Abstract
Here we provide the first genome-wide in vivo analysis of the Na+/Ca2+ exchanger family in the model system Caenorhabditis elegans. We source all members of this family within the Caenorhabditis genus and reconstruct their phylogeny across humans and Drosophila melanogaster. Next, we provide a description of the expression pattern for each exchanger gene in C. elegans, revealing a wide expression in a number of tissues and cell types including sensory neurons, interneurons, motor neurons, muscle cells, and intestinal tissue. Finally, we conduct a series of behavioral and functional analyses through mutant characterization in C. elegans. From these data we demonstrate that, similar to mammalian systems, the expression of Na+/Ca2+ exchangers in C. elegans is skewed toward excitable cells, and we propose that C. elegans may be an ideal model system for the study of Na+/Ca2+ exchangers.
Keywords: exchanger proteins, sodium calcium exchanger, sodium calcium potassium exchanger, comparative phylogeny, Caenorhabditis elegans, gene expression pattern
CALCIUM functions as a diverse signaling molecule in a variety of cell types through activation and conformational changes of proteins, as well as via modulation of cellular capacitance (Berridge et al. 2000, 2003; Bootman et al. 2001). Neurotransmitter release, muscular contraction, apoptosis, and lymphocyte activation are some of the many cellular processes mediated by calcium signaling, and accordingly, strict balance of calcium levels must be maintained to prevent cellular dysfunction. Cells accomplish this primarily by extruding calcium through plasma membrane-embedded plasma membrane Ca2+ ATPase (PMCA) pumps and utilizing exchanger ion transporters. PMCA proteins are high-affinity/low-capacity pumps that maintain calcium homeostasis over sustained periods of time by removing one Ca2+ ion for every ATP hydrolyzed (Tidow et al. 2012). Exchangers such as Na+/Ca2+ exchangers (NCX), Na+/Ca2+/K+ exchangers (NCKX), and calcium/cation exchangers (CCX) are low-affinity/high-capacity ion transporters that rapidly expel calcium ions (Philipson and Nicoll 2000; Philipson et al. 2002; Lytton 2007; Nicoll et al. 2013). The NCX, NCKX, and CCX families of exchangers comprise the three branches of the family of Na+/Ca2+ exchangers in animals (Cai and Lytton 2004a,b; Lytton 2007). Under normal physiological conditions, NCX ion transporters utilize the energy stored in the transmembrane gradient to allow influx of three Na+ ions and extrusion of one Ca2+ ion (Hilge 2012; Ottolia and Philipson 2013). In the case of the NCKX transporter, there is one Ca2+ and one K+ ion exchanged in return for Na+ ion influx, and in the case of the CCX exchangers, both Na+/Ca2+ and Li+/Ca2+ exchanges have been observed (Lytton. 2007; Visser and Lytton 2007). As of yet, the nematode Caenorhabditis elegans has not been used as an in vivo model organism to study the NCX, NCKX, CCX exchanger family. Here we provide a detailed description of the phylogeny of this family of transporters in C. elegans, examine the expression patterns of each member, and uncover roles for one NCX member and one CCX member in muscle contractions, lipid accumulation, and longevity in C. elegans. Our results show that exchanger proteins are widely expressed in a number of tissues and cell types in C. elegans including sensory neurons, interneurons, motor neurons, muscle cells, and intestinal tissue, and we propose that C. elegans may be an ideal in vivo model system to contribute to our understanding of Na+/Ca2+ exchanger biology.
Results
Phylogenetic analysis of NCX, NCKX, and CCX transporters in C. elegans
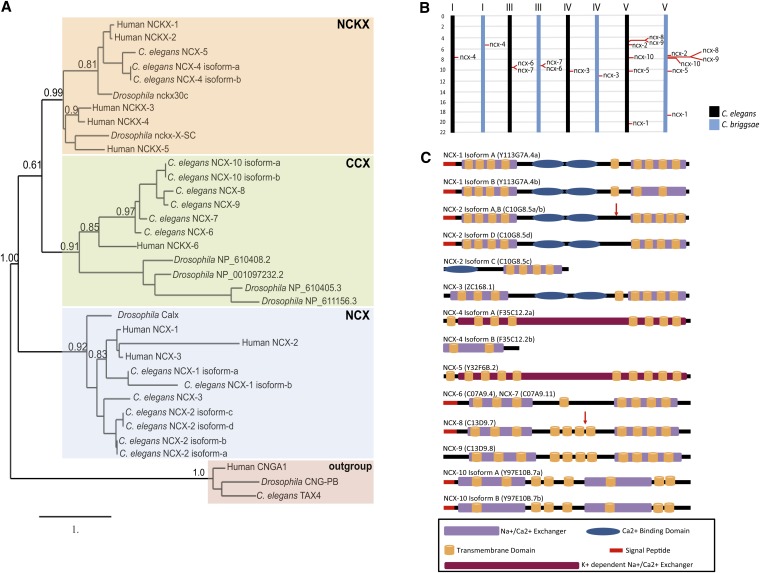
We first performed a detailed phylogenetic characterization of the NCX, NCKX, and CCX genes in C. elegans. To identify orthologs and paralogs of these exchangers, we interrogated genomes within the Caenorhabditis genus (C. briggsae, C. brenneri, C. japonica, C. remanei) and within Pristionchus pacificus, Drosophila melanogaster, and humans. A total of 10 Na+/Ca2+ exchanger genes were detected in the C. elegans genome and are designated ncx-1–ncx-10 at Wormbase (http://www.wormbase.org). Phylogenetic analysis revealed that ncx-1, ncx-2, and ncx-3 belong to the NCX family of exchangers (Figure 1A). Orthologs of these three NCX genes were detected throughout the Caenorhabditis genus (i.e., in C. briggsae, C. brenneri, C. japonica, and C. remanei) with one exception: a clear ortholog of ncx-3 was missing from C. brenneri, suggesting an example of gene loss. Orthologs of all three NCX genes were also detected in the Diplogastridae family member Pristionchus pacificus. For ncx-1 there are two predicted isoforms and for ncx-2 there are four predicted isoforms in C. elegans. In humans there are also three NCX loci (ncx1, ncx2, and ncx3), with ncx-1 in C. elegans being the most closely related NCX transporter to the human orthologs. NCX-2 and NCX-3 in C. elegans are more divergent from the human NCX/C.elegans NCX-1 cluster. In Drosophila we detected only a single NCX gene, called CalX, which is in keeping with previous reports (Cai and Lytton 2004a). Next, we found that ncx-4 and ncx-5 in C. elegans encode for proteins that belong to the NCKX branch (Figure 1A). Orthologs of the C. elegans NCKX members were detected in all Caenorhabditis members examined and also in the more divergent nematode species P. pacificus. We detected two NCKX orthologs in Drosophila, called nckx30c and nckx-X-SC. Both NCX-4 and NCX-5 in C. elegans are more closely related to nckx30c in Drosophila. Within humans there is an expansion of the NCKX branch to produce five members—NCKX1, NCKX2, NCKX3, NCKX4, and NCKX5. Human NCKX1 and NCKX2 are most closely related to the C. elegans NCKX members, and human NCKX3, NCKX4, and NCKX5 form a separate clade that includes the Drosophila nckx-X-SC gene. From our phylogenetic analysis we found that the remaining C. elegans calcium cation exchanger genes belong to the CCX branch (Figure 1A); these are ncx-6, ncx-7, ncx-8, ncx-9, and ncx-10. In humans there is one member of this branch named NCKX6, and we detected four uncharacterized members of this branch in Drosophila melanogaster: NP_610408.2, NP_001097232.2, NP_610405.3, and NP_611156.3. With the exception of ncx-8, which was detected only in C. briggsae (CB08638), orthologs of all the C. elegans CCX members were detected in all Caenorhabditis members examined: C. briggsae, C. brenneri, C. japonica, and C. remanei. In the case of P. pacificus, we detected orthologs for ncx-7, ncx-8, ncx-9, and ncx-10. Synteny of the NCX, NCKX, and CCX genes are highly conserved between C. elegans and C. briggsae (Figure 1B). The NCKX exchanger ncx-4 is located on chromosome I; ncx-6 and ncx-7 are located on chromosome III; ncx-3 is located on chromosome IV; and ncx-1, ncx-2, ncx-5, ncx-8, ncx-9, and ncx-10 are located on chromosome V for both species. The genomic positions of ncx-6 and ncx-7 and also of ncx-8 and ncx-9 are consecutive within both genomes, indicating the possibility of more recent gene-duplication events (Figure 1B).
Figure 1.
(A) Phylogenetic analysis of NCX, NCKX, and CCX exchangers. Na+/Ca2+ exchangers from C. elegans, D. melanogaster, and Homo sapiens were used to build a phylogeny for this superfamily. The cyclic nucleotide gated channel subunits were used as an outgroup. Genomes were downloaded from Wormbase (C. elegans), National Center for Biotechnology Information (Drosophila), and Ensembl (human) and interrogated for NCX, NCKX, and CCX exchanger sequences. Orthologs were aligned using the multiple sequence alignment software MUSCLE v3.8.31, and gaps were systematically stripped after alignment. Phylogenetic relationships were inferred by reconstructing trees by maximum likelihood using the PhyML command-line application. The appropriate model was selected using Prottest v.3 and determined to be the “WAG+I+G+F” model with a fixed gamma distribution parameter of 1.5, proportion of invariable sites set to 0.003, and four substitution rate categories. (B) Analysis of synteny between C. elegans and its sister species C. briggsae for NCX, NCKX, and CCX exchanger genes. Chromosomal coordinates were mined from WormBase. The C. briggsae ortholog of ncx-8 was inferred from bidirectional BlastP searches, phylogenetic analysis, and synteny position to be CBG08638. The y-axis numbers indicate mega-base- pair position. (C) Predicted protein structure of NCX, NCKX, and CCX exchangers. Protein structure was determined using SMART database, Interpro database, and the transmembrane prediction program TM Finder. Calcium-binding domains were detected only for the C. elegans NCX exchangers (NCX-1, NCX-2, NCX-3). The CCX exchangers (NCX-6, NCX-7, NCX-8, NCX-9, and NCX-10) lack predicted calcium-binding domains and have additional transmembrane segments positioned between the Na+/Ca2+ exchanger domains. The NCKX proteins, NCX-4 and NCX-5, are predicted to contain K+-dependent Na+/Ca2+ exchanger-like domains. The alternative isoform NCX-4(b) does not contain a predicted K+-dependent Na+/Ca2+ exchanger domain. Red arrowheads indicate the position of the genetic lesion in ncx-2(gk879849) and ncx-8(gk234217).
Predicted protein structure of NCX, NCKX, and CCX transporters in C. elegans
We examined the predicted protein structure of all 10 C. elegans Na+/Ca2+ exchanger genes and their isoforms using SMART, InterPro, and TM Finder programs (Figure 1C). The C. elegans NCX-1, NCX-2, and NCX-3 proteins that form the NCX branch from our phylogenetic analysis all have similar structure to the mammalian NCX exchangers. The C. elegans NCX exchanger proteins contain two Na+/Ca2+ exchanger domains that are spanned by multiple transmembrane segments. Between the Na+/Ca2+ exchanger sites is a large intracellular loop (between TM4 and TM5 in NCX-1 and NCX-2 isoforms As), which contains two calcium-binding domains (Figure 1C). NCX-1 isoform A, NCX-1 isoform B, and NCX-2 isoforms A, B, and D contain a signal peptide that is absent in NCX-2 isoform C and NCX-3 (Figure 1C). From our phylogenetic analysis we identified two NCKX exchangers in C. elegans: NCX-4 and NCX-5. Both NCKX exchangers have similar protein structures characterized by a large K+-dependent Na+/Ca2+ exchanger-like domain with multiple transmembrane segments separated by a hydrophilic middle region (Figure 1C). NCX-4 isoform B is the smallest exchanger found within the C. elegans Na+/Ca2+ exchanger gene family comprising 154 amino acids and interestingly does not contain a predicted K+-dependent Na+/Ca2+ exchanger domain but instead is predicted to contain only a single Ca2+ exchanger domain. This suggests a surprising level of functional complexity mediated at the transcriptional level within the NCKX branch. The C. elegans NCX-6, NCX-7, NCX-8, NCX-9, and NCX-10 exchangers are all structurally distinct from the NCX and NCKX proteins. Although NCX-6, NCX-7, NCX-8, NCX-9, and NCX-10 contain two Na+/Ca2+ exchanger domains similar to the NCX exchanger proteins, they lack calcium-binding domains and have multiple transmembrane segments positioned between both Na+/Ca2+ exchanger domains. NCX-6/NCX-7 and NCX-8/NCX-9 share very similar overall protein structures. A predicted signal peptide is found in NCX-6, NCX-7, NCX-8, and NCX-10 and is absent in NCX-9.
NCX, NCKX, and CCX expression patterns in C. elegans
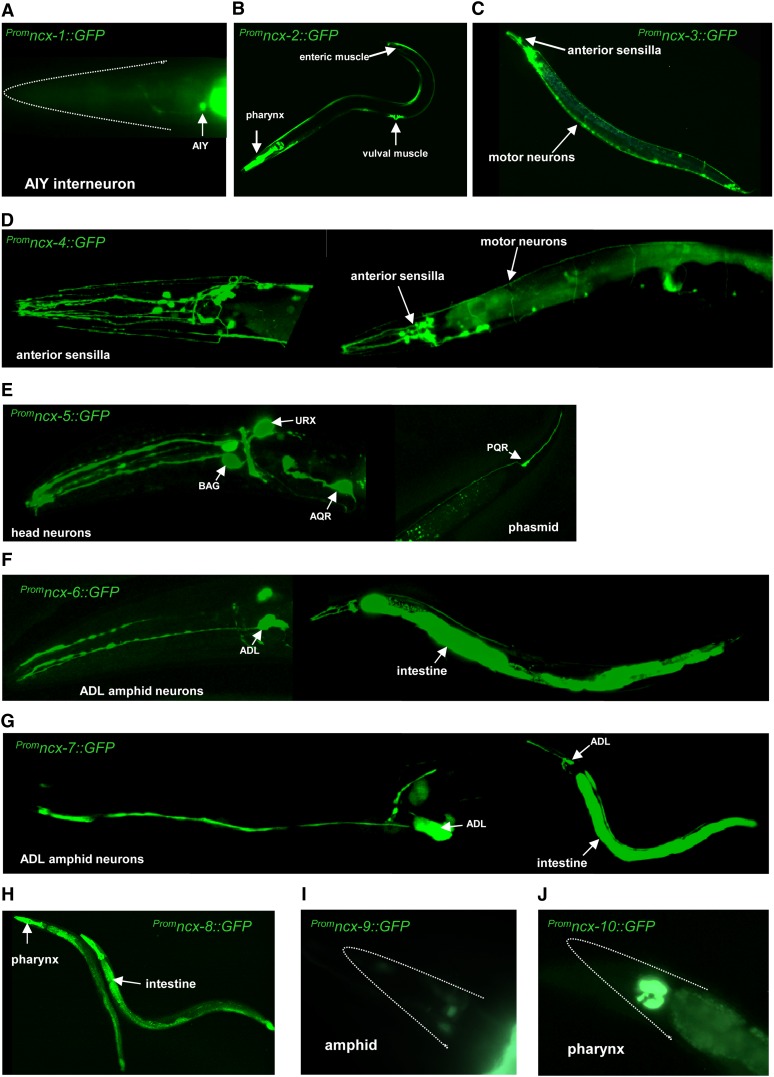
To examine the tissue and cell distribution of NCX, NCKX, and CCX genes in C. elegans, we generated reporter GFP fusions by fusing promoter sequences (Supporting Information File S1, Figure S1 and Table S1) to a GFP gene. Stable transgenic lines expressing these reporters were examined microscopically to identify the cells in which each gene was expressed (Table 1 and Figure 2). From this analysis we found that the Na+/Ca2+ exchanger genes in C. elegans are expressed in very diverse cell types including primary sensory neurons, interneurons, and motor neurons, as well as various pharyngeal, body-wall, and vulval muscle cells. Specific branches of the Na+/Ca2+ exchanger family also exhibited tissue specificity; for example, the NCKX members ncx-4 and ncx-5 are exclusively expressed in neuronal cells. These neuronal cells include sensory neurons, interneurons, and motor neuron cells (Table 1 and Figure 2). Interestingly, in the case of ncx-5 it is not very widely expressed, suggesting a more specific role in sensory neurons: ncx-5 is expressed in three pairs of amphid neurons called the BAG, URX, and AQR cells as well as in one pair of phasmid neurons called the PQR cells. These neurons are specialized oxygen-sensing cells that play a critical role in social feeding behavior (Coates and De Bono 2002; Gray et al. 2004, 2005). The NCX genes ncx-1, ncx-2, and ncx-3 are, as a family, not restricted to a specific tissue, but individually they are. The ncx-1 and ncx-3 are exclusively expressed in neurons, while ncx-2 is expressed only in non-neuronal cells including the pharyngeal tissue, enteric muscle, and vulval muscle (Figure 2B). The CCX genes exhibit much more diversity in their expression patterns, which is similar to the CCX transporter in humans (Cai and Lytton 2004a,b). The CCX class gene ncx-6 is expressed in a pair of head neurons termed the ADL and also in the intestine (Table 1 and Figure 2). The ADL amphid neurons play a role in detecting high concentrations of toxic heavy metals (Cu2+ and Cd2+) and repellant odors, avoiding high oxygen levels, and regulate social feeding behavior (Troemel et al. 1995; Sambongi et al. 1999). The ncx-7 gene is also expressed in the ADL neuron pair and was also detected more faintly in another pair of amphid neurons as well as the intestine and one pair of posterior neurons. The CCX exchanger ncx-8 is expressed strongly in the intestinal cells and also in the pharyngeal muscle, ncx-9 was faintly expressed in neurons particularly in the head region, and finally ncx-10 was detected only in pharyngeal muscle cells (Table 1 and Figure 2).
Table 1. Na+/Ca2+ exchanger gene expression patterns in C. elegans.
| Gene | Sequencea | Coordinatesb | Expression pattern in adult hermaphrodites |
|---|---|---|---|
| ncx-1 | Y113G7A.4 | V:20105601.0.20120644 | AIY interneurons |
| ncx-2 | C10G8.5 | V:5303671.0.5311230 | Pharyngeal muscle including procorpus, metacorpus, isthmus and terminal bulb, body-wall muscle, enteric muscle, vulval muscle |
| ncx-3 | ZC168.1 | IV:10716636.0.10721735 | Head neurons, dorsal/ventral nerve cord and commissures, phasmid neurons |
| ncx-4 | F35C12.2 | I:9808895.0.9813066 | AWC, ASE, two pairs of labial neurons, ventral/dorsal nerve cord and commissures, faint expression in one pair of nondye-filling posterior neurons |
| ncx-5 | Y32F6B.2 | V:10486450.0.10490025 | URX, AQR, and BAG anterior neurons and PQR phasmid neurons |
| ncx-6 | C07A9.4 | III:9710165.0.9713844 | ADL neuron, intestine |
| ncx-7 | C07A9.11 | III:9715799.0.9720207 | ADL neuron (faint expression in one other amphid neuron pair), intestine, one pair of posterior cells |
| ncx-8 | C13D9.7 | V:4983735.0.4987183 | Pharyngeal muscle including procorpus, metacorpus, isthmus and terminal bulb, intestine |
| ncx-9 | C13D9.8 | V:4991490.0.4995049 | Very faint expression in neurons |
| ncx-10 | Y97E10B.7 | V:7930455.0.7933078 | Pharyngeal muscle—predominately the terminal bulb |
Identifier at http://www.wormbase.org.
Indicates the chromosome number and genomic region.
Figure 2.
NCX, NCKX, and CCX exchanger genes are widely expressed in C. elegans. Stable transgenic lines expressing GFP reporter fusions were imaged, and representative images are shown from reporter lines expressing the following fusions: (A) (p)ncx-1::GFP; (B) (p)ncx-2::GFP; (C) (p)ncx-3::GFP; (D) (p)ncx-4::GFP; (E) (p)ncx-5::GFP; (F) (p)ncx-6::GFP; (G) (p)ncx-7::GFP; (H) (p)ncx-8::GFP; (I) (p)ncx-9::GFP; and (J) (p)ncx-10::GFP are shown to illustrate the diversity of tissues and cell types that express the NCX (ncx-1, ncx-2, ncx-3), NCKX (ncx-4 and ncx-5), and CCX (ncx-6, ncx-7, ncx-8, ncx-9, and ncx-10) genes in C. elegans. All images are from day 1 or day 2 adults. In A, I, and J, the white dotted line indicates the anterior head region of the animal. For all images, left is anterior and dorsal is up. In D, F, and G, whole-body images are to the right, and zoomed-in images of the anterior region are to the left.
Behavioral analysis of ncx-2 and ncx-8 mutant animals
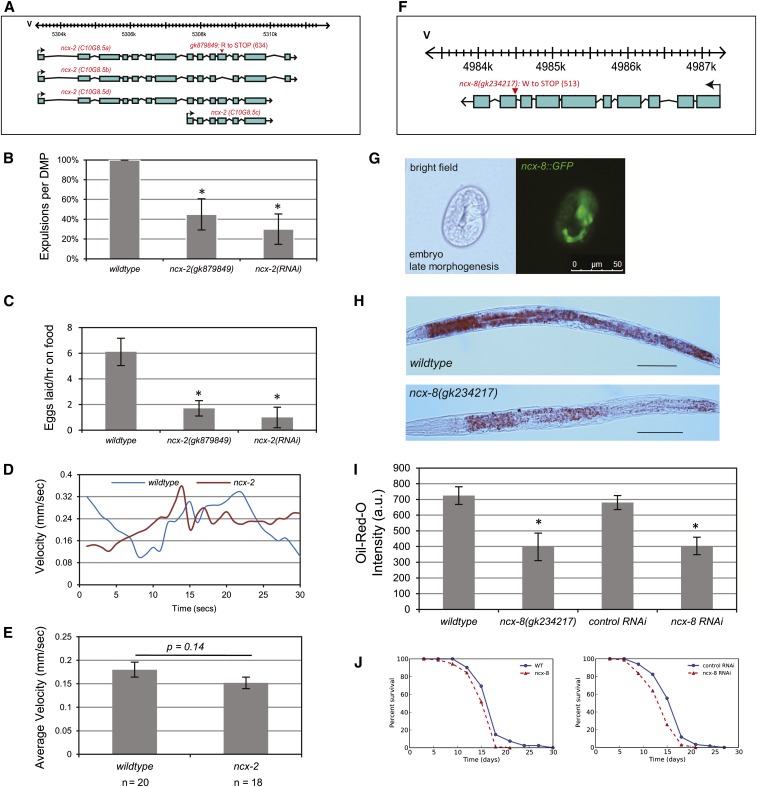
To functionally characterize Na+/Ca2+ exchangers in C. elegans, we obtained mutants in the ncx-2 (NCX) and ncx-8 (CCX) genes. The lesion and gene structure for each mutant is illustrated in Figure 3, A and F. In each case a single base-pair mutation resulting in a premature stop codon defines the lesion, and in each case it occurs before the C-terminal exchanger domain (see red arrowheads in Figure 1C). To understand the function of each gene, we examined behaviors based upon the expression pattern of each gene. In the case of ncx-2 we observed strong expression in the pharynx, body-wall muscle, vulval muscle, and enteric muscles (Figure 2B). We reasoned that examining the defecation motor program (DMP) cycle in these mutants may uncover a role for NCX-2 in regulating rhythmical motor programs. The DMP cycle is a stereotyped sequence of muscle contractions and occurs approximately every 45 sec (Thomas 1990). In the adult hermaphrodite the defecation program begins with the contraction of the posterior body-wall muscles, which increases internal pressure; this contraction lasts for only ∼1 sec, and upon relaxation of these muscles the intestinal contents flow posteriorly, thereby allowing the intestinal contents to collect in the pre-anal region. Finally, the contents of the pre-anal region are expelled by contraction of the enteric muscles. We assayed ncx-2(gk879849) and wild-type animals cultivated on bacteria expressing double-stranded RNA (dsRNA) targeting ncx-2, and in each case we observed a significant delay in the number of expulsions per DMP cycle when compared with wild-type animals [Figure 3B: P < 0.005 for wild-type vs. ncx-2(gk879849) mutants; P < 0.005 for wild-type vs. ncx-2(RNAi) animals]. We also observed strong expression of ncx-2 in vulval muscle, and so next we examined the rate of egg laying for ncx-2(gk879849) mutants and wild-type animals cultivated on bacteria expressing dsRNA targeting ncx-2 (Figure 3C). In each case, we observed significantly reduced egg-laying frequency in the ncx-2 mutant background [Figure 3C: P < 0.05 for wild-type vs. ncx-2(gk879849) mutants; P < 0.05 for wild-type vs. ncx-2(RNAi) animals]. We also examined the number of fertilized unlaid eggs for ncx-2(gk879849) mutants compared with those for wild-type animals and found that ncx-2 mutants do not lay fewer eggs due to a lack of eggs in the uterus but also do not accumulate more eggs [mean number of unlaid eggs for wild type = 12 ± 0.6 and mean for ncx-2(gk879849) = 9 ± 0.4]. We also observed expression of ncx-2 in body-wall muscle and tested a role for NCX-2 in locomotion behavior. To this end, we examined the speed of movement for ncx-2(gk879849) mutant animals (Figure 3, D and E). We recorded movies of wild-type and ncx-2 mutant animals moving while off food to compute their velocity. Representative plots of velocity of an individual wild-type and ncx-2(gk879849) mutant animal are plotted in Figure 3D, and bars of the average velocity for wild type and ncx-2(gk879849) are graphed in Figure 3E. We did not observe a significant difference in movement speed between wild-type and ncx-2 mutant animals. Next we attempted to characterize the ncx-8(gk234217) mutant (Figure 3F). We observed expression of ncx-8 in the pharynx and intestine during late embryonic morphogenesis (Figure 3G) and reasoned that NCX-8 may be contributing to metabolic function in C. elegans. To test this, we examined lipid accumulation in animals using Oil-Red-O stain. Oil-Red-O is used to stain lipids in C. elegans (Figure 3H), and quantification of Oil-Red-O staining is used to measure lipid accumulation (O’Rourke et al. 2009). Wild-type animals and ncx-8(gk234217) mutant animals were fixed and soaked in Oil-Red-O, followed by quantification of Oil-Red-O staining. In the case of the ncx-8(gk234217) mutant animals, we observed significantly lower Oil-Red-O staining (Figure 3I: P < 0.05). We also cultivated wild-type animals on plates with bacteria expressing dsRNA targeting the ncx-8 gene and also cultivated wild-type animals on control plates with bacteria transformed with empty control vectors. In the case of the animals cultivated on the RNA interference (RNAi) plates targeting ncx-8, we observed significant decreases in Oil-Red-O staining as compared with the animals cultivated on the control plates (Figure 3I: P < 0.05). Mutations in metabolic processes have been shown to affect longevity in C. elegans (Crawford et al. 2007; Wang et al. 2008), and so we investigated the life span of ncx-8(gk234217) mutant animals at 20°. Here we observed a significant difference in the percentage of survival of ncx-8(gk234217) mutant animals as compared with wild-type animals (Figure 3J: P < 0.005 for ncx-8 mutants vs. wild-type animals at 20°). We also cultivated wild-type animals on plates with bacteria expressing dsRNA targeting the ncx-8 gene and, as a control, cultivated wild-type animals on control plates with bacteria transformed with empty control vectors. In the case of the animals cultivated on the RNAi plates against ncx-8, we observed a significant decrease in the percentage of survival compared with the animals cultivated on the control plates (Figure 3J: P < 0.005).
Figure 3.
Characterization of the NCX transporter ncx-2 and the CCX transporter ncx-8. (A) Predicted gene structure of the ncx-2 splice forms on chromosome V. The amino acid position of the gk879849 lesion is shown. The lesion in gk879849 is defined by a premature opal stop codon at amino acid 634. (B) Bar chart of the percentage of expulsions per DMP cycle from 20 animals for wild-type animals, ncx-2(gk879849) mutant animals, and wild-type animals that were cultivated on bacteria expressing dsRNA targeting the ncx-2 locus [ncx-2(RNAi)]. In the case of the ncx-2 mutants, there is a significant defect in the number of expulsions per cycle when compared with wild-type animals at P < 0.005 for ncx-2(gk879849) mutant animals and ncx-2(RNAi) animals. (C) Bar chart of the average rate of egg laying per hour for wild-type, ncx-2(gk879849), and wild-type animals that were cultivated on bacteria expressing dsRNA targeting the ncx-2 locus [ncx-2(RNAi)]. Error bars represent the S.E.M; *P < 0.05. For each genotype a sample size of 10 animals was obtained and the mean was calculated. (D) Representative plots of the velocity (mm/sec) for wild-type and ncx-2(gk879849) mutant animals when off food. The animals were transferred to an unseeded plate and allowed to roam for 10 min and then movies of movement were taken. (E) Bar chart of the average velocity for wild-type and ncx-2(gk879849) mutant animals when off food. Error bars represent the S.E.M. P-values were calculated using the Student’s t-test. (F) Predicted gene structure of the ncx-8 gene on chromosome V. The amino acid position of the lesion is highlighted for ncx-8(gk234217), and the lesion in gk324217 is defined by a premature stop codon at amino acid 513. (G) Expression of the ncx-8 gene during the embryonic late morphogenesis stage. Bright-field image of an embryo (left) and GFP image of the same embryo (right). (H) Oil-Red-O staining in the intestine of a wild-type animal (top) and ncx-8(gk324217) mutant animal (bottom). The ncx-8(gk324217) mutant exhibits less Oil-Red-O staining. Oil-Red-O is used as a read-out for lipid accumulation in C. elegans (O’Rourke et al. 2009). Bar, 100 μm. (I) Quantification of Oil-Red-O staining in wild-type, ncx-8(gk234217) mutant animals, wild-type animals that were fed an empty control vector (L4440), and wild-type animals that were fed bacteria expressing dsRNA targeting the ncx-8 locus. In the case of the ncx-8(gk234217) mutants and the animals that were fed RNAi clones targeting the ncx-8 locus, the average Oil-Red-O staining is significantly lower when compared to wild-type animals or wild-type animals that were fed the control RNAi vector. P-values were calculated using the Student’s t-test. Error bars represent the S.E.M.; *P < 0.05. (J) Longevity assays of ncx-8(gk234217) mutant animals and wild-type animals at 20°C (left), and longevity assays of wild-type animals cultivated on control RNAi plates and RNAi plates targeting the ncx-8 locus (right). In each case a significant difference in survival was observed as compared with controls: P < 0.005 for ncx-8 mutants vs. wild-type animals at 20°C, and P < 0.005 for wild-type animals cultivated on control RNAi plates compared with wild-type animals cultivated on ncx-8 RNAi plates at 20°C. The P-values were calculated from log-rank tests.
Discussion
Here we analyze the family of Na+/Ca2+ exchangers in C. elegans. Our analysis places these transporters into a phylogenetic framework, provides a comprehensive guide to their expression patterns, and reveals details about their functional roles through mutant analysis. From these data it is clear that nematode NCX, NCKX, and CCX exchangers are widely expressed and evolutionarily conserved. Current data from mammalian NCX exchangers show that these proteins function in muscles, neuronal cells, and renal tissue. Mammalian NCX1 exhibits expression in cardiac muscle, neuronal tissue, and the kidneys (Philipson et al. 2002; Nicoll et al. 2007; Valsecchi et al. 2013). NCX2 and NCX3 are expressed in skeletal muscle and neuronal cells (Li et al. 1994; Nicoll et al. 1996; Lytton 2007). The mammalian NCKX exchangers are more widely expressed in various cell types including photoreceptor cells, retinal ganglion cells, platelets, vascular smooth muscle, uterine tissue, intestinal cells, lung tissue, thymus, and epidermal cells (Lytton et al. 2002; Cai and Lytton 2004a; Lytton 2007; Visser and Lytton 2007; Altimimi et al. 2013). The CCX protein in humans is also very widely expressed in neurons, cardiac cells, skeletal muscle, lungs, kidneys, intestinal cells, and testes (Cai and Lytton 2004b). There are examples of tissue and cell-type specificity within the NCKX branch of mammals: for example, NCKX2 has been reported only in photoreceptors cells, retinal ganglion cells, and other neurons of the brain (Li et al. 1994). So within both NCX and NCKX there are broadly utilized exchangers and also regionally specified exchangers. Within the C. elegans Na+/Ca2+ exchanger family we observe a similar pattern. Both NCX and CCX exchangers exhibit diverse expression patterns, but within each branch there are also more narrowly tuned exchangers. For example, the NCX gene ncx-2 was detected only in muscle cells, while ncx-1 and ncx-3 were observed only in neuronal cell types. Similarly within the CCX branch, the ncx-8 gene was observed in both muscle cells and intestinal cells. The other CCX genes, ncx-6, ncx-7, ncx-9, and ncx-10, were observed in both neuronal cells and other cell types (Table 1). Interestingly, in the case of the NCKX genes we observed tissue specificity as ncx-4 and ncx-5 were observed only in neuronal cells. Overall, the wide expression pattern observed for mammalian Na+/Ca2+ exchangers is also true for C. elegans and shows a similar overall bias toward excitable cells. Within Drosophila there are three characterized Na+/Ca2+ exchangers, two of which belong to the NCKX branch and one of which belongs to the NCX branch. In the case of the NCKX gene nckx-x (nckx-x-SC in Figure 1A), neuronal expression has been observed throughout the nervous system and also in the eye-antennal disc, mushroom body, embryonic brain, and ventral nerve cord (Winkfein et al. 2004). The NCKX gene nckx30c is also widely expressed in neuronal cells of the brain and also in the craniofacial tissue, renal cells, and abdomen (Haug-Collet et al. 1999). The NCX gene in Drosophila, Calx, has been reported to be expressed only in photoreceptor cells (Schwarz and Benzer 1997; Webel et al. 2002). Through our phylogenetic analysis we also detected four uncharacterized CCX candidates in Drosophila (Figure 1A), and the precise expression of these genes is yet to be determined. By examining the family of Na+/Ca2+ exchangers in three different systems, we now have data suggesting that Na+/Ca2+ exchangers are represented by both a broadly tuned more general cohort and a more narrowly tuned and specified subset. However, exactly how these differences are regulated and how these differences in expression pattern map onto functional specializations is an unresolved question.
Supplementary Material
Acknowledgments
We thank Anthony LaMantia, John Hawdon, Sally Moody, Mary Ann Stepp, and Piali Sengupta for advice and insightful comments; Theresa Stiernagle and Aric Daul for the CGC strains used; the Moerman and Waterston labs and Million Mutation Project team for mutants; our anonymous reviewers for suggestions and feedback; and Thomas Maynard and Anastas Popratiloff for imaging advice. Funding was provided by The George Washington University Department of Biological Sciences and Columbian College of Arts and Sciences (to V.S., C.H., Z.M., and D.O.); The George Washington University Luther Rice Undergraduate Research Fellowship (to E.B.); and The George Washington University Department of Biological Sciences Wilbur V. Harlan Scholarship Trust (to J.S.).
Footnotes
Communicating editor: P. Sengupta
Literature Cited
- Altimimi H. F., Szerencsei R. T., Schnetkamp P. P., 2013. Functional and structural properties of the NCKX2 na(+)-ca (2+)/K (+) exchanger: a comparison with the NCX1 na (+)/ca (2+) exchanger. Adv. Exp. Med. Biol. 961: 81–94. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Lipp P., Bootman M. D., 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1: 11–21. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Bootman M. D., Roderick H. L., 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4: 517–529. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Lipp P., Berridge M. J., 2001. The organisation and functions of local ca(2+) signals. J. Cell Sci. 114: 2213–2222. [DOI] [PubMed] [Google Scholar]
- Cai X., Lytton J., 2004a The cation/ca(2+) exchanger superfamily: phylogenetic analysis and structural implications. Mol. Biol. Evol. 21: 1692–1703. [DOI] [PubMed] [Google Scholar]
- Cai X., Lytton J., 2004b Molecular cloning of a sixth member of the K+-dependent na+/Ca2+ exchanger gene family, NCKX6. J. Biol. Chem. 279: 5867–5876. [DOI] [PubMed] [Google Scholar]
- Coates J. C., de Bono M., 2002. Antagonistic pathways in neurons exposed to body fluid regulate social feeding in Caenorhabditis elegans. Nature 419: 925–929. [DOI] [PubMed] [Google Scholar]
- Crawford D., Libina N., Kenyon C., 2007. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell 6: 715–721. [DOI] [PubMed] [Google Scholar]
- Gray J. M., Karow D. S., Lu H., Chang A. J., Chang J. S., et al. , 2004. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430: 317–322. [DOI] [PubMed] [Google Scholar]
- Gray J. M., Hill J. J., Bargmann C. I., 2005. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102: 3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug-Collet K., Pearson B., Webel R., Szerencsei R. T., Winkfein R. J., et al. , 1999. Cloning and characterization of a potassium-dependent sodium/calcium exchanger in Drosophila. J. Cell Biol. 147: 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilge M., 2012. Ca2+ regulation of ion transport in the na+/Ca2+ exchanger. J. Biol. Chem. 287: 31641–31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Matsuoka S., Hryshko L. V., Nicoll D. A., Bersohn M. M., et al. , 1994. Cloning of the NCX2 isoform of the plasma membrane na(+)-Ca2+ exchanger. J. Biol. Chem. 269: 17434–17439. [PubMed] [Google Scholar]
- Lytton J., 2007. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 406: 365–382. [DOI] [PubMed] [Google Scholar]
- Lytton J., Li X. F., Dong H., Kraev A., 2002. K+-dependent na+/Ca2+ exchangers in the brain. Ann. N. Y. Acad. Sci. 976: 382–393. [DOI] [PubMed] [Google Scholar]
- Nicoll D. A., Quednau B. D., Qui Z., Xia Y. R., Lusis A. J., et al. , 1996. Cloning of a third mammalian na+-Ca2+ exchanger, NCX3. J. Biol. Chem. 271: 24914–24921. [DOI] [PubMed] [Google Scholar]
- Nicoll D. A., Ren X., Ottolia M., Phillips M., Paredes A. R., et al. , 2007. What we know about the structure of NCX1 and how it relates to its function. Ann. N. Y. Acad. Sci. 1099: 1–6. [DOI] [PubMed] [Google Scholar]
- Nicoll D. A., Ottolia M., Goldhaber J. I., Philipson K. D., 2013. 20 years from NCX purification and cloning. Milestones. Adv. Exp. Med. Biol. 961: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke E. J., Soukas A. A., Carr C. E., Ruvkun G., 2009. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 10: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolia M., Philipson K. D., 2013. NCX1: Mechanism of transport. Adv. Exp. Med. Biol. 961: 49–54. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Nicoll D. A., 2000. Sodium-calcium exchange: A molecular perspective. Annu. Rev. Physiol. 62: 111–133. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Nicoll D. A., Ottolia M., Quednau B. D., Reuter H., et al. , 2002. The na+/Ca2+ exchange molecule: An overview. Ann. N. Y. Acad. Sci. 976: 1–10. [DOI] [PubMed] [Google Scholar]
- Sambongi Y., Nagae T., Liu Y., Yoshimizu T., Takeda K., et al. , 1999. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in caenorhabditis elegans. Neuroreport 10: 753–757. [DOI] [PubMed] [Google Scholar]
- Schwarz E. M., Benzer S., 1997. Calx, a na-ca exchanger gene of drosophila melanogaster. Proc. Natl. Acad. Sci. USA 94: 10249–10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., 1990. Genetic analysis of defecation in Caenorhabditis elegans. Genetics 124: 855–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidow H., Poulsen L. R., Andreeva A., Knudsen M., Hein K. L., et al. , 2012. A bimodular mechanism of calcium control in eukaryotes. Nature 491: 468–472. [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Chou J. H., Dwyer N. D., Colbert H. A., Bargmann C. I., 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218. [DOI] [PubMed] [Google Scholar]
- Valsecchi V., Pignataro G., Sirabella R., Matrone C., Boscia F., et al. , 2013. Transcriptional regulation of ncx1 gene in the brain. Adv. Exp. Med. Biol. 961: 137–145. [DOI] [PubMed] [Google Scholar]
- Visser F., Lytton J., 2007. K+ -dependent na+/Ca2+ exchangers: Key contributors to Ca2+ signaling. Physiology (Bethesda) 22: 185–192. [DOI] [PubMed] [Google Scholar]
- Wang M. C., O’Rourke E. J., Ruvkun G., 2008. Fat metabolism links germline stem cells and longevity in C. elegans. Science 322: 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel R., Haug-Collet K., Pearson B., Szerencsei R. T., Winkfein R. J., et al. , 2002. Potassium-dependent sodium-calcium exchange through the eye of the fly. Ann. N. Y. Acad. Sci. 976: 300–314. [DOI] [PubMed] [Google Scholar]
- Winkfein R. J., Pearson B., Ward R., Szerencsei R. T., Colley N. J., et al. , 2004. Molecular characterization, functional expression and tissue distribution of a second NCKX na+/Ca2+ -K+ exchanger from drosophila. Cell Calcium 36: 147–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.