Abstract
The Chagas disease vector Triatoma infestans is largely controlled by the household application of pyrethroid insecticides. Because effective, large-scale insecticide application is costly and necessitates numerous trained personnel, alternative control techniques are badly needed. We compared the residual effect of organophosphate-based insecticidal paint (Inesfly 5A IGR™ (I5A)) to standard deltamethrin, and a negative control, against T. infestans in a simulated natural environment. We evaluated mortality, knockdown, and ability to take a blood meal among fifth instar nymphs. I5A paint caused significantly greater mortality at time points up to 9 months compared to deltamethrin (Fisher's Exact Test, p < 0.01 in all instances). A year following application mortality among nymphs in the I5A was similar to those in the deltamethrin (χ2 = 0.76, df=1, p < 0.76). At months 0 and 1 after application, fewer nymphs exposed to deltamethrin took a blood meal compared to insects exposed to paint (Fisher's Exact Tests, p < 0.01 and p < 0.01 respectively). Insecticidal paint may provide an easily-applied means of protection against Chagas disease vectors.
Keywords: Inesfly 5A IGR™, Deltamethin, Chagas Disease, T. infestans, blood-meal
Introduction
Endemic regions in South America have relied on vector control for decades to reduce the incidence of Chagas disease (Dias et al. 2002, Lannes-Vierira et al. 2010). The focus has primarily been to eliminate triatomines from human living spaces to reduce human-vector contact and thus the potential transmission of Trypanosoma cruzi to humans. A significant reduction in the rate of household infestation by domestic triatomines leads to a rapid decrease in the frequency of acute cases of Chagas disease(Souza et al. 1984, Dias and Schofield 1999, Dias 2000, Dias et al. 2002, Vinhaes 2002, WHO 2009). Vector control may also indirectly contribute to reductions in congenital and transfusion-mediated T. cruzi transmission (Wendell 1997, Dias and Schofield 1999, Dias et al. 2002).
The current vector control strategy for Triatoma infestans, the principal vector of Chagas disease in South America, relies on large-scale indoor residual spray (IRS) campaigns using pyrethroid insecticides. Although these campaigns are vertically managed, they are often too expensive for limited disease control budgets. There is a great need for the development of vector control strategies that could be carried out by members of the community themselves (Woody and Woody 1955, Kroeger et al. 1995, Kroeger et al. 1997, Kroeger et al. 1999, Cecere et al. 2002, Herber and Kroeger 2003, Gentile et al. 2004, Reithinger et al. 2005, Reithinger et al. 2006, Levy et al. 2008, Amelotti et al. 2009b, a, Gurtler et al. 2009).
Insecticidal house paint is one option that has surfaced in recent years as a potential alternative to IRS. Amelotti and colleagues (2009) tested several formulations of insecticidal paint made by Inesfly™ on T. infestans. The investigators report that, in laboratory conditions, 100% of T. infestans placed in direct contact with Inesfly 5A IGR™ (I5A) painted surfaces died. This effect lasted up to one year after the first application.
Here we describe an evaluation of the effects of I5A insecticidal paint on T. infestans in a simulated natural environment. Our experiments addressed two objectives: 1.) To compare the effectiveness of I5A relative to the standard treatment of deltamethrin and no treatment (control); and, 2.) To quantify the residual effect of I5A on nymph mortality, knockdown, and their ability to obtain a blood meal.
Methods and Materials
Insecticidal Paint
I5A is a vinyl paint with an aqueous base. It contains three insecticides: diazinon (1.5%), chlorpyrifos (1.5%), and pyriproxyfen (0.063%) (Juvenile Growth Analogue, JGA). The active ingredients reside in CaCO+ resin microcapsules. The microcapsules have an active nucleus that is surrounded by a thin frame polymer that contains the active ingredients and pigments. The microcapsules allow for the gradual release of insecticides, which presumably increases duration of effectiveness and reduces the unwanted toxicity of the insecticides (Amelotti et al. 2009a). Toxicological information is available for diazinon(Diazinon) chlorpyrifos(Chlorpyrifos) and pyriproxyfen(Pyriproxyfen) and deltamethrin(Deltamethrin).
Setting and Insects
The study was conducted between November 2009 and June 2010 at the University of Pennsylvania/Universidad Peruana Cayetano Heredia Chagas Control Laboratory (Arequipa, Peru). Fifth instar T. infestans nymphs used in the experiments were from the UNSA strain. The UNSA strain was originally derived from field-collected samples from the district of Hunter, Arequipa, in 1973, prior to any insecticide application campaigns in the region. The strain has been used for years to evaluate the effectiveness of insecticide application and is consistently susceptible to deltamethrin (Maloney et al. 2011). Each insect used was partially starved, meaning that is was either a 1 or 2 on Montenegro’s Qualitative Feeding Scale (Montenegro 1983). All the insects used had molted 2 weeks prior to the initiation of the experiment. Throughout the experiment, the nymphs were kept under general temperature conditions in the area temperatures (19.8–27.5°C) and humidity (18.7–79.3%)
Setting
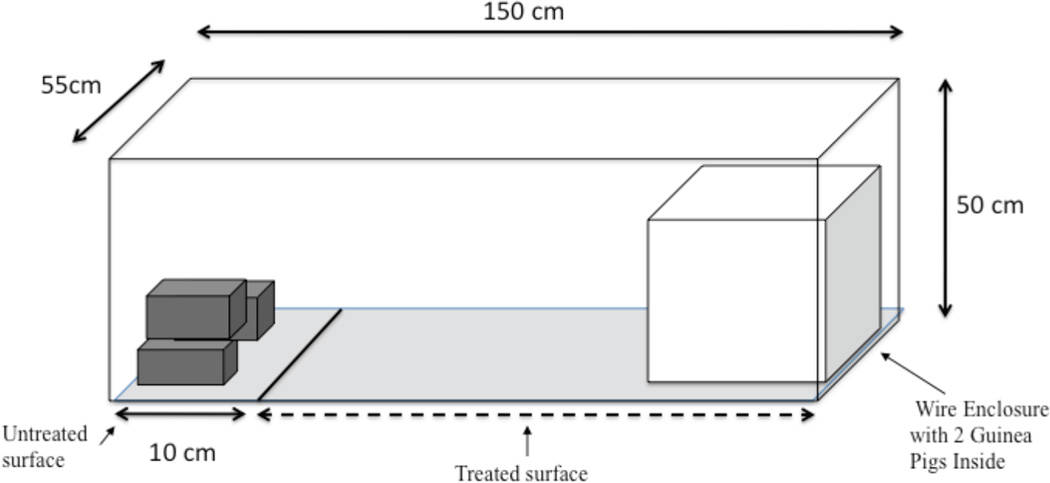
Three treatment settings were included in the experimental design. Each setting consisted of a separate glass aquarium measuring 150 cm × 55 cm × 50 cm (L × W × H). Each aquarium had a removable concrete floor to which the treatment was applied. One aquarium floor was painted with I5A insecticidal paint at a concentration of 320.8 grams/meter2. The insecticidal paint contained diazinon (1.5%), chlorpyrifos (1.5%), and pyriproxyfen (0.063%). To maintain natural field settings, no attempt was made to control the applied dose; a single coat of paint was applied with a paintbrush. The second floor was treated with Bayer™’s K-Othrine deltamethrin wettable powder, diluted in water and applied using a Hudson X-Pert compression sprayer at a target dose rate of 25 mg/m2. The third floor was left untreated. On each floor, a 15 cm wide section across one end of the floor was marked and left untreated. After treatment, each floor was allowed to dry for 24 hours. In the untreated area, three stacked bricks were placed to provide a chemical-free refuge for T. infestans triatomines. Opposite the untreated section in each setting, a wire cage was placed with two 400–500 gram guinea pigs (Cavia porcellus) inside. The aquaria and experimental design are shown in the diagram in Figure 1. Guinea pigs were fed concentrated pellets, water, and alfalfa ad libitum throughout the experiment. Tulane University provided IACUC approval for the experiment.
Figure 1.
A schematic of the experiment. Insects were released into stacked bricks at one end of the aquarium system. Two guinea pigs were placed on the opposite end of the aquarium; the floor between was treated with either I5A paint, deltamethrin, or left untreated.
Experimental Design
Fifteen 5th instar nymphs were individually coded and placed on the brick habitat. Feeding status for each nymph was measured on days 0, 1, 3, 7, and 14 with day 0 representing the day the nymphs were placed in the aquarium. At each time point the nymphs’ status was classified as alive, knocked-down, or dead. We defined “knocked-down” as an insect heavily impaired and unable to walk in a coordinated fashion (Palomino et al. 2005, Palomino et al. 2007). On day 14, all nymphs were removed and observed for 3 days in a chemical-free setting. The experiment was first performed 24 hours following insecticide application, and replicated at months 1, 3, 6, 9, and 12. All materials were stored in the experiment room, which closely mimicked the conditions of houses in infested areas of Arequipa. Using a second set of treated floors, the full experiment was repeated twice at each time period. In total there were two experiments per treatment setting at each time period.
The mortality and blood meal obtainment of the nymphs were our response variables. Each nymph was considered to have successfully obtained a blood meal if it was classified as a 3 or 4 according to Montenegro’s Qualitative Feeding Scale (Montenegro 1983). To compare overall mortality and feeding between treatment settings, the observations from both replicate experiments at months 24 hours, 1, 3, 6, 9, and 12 months were pooled. Cox proportional hazards rates (HR) were used to assess the odds of mortality and feeding over the course of the first 14 days of the experiment, controlling for the length of time since the treatment was applied. Within each treatment group, a two-sided Fisher’s exact test was used to compare the mortality, knockdown, and feeding each month after the first 14 days of the experiment. Analyses were performed using STATA 10.0 SE (StataCorp 2007).
Results
Mortality in insects exposed to I5A treated surfaces was significantly greater than that in insects exposed to deltamethrin (HR = 3.26, 95%, p < 0.01) and control surfaces (HR = 20.22, 95%, p < 0.01). However, significantly fewer nymphs crossed the deltamethrin-treated surface and successfully obtained a blood meal compared to nymphs exposed to both the I5A (HR = 0.47, 95%, p < 0.01) and control surfaces (HR = 0.28, 95%, p < 0.01).
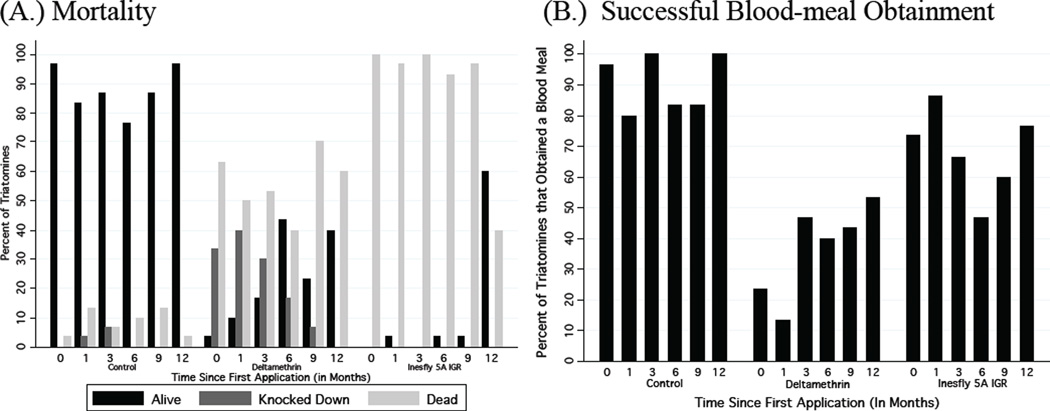
Figure 2.A shows the percent of nymphs that died, were knocked down, or remained alive at each month, stratified by treatment setting. Up until 9 months after application, mortality among nymphs in the I5A setting was consistently higher than that in the deltamethrin setting (p < 0.01 for all time points). However, by month 12, mortality among nymphs in the I5A setting was essentially equal to that in the deltamethrin setting (p < 0.20). The residual efficacy of deltamethrin in terms of mortality was similar throughout the 12 months (p < 0.24). At months 3, 6, and 9 after application, the I5A treatment left more insects knocked-down or dead compared to deltamethrin (p < 0.05 in all instances). By month 12, both I5A and deltamethrin performed similarly in terms of mortality (p < 0.20).
Figure 2.
Mortality and blood meal obtainment of insects in the experiment over time. (A.) Percent mortality, knocked down, and alive; (B.) percent of T. infestans that successfully obtained a blood meal at different durations following application (in months). The same insects were recorded for figures A and B.
Figure 2.B shows the percent of nymphs that obtained a blood meal at each time period, stratified by treatment setting. The residual efficacy for blood meal obtainment for I5A throughout the 12 months was equal to or better than at month 0. Early in the experiment (24 hours through 1 month following application) more nymphs in the I5A setting obtained a blood meal than nymphs in the deltamethrin setting (p < 0.01). However, after month 3, I5A and deltamethrin performed similarly in terms of preventing blood meal obtainment (p > 0.05 in all instances). At all time periods, both the deltamethrin and I5A performed better than the control group in terms of increased mortality and reduced blood meal obtainment.
Discussion
Here we demonstrate that insecticidal paint I5A applied between the refuge of a T. infestans nymph and its potential blood meal is equally effective, or more effective, at killing T. infestans than the standard treatment of deltamethrin. These results were consistent for up to 9 months following the initial application. Our findings can be attributed to two factors: excito-repellency and neurotoxicity. Deltamethrin has a well-documented repellant effect on triatomines (Wood et al. 1993, Diotaiuti et al. 2000). Triatomines may have been less willing to leave their refuge and cross the deltamethrin to obtain a blood meal than the I5A, the active ingredients of which are organophosphates that are not known to have an excito-repellent effect on insects (Mosqueira et al. 2010b). Deltamethrin is also a known contact neurotoxin and may affect T. infestans more rapidly or differently than the organophosphates in the I5A.
The death rates observed in this study were consistently lower than the death rates observed by Amelotti and colleagues (2009) who used controlled laboratory experiments to test I5A on T. infestans mortality (Amelotti et al. 2009a). Previous research shows that the surface on which insecticide is placed as well as the environmental conditions the insecticide is exposed to impact its effectiveness (Penna et al. 1985, Rojas de Arias et al. 1999, Rojas de Arias et al. 2003, Arias et al. 2004). In Amelotti’s experiment, one year after application 97% of insects placed in direct contact with a concrete tile painted with I5A diluted in water (50%) died within 24 hours. In our experiment, which more closely mimics natural conditions, triatomines were never forced to come into contact with the treated surface and likely were in contact with the paint for less than 24 hours.
Our study had several limitations. At month 3, structural problems in the experiment room delayed data collection of the second repetition by 10 days. Despite the delay, nymphs in the second repetition did not experience lower mortality or higher blood meal obtainment as compared to nymphs in the first repetition. We were only able to perform the experiment with fifth instar nymphs, and could not assess the effect of I5A on adult insects, where it may be the most important in terms of influencing transmission dynamics. Finally, the semi-field system was experimental and only roughly mimicked the habitat of triatomines in households.
Field tests over longer periods of time are needed to more conclusively describe the impact of insecticidal paint on domestic and peri-domestic insect populations, the effects of the paint on T. cruzi transmission, and the implications of insecticidal house paint for other vector-borne diseases. Currently, the effectiveness of insecticidal house paints is being tested on a variety of disease-causing insects including vectors of lymphatic filariasis (Mosqueira et al. 2010a), Chagas disease (Dias and Jemmio 2008, Amelotti et al. 2009a, Alarico et al. 2010), and malaria (Mosqueira et al., 2002, Mosqueira et al., 2010a). Paints are also being tested on several nuisance insects, such as red palm weevil (Llacer et al. 2010) and the common Argentine ant (Blasco et al. 2010). Since many insecticides work well at targeting a variety of vector and nuisance insects and could be mixed with paint, insecticidal paint may be one way that residents living in areas with multiple disease vectors could be proactive in reducing their risk of several diseases in a sustainable manner.
| Months after Treatment (n=30) |
Inesfly 5A IGR™ | Deltamethrin | Control | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| 0 | 30 | 100.00** | 19 | 63.33** | 1 | 3.33 |
| 1 | 29 | 96.67** | 15 | 50.00** | 4 | 13.33 |
| 3 | 30 | 100.00** | 16 | 53.33** | 2 | 6.67 |
| 6 | 28 | 93.33** | 12 | 40.00** | 3 | 10.00 |
| 9 | 29 | 96.67** | 21 | 70.00** | 4 | 13.33 |
| 12 | 12 | 40.00** | 18 | 60.00** | 1 | 3.33 |
| Total (n=180) | 158 | 87.78** | 101 | 56.11** | 15 | 8.33 |
Using Fisher’s Exact Test: **p < 0.01, * p < 0.05
Data are from observation of insects 14 days following each exposure
Significance testing compares each treatment setting to the control surface at a given time.
| Months after Treatment (n=30) |
Inesfly 5A IGR™ | Deltamethrin | Control | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| 0 | 22 | 73.33** | 7 | 23.33** | 29 | 96.67 |
| 1 | 26 | 86.67 | 4 | 13.33** | 24 | 80.00 |
| 3 | 20 | 66.67** | 14 | 46.67** | 30 | 100.00 |
| 6 | 14 | 46.67** | 12 | 40.00** | 25 | 83.33 |
| 9 | 18 | 60.00* | 13 | 43.33** | 25 | 83.33 |
| 12 | 23 | 76.67** | 16 | 53.33** | 30 | 100.00 |
| Total (n=180) | 123 | 68.33** | 66 | 36.67** | 163 | 90.56 |
Using Fisher’s Exact Test: **p < 0.01, * p < 0.05
Data are from observation of insects 14 days following each exposure
Significance testing compares each treatment setting to the control surface at a given time.
Acknowledgements
We thank the Ministerio de Salud del Perú (MINSA), Dirección General de Salud de las Personas (DGSP), Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxénicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), Dirección General de Salud Ambiental (DIGESA), Gobierno Regional de Arequipa, Gerencia Regional de Salud de Arequipa (GRSA), Organización Panamericana de la Salud (OPS) and the Canadian International Development Agency (CIDA). This work was supported by National Institutes of Health Grants P50 AI074285-04, P50 AI074285-05, 5K01 AI079162-03 and 5K01 AI079162-04. Additional funding was provided by the Institute for BioTechnology Futures. This study was approved by the Tulane University Institutional Animal Care and Use Board.
References
- Alarico A, Romero N, Hernández L, Catalá S, Gorla D. Residential effect of a micro-encapsulated formulation of organophosphates and piriproxifen on the mortality of deltamethrin resistant Triatoma infestans populations in rural houses of the Bolivian Chaco region. Mem. Inst. Oswaldo Cruz. 2010;105:752–756. doi: 10.1590/s0074-02762010000600004. [DOI] [PubMed] [Google Scholar]
- Amelotti I, Catala S, Gorla D. Experimental evaluation of insecticidal paints against Triatoma infestans (Hemiptera: Reduviidae), under natural climatic conditions. Parasites & Vectors. 2009;2(1):30. doi: 10.1186/1756-3305-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelotti I, Catala S, Gorla D. Effects of cypermethrin pour on applied on chicken over Triatoma infestans, under laboratory conditions. J. Med. Entomol. 2010;47:1135–1140. doi: 10.1603/me10116. [DOI] [PubMed] [Google Scholar]
- Arias A, Lehane M, Schofield C, Maldonado M. Pyrethroid Insecticide Evaluation on Different House Structures in a Chagas Disease Endemic Area of the Paraguayan Chaco. Mem. Inst. Oswaldo Cruz. 2004;99:657–662. doi: 10.1590/s0074-02762004000600022. [DOI] [PubMed] [Google Scholar]
- Blasco J, Tena A, Vanaclocha P, Cambra M, Urbaneja A, Monzo C. Efficacy of a micro-encapsulated formulation compared with a sticky barrier for excluding ants from citrus canopies. Journal of Applied Entomology. 2011;135:467–472. [Google Scholar]
- Cecere M, Gürtler R, Canale D, Chuit R, Cohen J. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Chlorpyrifos, MSDS No. sc-217887. Santa Cruz, CA: Santa Cruz Biotechnology; 2000. Jun 12, [accessed September 21, 2012]. [Online] http://datasheets.scbt.com/sc-217887.pdf. [Google Scholar]
- Deltamethrin, MSDS No. D9315. Saint Louis, MO: Sigma-Aldrich; 2012. Jul 13, [accessed September 21, 2012]. [Online] http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=D9315&brand=SIGMA&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsigma%2Fd9315%3Flang%3Den. [Google Scholar]
- Dias J. Chagas disease control and the natural history of human Chagas disease: a possible interaction? Mem. Inst. Oswaldo Cruz. 2000;95:14–20. [Google Scholar]
- Dias J, Schofield C. The evolution of Chagas Disease (American trypanosomiasis) control after 90 years since Carlos Chagas discovery. Mem. Inst. Oswaldo Cruz. 1999;94:103–121. doi: 10.1590/S0074-02761999000700011. [DOI] [PubMed] [Google Scholar]
- Dias J, Jemmio A. Sobre uma pintura inseticida para o controle de Triatoma infestans, na Bolivia. Rev. Soc. Bras. Med. Trop. 2008;41:79–81. doi: 10.1590/s0037-86822008000100016. [DOI] [PubMed] [Google Scholar]
- Dias J, Silveira A, Schofield C. The impact of Chagas disease control in Latin America: a review. Mem. Inst. Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Diazinon, MSDS No. 66222103-07-LPI. Greeley, CO: Loveland Products, Inc; 2007. Oct 15, [accessed September 21, 2012]. [Online] http://strawberry.ifas.ufl.edu/MSDS%20Labels%20for%20Pesticides/Diazinon.pdf. [Google Scholar]
- Diotaiuti L, Penido C, Araujo H, Schofield C, Pinto C. Excito-repellency effect of deltamethrin on triatomines under laboratory conditions. Rev. Soc. Bras. Med. Trop. 2000;33:247–252. doi: 10.1590/s0037-86822000000300002. [DOI] [PubMed] [Google Scholar]
- Gentile A, Sartini J, Campo M, Sánchez J. Efficacy of Fipronil in the control of the peridomiciliary cycle of Triatoma infestans in an area resistant to Deltamethrin. Cad. Saude Publica. 2004;20:1240–1248. doi: 10.1590/s0102-311x2004000500018. [DOI] [PubMed] [Google Scholar]
- Gurtler R, Cebellos L, Stariolo R, Kitron U, Reithinger R. Effects of tropical application of fipronil spot-on on dogs against the Chagas disease vector Triatoma infestans. Trans. R. Soc. Trop. Med. Hyg. 2009;103:298–304. doi: 10.1016/j.trstmh.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber O, Kroeger A. Pyrethroid-imregnated curtains for Chagas' disease control in Venezuela. Acta Trop. 2003;88:33–38. doi: 10.1016/s0001-706x(03)00193-1. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Mancheno M, Alarcon J, Pesse K. Insecticide-impregnated bednets for malaria control: varying experiences from Equador, Colombia, and Peru concerning acceptability and effectiveness. Am. J. Trop. Med. Hyg. 1995;53:313–323. doi: 10.4269/ajtmh.1995.53.313. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Ordoñez-Gonzalez J, Behrend M, Alvarez G. Bednet impregnation for Chagas disease control: a new perspective. Trop. Med. Int. Health. 1999;4:194–198. doi: 10.1046/j.1365-3156.1999.43370.x. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Meyer R, Mancheno M, Gonzalez M, Pesse K. Operational aspects of bednet impregnation for community-based malaria control in Nicaragua, Equador, Peru, and Colombia. Trop. Med. Int. Health. 1997;2:489–602. doi: 10.1046/j.1365-3156.1997.d01-319.x. [DOI] [PubMed] [Google Scholar]
- Lannes-Vierira J, deAraujo-Jorge T, Soeiro M, Gadelha P, Correa-Oliveira R. The Centennial of the Discovery of Chagas Disease: Facing the Current Challenges. PloS Negl. Trop. Dis. 2010;4:e645. doi: 10.1371/journal.pntd.0000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Quispe-Machaca V, Ylla-Velasquez J, Waller L, Richards J, Rath B, Borrini-Mayori K, Cornejo de Carpio J, Cordova-Benzaquen E, McKenzie F, Wirtz R, Maquire J, Gilman R, Bern C. Impregnated Netting Slows Infestation by Triatoma infestans. Am. J. Trop. Med. Hyg. 2008;79:528–534. [PMC free article] [PubMed] [Google Scholar]
- Llacer E, Dembilio O, Jacas J. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae) J. Econ. Entomol. 2010;103:402–408. doi: 10.1603/ec09310. [DOI] [PubMed] [Google Scholar]
- Maloney KM, Annca-Juarez J, Salazar R, Borrini-Mavori K, Pamo-Tito D, Keating JA, Levy MZ. Secondary kill effect of deltamethrin on Triatoma infestans. J Med. Entomol. 2011;48:929–933. doi: 10.1603/me10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro S. Determinación de las reservas alimenticias en Triatoma infestans Klug, 1834 (Hemiptera, Reduviidae) en base a caracteres externos. I. Adultos. Phys. Secc. C. 1983;41(101):159–167. [Google Scholar]
- Mosqueira B, Duchon S, Chandre F, Hougard J, Carnevale P, Mas-Coma S. Efficacy of an insecticide paint against insecticide-susceptible and resistant mosquitoes-Part 1: Laboratory evaluation. Malaria Journal. 2010;9:340. doi: 10.1186/1475-2875-9-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosqueira B, Chabi J, Chandre F, Akogbeto M, Hougard J, Carnevale P, Mas-Coma S. Efficacy of an insecticide paint against malaria vectors and nuisance in West Africa-Part 2: Field evaluation. Malaria Journal. 2010;9:341–348. doi: 10.1186/1475-2875-9-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino M, Villaseca P, García N, Mosqueda R, Vergaray M. Evaluación de campo de la residualidad de insecticidas piretroides versus Triatoma infestans en tres substratos del distrito Tiabaya – Arequipa. Octubre, 2004 – Abril, 2005. Ministerio de Salud – Instituto Nacional de Salud – Centro de Información y Documentación Científica. Serie Informes Técnicos N°58 [Google Scholar]
- Palomino M, Leon W, Valencia P, Cárdenas F, Ancca J. Evaluación de campo del efecto residual de la deltametrina sobre mortalidad y Knockdowni en Triatoma infestans, según tipo de supeficie en Arequipa, Perú. 2007 [Google Scholar]
- Penna R, Oliveira A, Ferreira M, Jonhnson C, Bosworth A, Marsden P. The influence of building materials on the residual action of BHC. Mem. Inst. Oswaldo Cruz. 1985;80:443–445. doi: 10.1590/s0074-02761985000400010. [DOI] [PubMed] [Google Scholar]
- Pyriproxyfen, Document No. 098UM. Kempton Park, South Africa: Universal Crop Protection; 2010. Jul, [accessed September 21, 2012]. [Online] http://www.villacrop.co.za/produkte/docs/Pyriproxyfen%20100EC_UCP_MSDS.pdf. [Google Scholar]
- Reithinger R, Ceallos L, Stariolo R, Davies C, Gurtler R. Chagas disease control: deltamethrin-treated collars reduce Triatoma infestans feeding success on dogs. Trans. R. Soc. Trop. Med. Hyg. 2005;99:502–508. doi: 10.1016/j.trstmh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Ceallos L, Stariolo R, Davies C, Gurtler R. Extinction of experimental Triatoma infestans populations following continuous exposure to dogs wearing deltamethrin-treated collars. Am. J. Trop. Med. Hyg. 2006;74:766–771. [PMC free article] [PubMed] [Google Scholar]
- RojasdeArias A, Ferro E, Ferreira M, Simancas L. Chagas disease vector control through different interventions modalities in endemic localities of Paraguay. Bull World Health Organ. 1999;77:331–339. [PMC free article] [PubMed] [Google Scholar]
- Rojas de Arias A, Lehane M, Schofield C, Fournet A. Comparative evaluation of pyrethroid insecticide formulations against Triatoma infestans (Klug): residual efficacy on four substrates. Mem. Inst. Oswaldo Cruz. 2003;98:975–980. doi: 10.1590/s0074-02762003000700020. [DOI] [PubMed] [Google Scholar]
- Souza A, Wanderley D, Buralli G, Andrade J. Consolidation of the control of Chagas disease vectors in the State of Sao Paulo. Mem. Inst. Oswaldo Cruz. 1984;79:125–131. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. computer program, version By StataCorp. College Station, TX: 2007. [Google Scholar]
- Wendell S. Doenca de Chagas transfusional. In: Dias JCP, Silviera AC, Schofield CJ, editors. Clínica e Terapêutica da Doença de Chagas: uma Abordagem Prática para o Clínico Geral. Fiocruz, Rio de Janeiro; 1997. pp. 411–427. [Google Scholar]
- World Health Organization. Geneva, Switzerland: 2009. Chagas disease: control and elimination, Sixty-Second World Health Assembly Conference Proceedings: Provisional Agenda item 12.12. [Google Scholar]
- Wood E, Licastro S, Casabe N, Sivori J, Zerba E. Evaluation of the flushing out activity of pyrethroids on Triatoma infestans. Rev Panam Salud Publica. 1993;1:133–137. [Google Scholar]
- Woody N, Woody H. American trypanosomiasis (Chagas'disease): first indigenous case in the United States. JAMA. 1955;159:676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]