD24+ cells (61% versus 15 % in the CD24−) (Yu and Cantorna, 2011). Conversely, only 60% of the D− DPdim iNKT cells were the mature CD24− type and there was an equal rate of apoptosis in the CD24+ and CD24− iNKT cells (60%) (Yu and Cantorna, 2011). In the absence of vitamin D iNKT cell precursors undergo a high rate of apoptosis that resulted in fewer and less mature iNKT cells (Fig. 2A, (Yu and Cantorna, 2011)). After proliferation and expansion the now DP iNKT cells undergo further maturation. The iNKT cell precursors begin as CD44−NK1.1− stage (S)1 iNKT cells that first upregulate CD44 (S2) and then fully mature (S3) into CD44+NK1.1+ iNKT cells that are either CD4− or CD4+ and don’t express CD8 (Benlagha et al., 2002; Gapin et al., 2001). In the VDR KO thymus the iNKT cells failed to completely mature and were blocked at the S2 stage (Fig. 2A, (Yu and Cantorna, 2008)). The resulting VDR KO iNKT cells were functionally defective in the amount of cytokine they secreted (Fig. 2A, (Yu and Cantorna, 2008)). The maturational defect was different between VDR KO and D− mice since both mice had significantly fewer iNKT cells but only the VDR KO mice had the additional functional block in development (Fig. 2A, (Yu and Cantorna, 2011)). Vitamin D deficient mice had fewer but functionally normal iNKT cells (Fig. 2A, (Yu and Cantorna, 2011)), suggesting differential effects of the VDR and the vitamin D ligand in regulating iNKT cell number and function.
Figure 2. Vitamin D regulated control of iNKT cell and TCRαβCD8αα cell numbers.
iNKT cells and TCRαβCD8αα cells develop in the thymus following rearrangement of the TCR and diverge from conventional T cells at the CD4/CD8 double positive (DP) stage. A) iNKT cells that express the TCR start out low for CD4 and CD8 (DPdim), CD1d Tetramer+, and CD24+. Maturation of the iNKT cell precursor results in apoptosis of the CD24+ cells, downregulation of CD24 and proliferation of the precursors. Vitamin D deficiency results in more apoptosis and fewer of the early iNKT cells that are CD24−/Tetramer+. The iNKT cells go on to proliferate and through 3 maturation stages (S). S1 iNKT cells are CD44−, S2 iNKT cells are CD44+, and S3 iNKT cells are both CD44+ and NK1.1+. VDR KO iNKT cells are blocked at the S2 to S3 transition and therefore the iNKT cells from VDR KO mice fail to fully mature. B) TCRαβCD8αα cell precursors are triple positive (TP, express CD4/CD8αβ/CD8αα). The TP thymocytes rearrange the TCRαβ and downregulate all forms of CD4 and CD8 (double negative, DN). There are fewer of the TCRαβCD8αα precursors in the thymus of vitamin D deficient and VDR KO mice. The DN TCRαβ cells migrate to the gastrointestinal tract and IL-15 induces the proliferation and upregulation of CD8αα. VDR KO DN/TCRαβ express low levels of the IL-15 receptor and proliferate poorly and as a result there are fewer TCRαβCD8αα T cells in the small intestine of VDR KO mice.
1.6 Mechanisms underlying the effects of vitamin D on CD8ααTCRαβ cells
The CD8αα/TCRαβ+ T cells also diverge from conventional T cells at the DP stage of thymic development (Fig. 2B, (Bruce and Cantorna, 2011a)). In the thymus the CD8αα/TCRαβ+ precursors transiently express CD8αα, CD4, and CD8αβ or become triple positive (TP) (Bruce and Cantorna, 2011a). TP cells can be detected in the thymus of WT mice as early as embryonic day (E)16 (Gangadharan et al., 2006). The frequency of TP cells was not different between VDR KO and WT mice from E16 through the first day of life (Bruce and Cantorna, 2011a). There were significantly less TP thymocytes in VDR KO than WT mice between 1 and 3 weeks of age but not at 6 weeks of age when the TP thymocytes were lowest in all mice (Fig. 2B, (Bruce and Cantorna, 2011a)). The CD8αα/TCRαβ+ T cell precursor go on to down regulate all three receptors and become double negative (DN). The CD8αα/TCRαβ+ T cells were detected in the IEL at very low levels at 1 week of age. There were very few CD8αα/TCRαβ+ T cells in the IEL of WT and VDR KO mice at week 1–2 (Bruce and Cantorna, 2011a). Between week 2 and week 3 of life the WT CD8αα/TCRαβ+ T cells expanded and were 40–50% of all of the IEL in those mice while the VDR KO mice failed to expand the CD8αα/TCRαβ+ T cell population (less than 20% of the IEL, (Bruce and Cantorna, 2011a)). VDR KO and WT mice were treated with bromodeoxyuridine to measure in vivo proliferation (Bruce and Cantorna, 2011a). The data showed that VDR KO IEL proliferated less than WT and in particular the TCRβ+ IEL (Bruce and Cantorna, 2011a). In the WT IEL TCRβ+ DN cells were rapidly proliferating and upregulated CD8αα in response to IL-15 (Fig. 2B, (Bruce and Cantorna, 2011a)). VDR KO mice had fewer TCRβ+ DN IEL and the ones that remained were proliferating very poorly (Bruce and Cantorna, 2011a). The fully mature CD8αα/TCRαβ+ T cells proliferated slowly in both the WT and VDR KO mice (Bruce and Cantorna, 2011a). The reduced proliferation of VDR KO TCRβ+ DN IEL was a result of reduced expression of the IL-15R on this population of cells (Bruce and Cantorna, 2011a). The reduced population of CD8αα/TCRαβ+ T cells in the VDR KO gut was a result of the failure of the DN precursor to migrate out of the thymus and into the gut, express the IL-15R and both expand and upregulate CD8αα (Fig. 2B (Bruce and Cantorna, 2011a)).
1.7 Conclusions
There are several mechanisms that account for the increased severity and incidence of IBD in the VDR KO and vitamin D deficient mouse. Th17 and Th1 cells are highly pathogenic in the absence of the VDR. In addition, vitamin D is required for iNKT cells and CD8αα T cells to develop. The selective requirement for vitamin D and the VDR in the development of these two populations of regulatory T cells and not on conventional T cell development suggests that there may be some common mechanism that are yet to be appreciated. In both cell types expansion and development require the VDR. The data thus far suggest that immune responses where iNKT cells and CD8αα T cells are important will be impacted by changes in vitamin D. Determining which of the vitamin D regulated pathways also occur in humans would help understand the potential impact of vitamin D on patients with IBD.
Acknowledgments
We thank the National Institute of Health Tetramer Core Facility for the CD1d and TL tetramers used in the experiments reviewed here and the members of the Center for Molecular Immunology and Infectious Diseases for lively discussion. This work was supported by National Institutes of Health/National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements AT005378 and National Institute of Neurological Disorders and Stroke NS067563.
Biographies
Dr. Margherita Cantorna is a Professor of Molecular Immunology and Nutrition in the Department of Veterinary and Biomedical Sciences at the Pennsylvania State University. Dr. Cantorna received her BS in Chemistry from the University of Illinois, her PhD at the University of Wisconsin in the Department of Medical Microbiology and Immunology and her post-doctoral fellowship also at the University of Wisconsin in the Biochemistry Department. Dr. Cantorna’s research has determined the mechanisms whereby vitamin D regulates T cells.
Jot Hui Ooi is a fourth year graduate student of the Graduate Program in Pathobiology at the Pennsylvania State University. She earned her BS in Biotechnology and Life Sciences from the Tokyo University of Agriculture and Technology in Japan. Her thesis project is to understand how vitamin D regulates gastrointestinal homeostasis and inflammation.
Jing Chen graduate student of the Graduate Program in Pathobiology at the Pennsylvania State University. She earned her BS in Biological Sciences from Wuhan University in China. Her thesis project is to determine the mechanisms by which vitamin D regulates CD8 T cells in the gastrointestinal tract during health and disease.
Footnotes
The authors declare no financial or commercial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9 (5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195 (5):603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296 (5567):553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202 (4):485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57 (6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167 (9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Bruce D, Cantorna MT. Intrinsic requirement for the vitamin D receptor in the development of CD8alphaalpha-expressing T cells. J Immunol. 2011a;186 (5):2819–2825. doi: 10.4049/jimmunol.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce D, Cantorna MT. Vitamin D and Inflammatory Bowel Disease. In: Feldman D, Pike WJ, Adams JS, editors. Vitamin D. 3. Academic Press; London: 2011b. pp. 1879–1890. [Google Scholar]
- Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011 doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229 (11):1136–1142. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130 (11):2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- Chandran P, Satthaporn S, Robins A, Eremin O. Inflammatory bowel disease: dysfunction of GALT and gut bacterial flora (II) Surgeon. 2003;1 (3):125–136. doi: 10.1016/s1479-666x(03)80091-4. [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324 (1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100 (9):5324–5329. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresner-Pollak R, Ackerman Z, Eliakim R, Karban A, Chowers Y, Fidder HH. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8 (4):417–420. doi: 10.1089/gte.2004.8.417. [DOI] [PubMed] [Google Scholar]
- Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17 (12):2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25 (4):631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2 (10):971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21 (11):573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51 (5):1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195 (5):625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12 (23):3668–3672. doi: 10.3748/wjg.v12.i23.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13 (3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211 (4482):590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- Imazeki I, Matsuzaki J, Tsuji K, Nishimura T. Immunomodulating effect of vitamin D3 derivatives on type-1 cellular immunity. Biomed Res. 2006;27 (1):1–9. doi: 10.2220/biomedres.27.1. [DOI] [PubMed] [Google Scholar]
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474 (7351):307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49 (1):26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2 (3):204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89 (5):922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55 (2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J Allergy Clin Immunol. 2003;112 (3):585–592. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- Middendorp S, Nieuwenhuis EE. NKT cells in mucosal immunity. Mucosal Immunol. 2009;2 (5):393–402. doi: 10.1038/mi.2009.99. [DOI] [PubMed] [Google Scholar]
- Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52 (1):12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221 (4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79 (6):1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34 (3):293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, Exley M, Snapper S, Balk SP, Hagen SJ, Kanauchi O, Motoki K, Sakai T, Terhorst C, Koezuka Y, Podolsky DK, Blumberg RS. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119 (1):119–128. doi: 10.1053/gast.2000.9114. [DOI] [PubMed] [Google Scholar]
- Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47 (2):211–214. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1 (4):1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277 (5333):1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, Su SB, Chan CC, Adorini L, Caspi RR. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182 (8):4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Topilski I, Flaishon L, Naveh Y, Harmelin A, Levo Y, Shachar I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34 (4):1068–1076. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8 (7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J Immunol. 2004;173 (5):3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105 (52):20834–20839. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008;105 (13):5207–5212. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol. 2011;186 (3):1384–1390. doi: 10.4049/jimmunol.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]