Abstract
Pomegranate has been shown to prolong PSA doubling time in early prostate cancer, but no data from a placebo controlled trial has been published yet. The objective of this study was to prospectively evaluate the impact of pomegranate juice in patients with prostate cancer.
We conducted a phase IIb, double blinded, randomized placebo controlled trial in patients with histologically confirmed prostate cancer. Only patients with a PSA value ≥ 5ng/ml were included. The subjects consumed 500 ml of pomegranate juice or 500 ml of placebo beverage every day for a 4 week period. Thereafter, all patients received 250 ml of the pomegranate juice daily for another 4 weeks. PSA values were taken at baseline, day 14, 28 and on day 56. The primary endpoint was the detection of a significant difference in PSA serum levels between the groups after one month of treatment. Pain scores and adherence to intervention were recorded using patient diaries.
102 patients were enrolled. The majority of patients had castration resistant prostate cancer (68%). 98 received either pomegranate juice or placebo between October 2008 and May 2011. Adherence to protocol was good, with 94 patients (96%) completing the first period and 87 patients (89%) completing both periods. No grade 3 or higher toxicities occurred within the study. No differences were detected between the two groups with regard to PSA kinetics and pain scores.
Consumption of pomegranate juice as an adjunct intervention in men with advanced prostate cancer does not result in significant PSA declines compared to placebo.
Keywords: Pomegranate juice, PSA, prostate cancer, nutraceutical, ellagig acids, polyphenols.
Introduction
Prostate cancer is the most commonly diagnosed cancer in men accounting for 29% of all male cancers and is the second leading cause of cancer-related death in the United States (American Cancer Society: Cancer Facts and Figures 2011. Atlanta, Ga: American Cancer Society, 2011). Therefore, the identification of effective preventive strategies for prostate cancer is of particular clinical importance. Since free radicals seem to play a pivotal role in the development of prostate cancer reduction of intracellular free radicals by antioxidants hold promise for disease prevention. However, in a large randomized trial (SELECT) the anti-oxidant vitamin E has failed to show any benefit in the prevention of prostate cancer 1. Polyphenols are anti-oxidants that have been shown to positively influence inflammation and cancer 2, 3. A study utilizing capsules containing polyphenols equivalent to 12 cups of green tea found a significant reduction in serum levels of PSA, hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) in men with prostate cancer after a short treatment period 4. Pomegranate juice (PJ) is a natural source of bioavailable 5 ellagitannins and has been demonstrated to reduce redox activity 6. As compared to red wine or green tea, pomegranate juice from commercial sources has an antioxidant capacity that is 3 times greater 7.
Pomegranate extracts contain ellagic acid, caffeic acid, luteolin and punicic acid, all of which have been associated with anti-cancer effects in preclinical models 6, 8.
Ellagic acids are considered the most active component of PJ, accounting for more than 50% of its antioxidant effects 7. In a carcinogenic mouse model (TRAMP) oral pomegranate extract supplementation results in significant tumor control by inhibiton of IGF-I/Akt/mTOR pathways in the prostate tissues and tumors 9. Pantuck and colleagues performed a single center, open-label study, examining the effect of PJ in men with rising PSA levels after surgery or radiation 10. The investigators found an impressive increase in the PSA doubling time (PSADT) from 15 to 54 months under the treatment (p< 0.001). Another study 11 tested pomegranate juice extract capsules in men with rising PSA levels after local therapy for prostate cancer (NCT01220817). Again, a statistically significant increase of the PSADT for one or three capsules daily (overall 11.9 to 18.5 months, p<0.001) was observed. In both studies a relevant proportion of men even showed a decrease of PSA serum levels due to consumption of PJ (15% and 13%). An on-going phase III study (NCT00413530) is examining whether the pomegranate liquid extract prolongs PSADT in patients who have rising serum PSA levels after primary therapy for localized prostate cancer. That randomized, double-blind, placebo-controlled study with an estimated enrollment of 200 patients was scheduled to close on December 2012. All of the above studies have focused on the role of PJ in patients with early biochemical failure after initial treatment. No data about a therapeutic impact of PJ in patients with more advanced or metastatic prostate cancer has been published yet. A standard procedure for patients having failed hormone ablation therapy would be the initiation of chemotherapy 12, 13. In the absence of symptoms the time point of starting chemotherapy is debatable hence patients and physicians often prefer to delay the start of this rather aggressive treatment. In our trial we report the influence of PJ compared to placebo in a cohort of asymptomatic or oligosymptomatic patients, the majority (68%) of which had castration resistant prostate cancer.
Patients and Methods
Eligibility criteria
Patients with histologically proven prostate cancer and a pathologic serum PSA (entry level at least 5 ng/ml) were eligible for the study. While patients were on study they had to continue their baseline treatment (e.g. androgen deprivation, zoledronic acid). The initiation of any new potential active treatment was not allowed. Exclusion criteria were any planned therapeutic intervention such as surgery, irradiation or any alteration of the ongoing therapy, e.g. withdrawal of medication or introduction of new drugs during the study period.
Design and assessments
The study was conducted at two Swiss centers (University Hospital Zurich and KS Graubünden) and designed as a phase IIb, double-blind, placebo-controlled randomized trial. Consenting patients with a documented PSA above 5 ng/ml and histologically confirmed prostate cancer were randomized into two groups. Group A received 500 ml placebo juice per day and Group B 500 ml of PJ (equivalent to 1147 mg polyphenol gallic acid) per day. PSA serum levels were measured the day before treatment started, and on days 14 and 28. Patients were given a diary to document their daily juice intake, any adverse events and their pain score with time and date. The intensity of pain was self-documented by the previously instructed patients by choosing a value from a non-linear scale (range 0-10). At day 29 patients entered the open label phase of the trial. In this second period of the study, 250 ml of PJ (equivalent to 573 mg polyphenol gallic acid) was consumed by all patients (bottles labelled C) and the diaries were continued as before. A final PSA serum value was taken on day 56. While on study, especially in the first period, patients were not informed about their current PSA values. No other diagnostics, e.g. CT-scans or laboratory values were part of this study. The relatively short observational period of 8 weeks was chosen because the projected patient collective often converts from watchful waiting to intervention due to disease progression.
Treatment
Block randomization (block size 4) was used to assign patients to the treatment groups 500 ml PJ or placebo daily. The patients were instructed to drink one bottle of 500 ml per day; the timing was left to the patient´s discretion. Consumption was self- monitored by daily recording using diaries. In addition, episodes of pain and usage of analgesics were also to be recorded by the patients in their diary.
The producer of the beverage (Biotta AG, Egnach, Switzerland) provided the pomegranate juice and the placebo. Both beverages had a very similar taste and color (Supplementary figure 1). The juices contained the same basic ingredients, pear purée, white tea, agave concentrate and aronia berry juice. In the placebo drink the 27.5% pomegranate extract was replaced with an artificial pomegranate flavoring substance. The pomegranate juice contained 2294 mg/l polyphenol gallic acid. The daily intake in the intervention arm was 1147 mg/day polyphenol gallic acid during the first study period and 573 mg/day polyphenol gallic acid in the second period of the study.
The company supplied PJ and placebo and gave limited financial support. The company had neither influence on the acquisition of the data nor on the statistical analysis. The investigational sites received identical bottles labelled with A, B and C. Bottles A and B were distributed to the patients according to the predefined randomization scheme in the first study period. Bottles labelled C were given to all patients in the second study period. Information which letter corresponded to pomegranate or placebo was kept secret at the company and was not revealed to the investigators and the study personnel before the end of the study. Unblinding was not done before all statistical analysis had been performed.
PSA testing
The majority of patients´ PSA serum levels (75%) was determined by a third generation assay (chemiluminescence-enzyme-immunoassay, third generation Immulite 2500, Siemens healthcare diagnostics, Eschborn, Germany) at the Institute of Clinical Chemistry, University Hospital Zurich, Switzerland. All patient sera from Chur (n=23) were tested with an assay according to the ECLIA principle (ElectroChemiLuminescenceImmunoAssay, cobas e411, Roche diagnostics, Mannheim, Germany) at the central laboratory of Kantonsspital Graubünden, Chur, Switzerland.
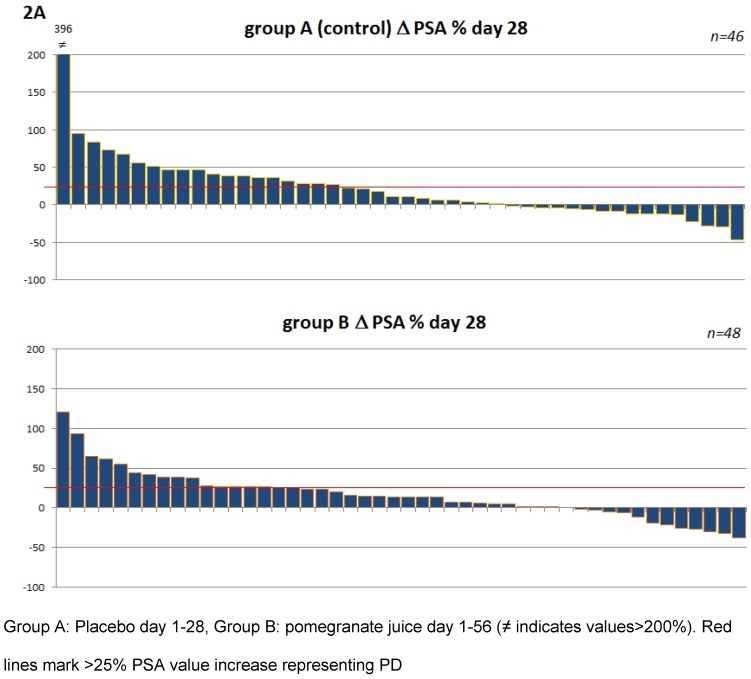
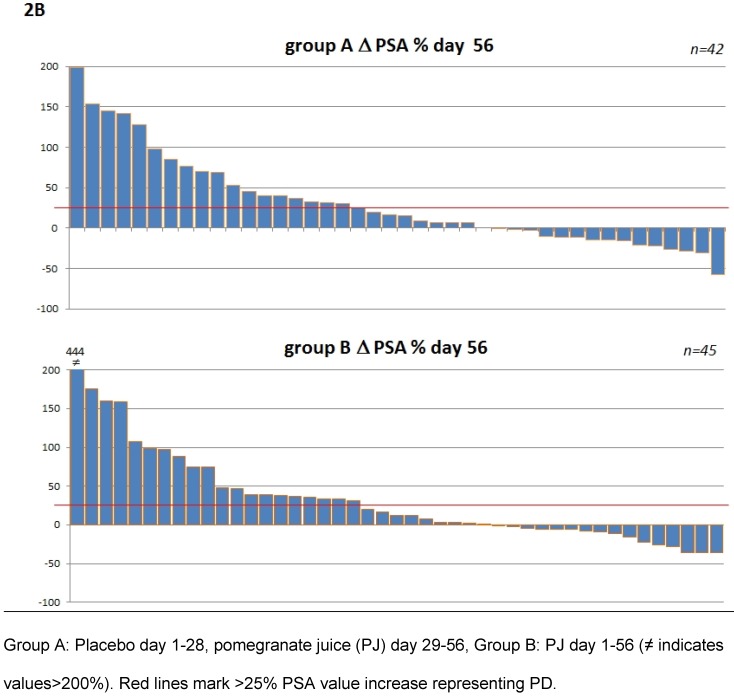
For the report of this study PSA progression was defined as PSA increase ≥25% 14 and PSA response was defined as a decline of PSA ≥50% from baseline according to the recommendations from the PSA working group 15. Patients with a PSA decline >30% are also reported here, as a PSA decrease of >30% under active treatment within the SWOG 99-16 study has been associated with a 50% reduction in the risk of death from prostate cancer 16. Following the recommendation of the Prostate Cancer Clinical Trials Working Group PSA responses of the individual patients are shown in waterfall plots 17.
Statistical analyses
Fisher's exact test was used to compare categorical data between groups and Mann-Whitney test for continuous data. Applying Wilcoxon signed rank test PSA levels between follow-up and baseline in the same group were evaluated. The aim was to show a difference of change scores before and after intervention as well as before and in between the treatment. With an estimation of 47 patients in each arm the study was calculated to have a power of 85% to detect a difference of 5ng/ml (the difference of a group A mean of 0 ng/ml and group B of - 5 ng/ml) under the assumption of a standard deviation of 8 ng/ml, if a two group t-test with a double sided significance of 0.05 was used. Statistical comparisons of patient characteristics were performed with Graphpad Prism 5 software (Graphpad Inc. La Jolla, USA).
Ethics and registration
This study was approved by the local Ethics Committee (# EK-1545) and registered at “Deutsches Krebsstudienregister der Deutschen Krebsgesellschaft” (www.studien.de) under the study ID No.555.
Results
Patient characteristics
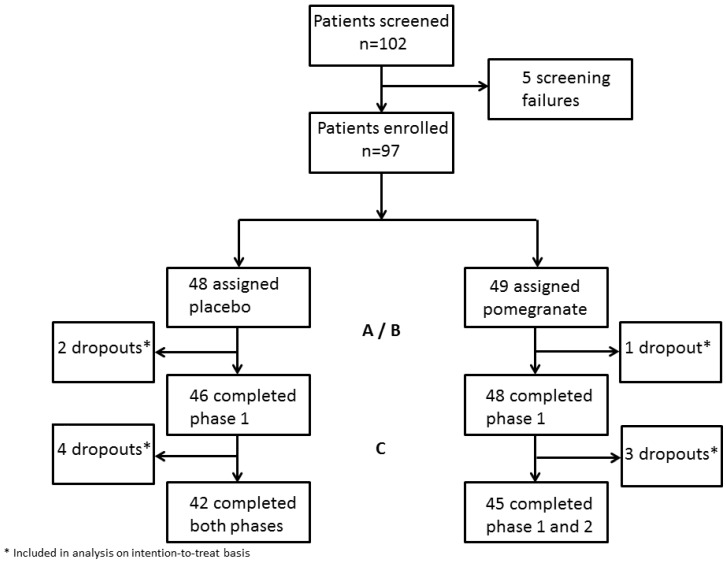
Between October 2008 and May 2011 a total of 102 patients with prostate cancer gave written consent and were enrolled. Of those, 5 patients were found ineligible as they did not fulfill the inclusion criteria. The trials profile is shown in Figure 1. Baseline characteristics of the 97 patients in the full analysis population are summarized in Table 1. With respect to ongoing treatment, 38% (18/47) of patients in the placebo group and 46% (22/48) in the verum group were under anti-hormonal treatment (LHRH analogues). Some patients received bicalutamid in combination or as a sole antihormonal treatment (22% in group A and 27% in group B). Low dose steroids (prednisone 5-10mg) were recorded in 4% of all patients (2% vs. 6%). 27% (25/94) study patients received no treatment but were under a watch and wait strategy. No patient received chemotherapy or underwent surgery or irradiation while on study. Statistically significant differences in tumor size (T3; 31% in the control vs. 51% in the intervention group, p=.036) and distant tumor spread (M1; 18% in controls vs. 44%, p=.032) at the time of initial diagnosis were observed (Table 1). Apart from these two variables the study was well balanced.
Figure 1.
Trial profile.
Table 1.
Patients' characteristics.
| Characteristics | Placebo (n= 46) | Pomegranate (n= 48) | P |
|---|---|---|---|
| Age (y) | 72±8.4 | 73±8.6 | NS |
| Age > 64 yrs | 40 | 36 | NS |
| Age > 69 yrs | 29 | 29 | NS |
| ECOG PS 0 | 74% | 75% | NS |
| ECOG PS 1 | 20% | 21% | NS |
| ECOG PS 2 | 7% | 4% | NS |
| Gleason Score <8 | 57% (20/35) | 46% (16/35) | NS |
| Gleason Score ≥8 | 43% (15/35) | 54% (19/35) | NS |
| TNM at diagnosis | |||
| T0 | 4% (1/29) | 0% (0/35) | NS |
| T1 | 17% (5/29) | 17% (6/35) | NS |
| T2 | 41% (12/29) | 17% (6/35) | NS |
| T3 | 31% (9/29) | 51% (18/35) | .036 |
| T4 | 7% (2/29) | 15% (5/35) | NS |
| N0 | 78% (22/28) | 55% (17/31) | NS |
| N1 | 22% (6/28) | 45% (14/31) | NS |
| M1 | 18% (5/28) | 44% (15/34) | .032 |
| At study entry | |||
| PSA (Median, Mean, SD) | 19, 90±222 ng/ml | 21, 60±82 ng/ml | NS |
| CRPC | 67% (31/46) | 63% (30/48) | NS |
| Treatment (previous and ongoing)* | |||
| RP | 17% (8/46) | 15% (7/48) | NS |
| RT | 24% (11/46) | 17% (8/48) | NS |
| RP+RT | 22% (10/46) | 11% (5/48) | NS |
| Watchful waiting | 26% (13/46) | 29% (14/48) | NS |
| ADT (continued) | 39% (18/46) | 46% (22/48) | NS |
| Docetaxel | 15% (7/46) | 10% (5/48) | NS |
NS= not significant, CRPC= castration resistant prostate cancer.
Data presented as mean ± standard deviation or n (%).
n=0 for ECOG> and n=0 for Gleason Score <5 and n=0 for N3 status.
RP radical prostatectomy; RT, radiotherapy; ADT, Androgen deprivation therapy.
TNM documented at diagnosis.
* percentages in the treatment section do not add up to 100%, as patients may have had multiple interventions.
Safety
Pomegranate juice was generally well tolerated with no grade 3 or 4 toxicities. Bowel disturbances were the most frequent adverse event reported. Obstipation was observed in both groups: 2/46 (4%) in the placebo group and 1/48 (2%) in group B. One patient on placebo withdrew his consent due to a CTC grade 2 obstipation. One patient in the PJ group reported a CTC grade 1 diarrhea (1/48; 2%) but no diarrhea was reported from patients in the placebo arm.
The adherence to the protocol was good, with 94 patients (96%) completing the first period (days 1-28) and 87 patients (89%) completing both periods (days 1-56). Three patients dropped out within the first 28 days due to unrelated medical problems. The other seven patients were unable or unwilling to complete the second period. Thus, 94 patients were eligible for analysis of the randomized part of the study and 87 patients (42 patients in the placebo group A or 45 in the PJ group B) completed the full trial protocol. 45 patients of group B could be analyzed for both dosages of the pomegranate juice.
Efficacy
The number of patients experiencing a PSA decrease during the placebo-controlled period of the study was not statistically significant between the two arms (table 2). PSA progression within the first four weeks was observed in 41% in the control group compared to 38% in the pomegranate group (p=0.83). There were no responses with PSA decline >50% in either group, but 1 patient in the placebo group and 3 patients in the pomegranate group showed a decline ≥30% (Table 2A). In the second period of the study, when all patients consumed 250 ml of PJ, PSA progression was observed at a rate of 24% in the former placebo group and 29% in those patients that had been given 500 ml PJ in the first period (group B), (p=0.63, Table 2A). Despite the nominal reduction of patients with PSA progression in the placebo group the difference between the non-interventional period (day 1-28) and the pomegranate consumption period (day 29-57) was not significant (p=0.11).
Table 2.
Response evaluation.
| treatment | PSA Progression | PSA Stabilisation | response* |
|---|---|---|---|
| 2A | |||
| intention to treat | |||
| Day 1-28** | |||
| A Placebo (n=46) | 19 (41%) | 26 (57%) | 0 (0%), 1 (2%) |
| B PJ high (n=48) | 18 (38%) | 27 (56%) | 0 (0%), 3 (6%) |
| Day 28-56 | |||
| A PJ low (n=42) | 10(24%) | 31 (74%) | 0 (0%), 1 (2%) |
| B PJ low (n=45) | 13 (29%) | 31 (69%) | 0 (0%), 1 (2%) |
| Day 1-56 | |||
| A Placebo+PJ low (n=42) | 18 (43%) | 21 (53%) | 1 (2%), 1 (2%) |
| B PJ high+low (n=45) | 18 (40%) | 24 (53%) | 0 (0%), 3 (7%) |
| 2B | |||
| patients with CRPC | |||
| Day 1-28** | |||
| A Placebo (n=31) | 16 (52%) | 14 (45%) | 0 (0%), 1 (3%) |
| B PJ high (n=30) | 12 (40%) | 16 (53%) | 0 (0%), 2 (7%) |
| Day 28-56 | |||
| A PJ low (n=27) | 10(37%) | 16 (59%) | 0 (0%), 1 (4%) |
| B PJ low (n=28) | 12 (43%) | 15 (54%) | 0 (0%), 1 (3%) |
| Day 1-56 | |||
| A Placebo+PJ low (n=27) | 15 (56%) | 11 (40%) | 1 (4%), 0 (0%) |
| B PJ high+low (n=28) | 16 (57%) | 11 (39%) | 0 (0%), 1 (4%) |
| 2C | |||
| patients without CRPC | |||
| Day 1-28** | |||
| A Placebo (n=15) | 3(20%) | 12 (80%) | 0 (0%), 0 (0%) |
| B PJ high (n=18) | 4 (22%) | 12 (67%) | 0 (0%), 2 (11%) |
| Day 28-56 | |||
| A PJ low (n=15) | 3(20%) | 11 (73%) | 1 (7%), 0 (0%) |
| B PJ low (n=17) | 1 (6%) | 16 (94%) | 0 (0%), 0 (0%) |
| Day 1-56 | |||
| A Placebo+PJ low (n=15) | 3(20%) | 11 (73%) | 0 (0%), 1 (7%) |
| B PJ high+low (n=17) | 3 (17%) | 12 (71%) | 0 (0%), 2 (12%) |
*Objective response (PSA) defined as PSA decline >50% in bold, responses ≥ 30% in italics **Day 1-28 represents the placebo-controlled phase of the studyProgressive disease was defined as a PSA increase of ≥ 25%Stable disease were all PSA values between OR and PD.
PJ high refers to a daily intake of 1147 mg/day polyphenol gallic acid (first period) PJ low 573 mg/day polyphenol gallic acid consumed by all subjects from day 28 on (second period)
p-values not displayed as there were no significant differences
To address the question whether heterogeneity within the patient populations may have had an influence subgroup analysis were performed (Table 2B and 2C). Patients were pooled according to their status of disease at study entry e.g. castration resistant prostate cancer (CCRPC) (n=61) and non CCRPC (n=33). These analyses as shown in table 2B and 2C revealed no difference for PJ or placebo in patients with regard to their hormonal response. The corresponding waterfall plots depicting individual PSA changes are shown in figure 2.
Figure 2.
Waterfall plots of percentage PSA changes from patients within the first period (day 1-28) 2A and the total period (day 1-56) 2B.
A combined PSA level analysis of both study periods showed PSA stabilization in 74% of patients starting with placebo versus 71% of those patients that had been under PJ continuously. The patients reported pain values in the range from 0-5 at study entry without significant group differences (mean pain score A=0.97 and B=0.98; p=0.49). After the randomized phase of the study, at day 28, no relevant pain decrease was found in either group. A non-significant trend toward lower pain scores was seen in group B (mean day 1: 0.98 ±1.25 versus mean day 28: 0.62 ±1.13; p=0.092).
Discussion
The objective of this study was to assess the impact of pomegranate juice on PSA levels in patients with advanced prostate cancer. There were no significant differences with regard to PSA levels or pain intensity between the observed groups in this trial. The study design included two treatment periods: Initially, patients were randomized to placebo (group A) or pomegranate juice consumption (group B). The results from the double-blinded, randomized controlled part of the study show no differences with regard to PSA level rise or decline, pain, frequency of dropout or adverse effects. The fact that 35% of men in the control group and 25% in the intervention group had a decline in their PSA value during the first phase of the study is remarkable. A possible explanation for this phenomenon could be a natural fluctuation of serum PSA levels in a four-week period in men with advanced prostate cancer that has not been recorded to this extend until now. The latter is particularly important when interpreting results from earlier and on-going studies that report on declining PSA levels due to specific interventions. An alternative explanation for the high percentage of men experiencing a PSA decline in both groups could be an unanticipated PSA test-to-test variability. With regard to this, an intermediate PSA level measurement had been predefined in the study at day 14. The majority of PSA levels obtained at day 14 and at day 28 were in accordance with the individual PSA course, e.g. if day 14 and 28 values were not pointing in the same direction, no more than a 10% difference of the day 14 value versus one of the other values was observed. Furthermore, test variability was controlled by processing all individual blood samples at the enrolling institution. Thus, there was no variability regarding the test used or the laboratory where the set of four patient's samples was analyzed. We conclude that test instability did not influence the final results. Another factor in this study that might have had an impact on patients with declining PSA values regardless of their group was the controlled intake of 500 ml of fluids (either pomegranate juice or placebo) in addition to other daily fluids. In the absence of a non-interventional control this influence cannot be ruled out. Regarding the value of pomegranate in the setting of advanced prostate cancer this variable plays no role as it appeared in both groups.
Due to the design of a verum-containing treatment period for all patients (second part of the trial) we were able to analyze PSA levels of all men who started with placebo in comparison to the PSA levels after a subsequent four-week consumption phase of 250 ml of PJ, equivalent to 573 mg/ml phenol gallic acids (Table 2A placebo day 1-28 versus placebo day 28-56 panel). Here, no difference between the placebo and the verum period in group A was detected (41% vs. 24% progress, 57% vs. 74% stabilization, 2% vs. 2% response; p=0.08) indicating that the pomegranate ingredients of the drink were not responsible for any change.
The absolute PSA level declines observed in this study did not reach clinical significance in either cohort when a PSA level decrease of ≥ 50% is defined as objective response. Overall, 5 responses of PSA level decline ≥ 30%, with a non-significant difference between the groups, were recorded at the end of the randomized part of the trial.
With respect to the impact of pomegranate juice in early prostate cancer patients there have been two reports 11 and a phase III study is currently underway with reports expected in 2012 (NCT00413530). Table 3 illustrates the differences between the clinical data available to date. The major difference of the studies is that the two previously reported studies in early prostate cancer patients focussed on PSADT and had longer observational periods. Within the context of low PSA values (<5 ng/ml, median 2.2) and minimal active disease this is an adequate approach. Our study included patients with more advanced disease and higher PSA values (median 19.7 ng/ml). These patients are often to start their next therapy within a short period of time. Thus, these patients cannot be observed for an extended time period. Hence, PSADT would not have been a good end point. However, with a half-life of PSA of 2.2 days (18) we calculated that any meaningful changes in the PSA course of these patients should be readily detectable by 4 independent measurements at least 14 days apart and within a follow-up of two months.
Table 3.
Comparison of this study with previously reported clinical studies.
| Criteria | Pantuck et al. | Paller et al. | Stenner et al. |
|---|---|---|---|
| Study design | Single arm Phase II | 2 intervention Arms | Placebo controlled |
| Observation period | 18months/progression | 18months/progression | 2months |
| PSA (ng/ml) | 0.5-5 | 0.5-5 | >5 |
| [Median; Mean,SD] | [1.05; 2.23±2.58] | [NR] | [19.7; 74.5±166] |
| Metastatic disease allowed | No | No | Yes[23%] |
| Grading | Gleason ≤7 | Gleason any | no restriction |
| [% Gleason ≥8] | [0%] | [10%] | [49%] |
| Pretreatment allowed | S, RT | S, RT, B, C | S, RT, B, C |
| PJ, Gallic Acid equivalent | 570mg/ml (8 oz.) | 1 and 3 Caps* | 1146mg/ml (phase1) |
| Daily intake | (1 caps= 8 oz.) | 573mg/ml (phase2) | |
| Duration of treatment | 12 months | 18months+ | 2months |
Abbreviations: S, surgery; RT, radiotherapy; B, brachytherapy; C, cryotherapysquare brackets [] indicate data collected within respective study. NR data not reported
*One POMx capsule is equivalent to an 8 oz. glass of juice
An unexpected limitation of our study is a certain heterogeneity of the included patient cohort. For example the recorded number of metastases in our study seems to indicate an imbalance in favor of the placebo arm. In this regard it has to be considered that no imaging diagnostics were performed at study entry. Baseline diagnostics included clinical examination and PSA level determination. Metastatic status was derived from previous clinical reports. Data concerning initial metastases (M1 at operation) was available in 58% (A) and 69% (B) of patients. The apparent imbalance could be due to under-diagnosing or under-reporting in the placebo group. Further supporting this notion is the fact that the average entry PSA level showed no difference with a median of 19 ng/ml (mean 90) in the placebo group and a median of 21 ng/ml (mean 60) in the interventional arm. When we examined responses of those men with castration resistant prostate cancer only (68% of all study participants), no difference between the groups was noted (Table 2B). Taken together, this imbalance, if present, should have had no impact on the results.
The molecular pathway of pivotal importance in prostate cancer is the IGF-1/AKT/mTOR pathway. Bi-allelic loss of PTEN in about 50% of metastatic prostate cancers 19 results in over activation of this pathway, rendering targeted therapy an attractive concept. Everolimus like pomegranate is an inhibitor of the IGF-1/AKT/mTOR pathway (9). Single agent everolimus in a patient collective comparable to the one in this study has yielded PSA response rates in the range of 3% (PSA decrease ≥50%) and 11% (≥30%) 20. This result indicates some activity but also the need of refining the therapeutic approaches directed against this pathway. In a preclinical model IGF-1/AKT/mTOR stimulation drives cancer cells into hormone insensitivity and leads to neuroendocrine differentiation 21. This could explain why everolimus and pomegranate as inhibitors of this pathway may not be very effective in patients that have failed anti-hormonal therapy and should rather be considered earlier in a treatment algorithm.
The analysis of our prospective study shows that pomegranate in the form of a daily beverage does not alter the course of PSA in patients with advanced prostate cancer regardless of the dosage. The maximum dose used here was twice as high as in the previously published study 10. However, the dosage of pomegranate does not seem to be of utmost importance. Two studies 10, 11 have shown no differences in the increase of PSADT when comparing 8 oz to a higher dose of 24 oz (6.9 months versus 5.3 months, p=0.92).
In conclusion, pomegranate beverage had no significant impact on PSA progression in patients with recurrent and advanced prostate cancer when compared to placebo. The hitherto published benefit of pomegranate juice seems to be restricted to early and well differentiated prostate cancer. Our results provide important information for the design and patient selection within further trials with pomegranate.
Supplementary Material
Acknowledgments
We thank Anja Kunz, Gabriela Manetsch-Dalla Torre and Stephan Malzacher for the excellent technical assistance regarding acquisition, processing, and evaluation of the data of the patients.
FSL, HL, RC, PS and MM designed the study, collected and analyzed the data and wrote the manuscript. HS, UP, TS, CR, AK, and RTS participated in the patients´ care, the critical data analysis and the writing of the manuscript.
All authors were involved in the revision process and approved the final manuscript. The study was partially funded by the Biotta AG Company as mentioned in the methods. No conflicts of interest of the authors are present.
Abbreviations
- PCa
prostate cancer
- PJ
pomegranate juice
- PSA
prostate specific antigen
- PSADT
PSA doubling time
- RP
radical prostatectomy
- RT
radiotherapy
- CRPC
castration resistant prostate cancer
- CTC
common toxicity criteria
- PD
progressive disease
References
- 1.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG. et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. doi:2008.864 [pii] 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longtin R. The pomegranate: nature's power fruit? J Natl Cancer Inst. 2003;95:346–8. doi: 10.1093/jnci/95.5.346. [DOI] [PubMed] [Google Scholar]
- 3.Ghiringhelli F, Rebe C, Hichami A, Delmas D. Immunomodulation And Anti-Inflammatory Roles Of Polyphenols As Anticancer Agents. Anticancer Agents Med Chem. 2012. doi:ACAMC-EPUB-20120131-024 [pii] [DOI] [PubMed]
- 4.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. doi:1940-6207.CAPR-08-0167 [pii] 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 5.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–8. doi: 10.1016/j.cccn.2004.04.029. doi:10.1016/j.cccn.2004.04.029 S0009898104002311 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Lansky EP, Harrison G, Froom P, Jiang WG. Pomegranate (Punica granatum) pure chemicals show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across Matrigel. Invest New Drugs. 2005;23:121–2. doi: 10.1007/s10637-005-5856-7. doi:10.1007/s10637-005-5856-7. [DOI] [PubMed] [Google Scholar]
- 7.Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–9. doi: 10.1021/jf000404a. doi:jf000404a [pii] [DOI] [PubMed] [Google Scholar]
- 8.Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A. et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 2004;7:274–83. doi: 10.1089/jmf.2004.7.274. doi:10.1089/1096620041938704. [DOI] [PubMed] [Google Scholar]
- 9.Adhami VM, Siddiqui IA, Syed DN, Lall RK, Mukhtar H. Oral infusion of pomegranate fruit extract inhibits prostate carcinogenesis in the TRAMP model. Carcinogenesis. 2012;33:644–51. doi: 10.1093/carcin/bgr308. doi:bgr308 [pii] 10.1093/carcin/bgr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ. et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–26. doi: 10.1158/1078-0432.CCR-05-2290. doi:12/13/4018 [pii] 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 11.Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, Hertzman BL, Efros MD, Roper RP, Liker HR, Carducci MA. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2012 doi: 10.1038/pcan.2012.20. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. The New England journal of medicine. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. doi:351/15/1502 [pii] 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 13.Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. doi:351/15/1513 [pii] 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 14.Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G. et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27:2450–6. doi: 10.1200/JCO.2008.19.9810. doi:JCO.2008.19.9810 [pii] 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M. et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–7. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 16.Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN Jr. et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98:516–21. doi: 10.1093/jnci/djj129. doi:98/8/516 [pii] 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. doi:26/7/1148 [pii] 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. The New England journal of medicine. 1987;317:909–16. doi: 10.1056/NEJM198710083171501. doi:10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 19.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–74. doi: 10.1038/sj.onc.1209096. doi:1209096 [pii] 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 20.Templeton A, Rothermundt C, Cathomas R, Baertschi D, Droege C, Gautschi O, Borner MM, Fechter E, Stenner F, Winterhalder RC, Mueller B, Dutoit V, Dietrich P, Schiess P, Wild P, Thalmann GN, Klingbiel D, Gillessen S. Everolimus as first-line therapy in nonrapidly progressive metastatic castration-resistant prostate cancer (mCRPC): A multicenter phase II trial (SAKK 08/08) J Clin Oncol. 2011;29(suppl):abstr4588. [Google Scholar]
- 21.Wu C, Huang J. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem. 2007;282:3571–83. doi: 10.1074/jbc.M608487200. doi:M608487200 [pii] 10.1074/jbc.M608487200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.